Open Access Article
Open Access ArticleWater-assisted metal-free catalyzed cyclization of 2-alkynylarylketones: a facile approach to indenones†
Shuai Zhang,
Xue-Ting Bai,
Dan-Yun Chen,
Pei Chen,
Qian-Qian Zhang and
Yan-Bo Wang *
*
Henan Engineering Laboratory of Flame-Retardant and Functional Materials, Institute of Fine Chemistry and Engineering, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, 475004, China. E-mail: wangyanbokf@henu.edu.cn
First published on 16th June 2017
Abstract
A simple and directed synthetic strategy starting from 2-alkynylarylketones was developed for the construction of various indenones under metal-free and water-assisted conditions. This intramolecular cyclization reaction could well tolerate a wide range of functional groups, and the corresponding functionalized indenones were obtained in moderate to excellent yields (up to 94%). In addition, the possible mechanism of this reaction may involve isobenzofuranium intermediates.
Introduction
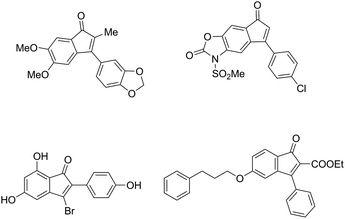
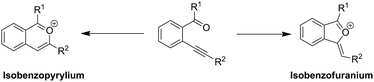
Indenones, prevalent in natural products and materials science, have drawn a considerable amount of attention (Fig. 1).1 Because of the importance of indenones, various synthetic methods for substituted indenones have already been reported. The traditional synthesis of functional indenones included multistep intramolecular Friedel–Crafts acylation or used organometallic reagents.2,3 The use of transition metal species including Rh,4 Co,5 Pd6 or other metals7 as a catalyst has been developed by intermolecular or intramolecular cyclization reactions in recent decades. In contrast, metal-free synthetic strategies for constructing indenones have been less elucidated. Methyl trifluoromethanesulfonate (MeOTf)-mediated annulation of arylnitriles or isothiocyanates with aromatic alkynes to synthesize indenones has been demonstrated under mild conditions by Xi and coworkers.8 An efficient acid catalyzed rearrangement of a tetrahalo-7,7-dimethoxybicyclo[2.2.1] heptenyl system leading to indenones was described by Khan et al.9 Superacid-induced intramolecular cyclization of 1,3-diarylpropynones was described for the synthesis of indenones.10 An efficient I2-catalyzed access to the synthesis of indenones from 2-alkynylbenzyl alcohols has been studied by Li et al.11 Benzoyl peroxide (BPO)-promoted radical cyclizations were also reported.12 Although the above elegant metal-free methods have been made to date, the development of a simple and efficient metal-free synthetic approach to indenones is highly desirable.2-Alkynylarylketones as an important class of substrates were widely investigated due to their convenient synthesis via the Sonogashira reaction13 and functionalized transformations to corresponding cyclic compounds.14 Usually, the intramolecular cyclization of 2-alkynylarylketones infer to two active intermediates isobenzopyrylium I and isobenzofuranium II (Scheme 1). Owing to the aromatization of the heterocyclic ring, isobenzopyrylium ions as stable oxonium cations has been extensively studied in both nucleophilic addition reactions and cycloaddition reactions.15 However, the reactivity of isobenzofuranium intermediates has been less explored.16 Based on the above studies, great interest has been aroused to further explore isobenzofuranium intermediates involved in the reaction. Herein, we are the first time to report a simple and effective access to various indenones by water-assisted metal-free catalyzed intramolecular cyclization of 2-alkynylaryl-ketones under mild reaction conditions, and the possible path of this reaction may involve in isobenzofuranium intermediates.
Results and discussion
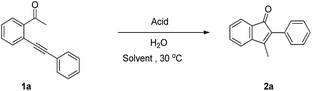
At the outset of our study, 2-alkynylarylketone 1a was chosen as a model substrate to optimize the reaction conditions and the experimental results are summarized in Table 1. When the intramolecular cyclization of 2-alkynylarylketones 1a using one equivalent amount of CH3COOH or CF3COOH as catalyst and 3 equiv. H2O in CH3CN under 30 °C was attempted, no desired product 2a was obtained (Table 1, entries 1 and 2). Treatment of 1a with p-CH3C6H4SO3H, CH3SO3H or CF3SO3H under same conditions afforded product 2a in 18%, 33%, or 40% yields, respectively (Table 1, entries 3–5). To our delight, a simple and typical inorganic acid HCl (4N in 1,4-dioxane) was used as catalyst for the intramolecular cyclization of 1a to give a yield of 85% at 30 °C (Table 1, entry 6). However, using another inorganic acid H2SO4 as catalyst led to a highly decreased yield (Table 1, entry 7). These observations clearly indicate that the properties of the acid play an important role in this transformation. Encouraged by the above results, further condition optimization of reaction solvents was conducted. In high polar solvents such as DMF or DMSO, no product 2a was obtained (Table 1, entries 8 and 9). Other solvents such as 1,4-dioxane, THF and CH2Cl2 afforded the product 2a in 43%, 36% or 44% yield, respectively (Table 1, entries 10–12). A slight decline in the yield of 2a appeared when the loading of HCl was decreased from 1.0 to 0.5 equiv. (Table 1, entry 13). In addition, the loading of H2O was decreased from 3.0 to 1.0 equiv. to give an apparent decline in the yield of 2a (Table 1, entry 14). On the contrary, increasing the loading of H2O from 3.0 to 5.0 equiv. can accelerate the reaction and a satisfying 91% yield of 2a was obtained (Table 1, entry 15). Thus, the optimal reaction conditions were obtained as follows: 0.3 mmol 1a, 1 equiv. HCl, 5 equiv. H2O in 2.0 mL CH3CN at 30 °C.| Entry | Acid (X equiv.) | H2O (X equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a General reaction conditions: 1a (0.3 mmol), catalyst, solvent (2.0 mL), 24 h at 30 °C.b Isolated yield.c HCl in 1,4-dioxane (4N). | ||||
| 1 | CH3COOH (1) | 3 | CH3CN | — |
| 2 | CF3COOH (1) | 3 | CH3CN | — |
| 3 | p-CH3C6H4SO3H (1) | 3 | CH3CN | 18 |
| 4 | CH3SO3H (1) | 3 | CH3CN | 33 |
| 5 | CF3SO3H (1) | 3 | CH3CN | 40 |
| 6 | HCl (1)c | 3 | CH3CN | 85 |
| 7 | H2SO4 (1) | 3 | CH3CN | 25 |
| 8 | HCl (1)c | 3 | DMF | — |
| 9 | HCl (1)c | 3 | DMSO | — |
| 10 | HCl (1)c | 3 | 1,4-Dioxane | 43 |
| 11 | HCl (1)c | 3 | THF | 36 |
| 12 | HCl (1)c | 3 | CH2Cl2 | 44 |
| 13 | HCl (0.5)c | 3 | CH3CN | 80 |
| 14 | HCl (1)c | 1 | CH3CN | 49 |
| 15 | HCl (1)c | 5 | CH3CN | 91 |
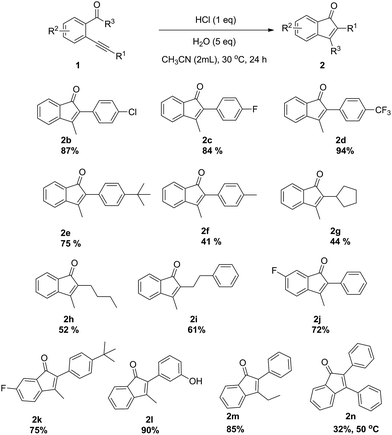
With the optimized conditions established, various substituted substrates for this intramolecular cyclization promoted by HCl were investigated and the results were shown in Scheme 2. It is clear that the electronic property of substituents on the phenyl ring in the R1 group exerts an obvious influence on the product yield. Substrates 1b–d with electron-withdrawing 4-Cl, 4-F and 4-CF3 groups showed higher reactivity in comparison with substrates 1e–f with electron-donating 4-tBu and 4-Me groups. The corresponding products 2g, 2h and 2i were obtained in middle yields, when the alkyl-substituted alkynes 1g, 1h and 1i were as substrates. The cyclization reactions of substrates having 4-F (1j or 1k) substituents on the phenyl ring in the R2 group were efficiently catalyzed to give the corresponding products 2j–k in high yields. In addition, substrate having 3-OH (1l) substituent on the phenyl ring in the R1 group was smoothly transformed into the corresponding product 2l in excellent yield. The steric effect of the R3 group on the reaction was also investigated. The reaction of substrate 1m with an ethyl group gave a good yield, whereas that of substrate 1n with phenyl group gave desired product 2n in only 35% yield at 50 °C. The structure of 2n was unambiguously determined by X-ray crystallography (see ESI†).
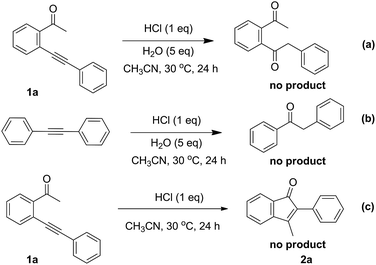
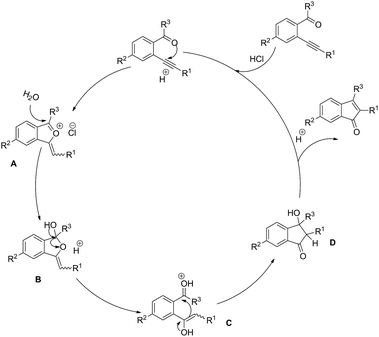
To understand the mechanistic pathway more clearly, some control experiments were carried out (Scheme 3). To exclude the hydration of the triple of substrates 1, 1a and 1,2-diphenylethyne failed to afford the corresponding hydration product under the standard conditions (Scheme 3a and b). Additionally, the experimental result showed that no product 2a was given under anhydrous conditions (Scheme 3c). This result indicates that the water is necessary to participate in this reaction. On the basis of the above results and previous work,7f a possible mechanism was proposed and shown in Scheme 4. When substrate is firstly activated by HCl, a 5-exo-dig cyclization takes place to form isobenzofuranium intermediate A. Then the new intermediate B is produced by the nucleophilic addition of H2O to A. The carbon–oxygen bond cleavage could form enol intermediate C by protonation of the oxygen atom of isobenzofuran B. Subsequently, the intermediate D can be obtained via an intramolecular ring-closing reaction of the intermediate C. Finally, after elimination of H2O in the presence of HCl, the product indenone is released together with the regenerated catalyst HCl for finishing a catalyst cycle. Furthermore, ESI-HRMS detection of the reaction mixture using 1a as substrate with 1 h was conducted to capture the information of reaction intermediates. The species of m/z 221.0961 ascribed to the intermediate A (R1 = Ph, R2 = H, R3 = Me; [MA]+) and the species of m/z 239.1067 ascribed to the intermediates B, C and D (R1 = Ph, R2 = H, R3 = Me; [MB + H]+, [Mc]+ and [MD + H]+) were observed (see ESI Fig. S1† for details).
Conclusions
In summary, a simple and efficient HCl mediated water-assisted method to the construction of useful indenones by the intramolecular cyclization reaction of 2-alkynylarylketones is described under mild conditions. Various functional substrates could smoothly apply to this cyclization reaction. The reaction process may be initiated by 5-exo-dig cyclization of the carbonyl group with the alkyne triple bond, leading to isobenzofuranium intermediates.Experimental
All chemicals and reagents were purchased from commercial suppliers without further purification unless otherwise stated. NMR spectra were recorded with tetramethylsilane as the internal standard. NMR spectrawere recorded on a Bruker Avance II 400M type (1H NMR, 400 MHz; 13C NMR, 100 MHz) spectrometer. High resolution mass spectra (HRMS) were recorded on a Q-TOF mass spectrometry (Micromass, Wythenshawe, UK) equipped with Z-spray ionization source. Infrared spectra (IR) was measured using a Nicolet NEXUS FT-IR spectrophotometer. Substrates 2-alkynylarylketones were prepared by Sonogashira coupling reaction of corresponding 2-bromoacetophenones with alkyne according to the relate literature.7f,15m,16e,17Representative experimental procedure for the intramolecular cyclization of 2-alkynylarylketones
Taking the intramolecular cyclization of 2-1-(2-(phenylethynyl)-phenyl)ethanone (1a) as example: A 10 mL vial was charged with 1-(2-(phenylethynyl)phenyl)ethanone 1a (66.1 mg, 0.30 mmol) and acetonitrile (2 mL), then H2O (27.0 mg, 1.5 mmol) and 0.30 mmol HCl (4N in 1,4-dioxane) was sequentially added into above solution. The vial was sealed and the reaction mixture was stirred at 30 °C for 24 h. After cooling to room temperature, the resulting mixture was diluted with ethyl acetate (10 mL) and washed with brine (10 mL). The aqueous phase was extracted with ethyl acetate (2 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The crude reaction mixture was purified by column chromatography on silica gel (PE/EtOAc) to give 2a in 91% yield.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. U1504205) and the Key Research Project of Education Department of Henan Province (No. 17A150002).Notes and references
- (a) G. M. Anstead, S. R. Wilson and J. A. Katzenellenbogen, J. Med. Chem., 1989, 32, 2163 CrossRef CAS PubMed; (b) J. H. Ahn, M. S. Shin, S. H. Jung, S. K. Kang, K. R. Kim, S. D. Rhee, W. H. Jung, S. D. Yang, S. J. Kim, J. R. Woo, J. H. Lee, H. G. Cheon and S. S. Kim, J. Med. Chem., 2006, 49, 4781 CrossRef CAS PubMed; (c) A. Morrell, M. Placzek, S. Parmley, B. Grella, S. Antony, Y. Pommier and M. Cushman, J. Med. Chem., 2007, 50, 4388 CrossRef CAS PubMed; (d) J. L. Jeffrey and R. Sarpong, Org. Lett., 2009, 11, 5450 CrossRef CAS PubMed; (e) X. Chen, J. Jin, N. Wang, P. Lu and Y. Wang, Eur. J. Org. Chem., 2012, 2012, 824 CrossRef CAS.
- (a) J. F. Feeman and E. D. Amstutz, J. Am. Chem. Soc., 1950, 72, 1522 CrossRef CAS; (b) M. B. Floyd and G. R. J. Allen, J. Org. Chem., 1970, 35, 2647 CrossRef CAS; (c) H. Martens and G. Hoornaert, Synth. Commun., 1972, 2, 147 CrossRef; (d) G. Jammaer, H. Martens and G. Hoornaert, Tetrahedron, 1975, 31, 2293 CrossRef CAS; (e) M. Rostami, A. R. Khosropour, V. Mirkhani, M. Moghadam, S. Tangestaninejad and I. Mohammadpoor-Baltork, Tetrahedron Lett., 2011, 52, 7149 CrossRef CAS.
- (a) R. L. Frank, H. Eklund, J. W. Richter, C. R. Vanneman and A. N. Wennerberg, J. Am. Chem. Soc., 1944, 66, 1 CrossRef CAS; (b) C. Manning, M. R. McClory and J. J. McCullough, J. Org. Chem., 1981, 46, 919 CrossRef CAS; (c) K. Katsumoto, C. Kitamura and T. Kawase, Eur. J. Org. Chem., 2011, 2011, 4885 CAS.
- Selected examples: (a) K. Kokubo, K. Matsumasa, M. Miura and M. Nomura, J. Org. Chem., 1996, 61, 6941 CrossRef CAS PubMed; (b) T. Fukuyama, N. Chatani, F. Kakiushi and S. Murai, J. Org. Chem., 1997, 62, 5647 CrossRef CAS; (c) T. Miura and M. Murakami, Org. Lett., 2005, 7, 3339 CrossRef CAS PubMed; (d) Y. Harada, J. Nakanishi, H. Fujihara, M. Tobisu, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2007, 129, 5766 CrossRef CAS PubMed; (e) T. Morimoto, K. Yamasaki, A. Hirano, K. Tsutsumi, N. Kagawa, K. Kakiuchi, Y. Harada, Y. Fukumoto, N. Chatani and T. Nishioka, Org. Lett., 2009, 11, 1777 CrossRef CAS PubMed; (f) B.-J. Li, H.-Y. Wang, Q.-L. Zhu and Z.-J. Shi, Angew. Chem., 2012, 124, 4014 (Angew. Chem. Int. Ed., 2012, 51, 3948) CrossRef; (g) S. Chen, J. Yu, Y. Jiang, F. Chen and J. Cheng, Org. Lett., 2013, 15, 4754 CrossRef CAS PubMed; (h) Z. Qi, M. Wang and X. Li, Org. Lett., 2013, 15, 5440 CrossRef CAS PubMed; (i) X. Yu, Y. Duan, W. Guo, T. Wang, Q. Xie, S. Wu, C. Jiang, Z. Fan, J. Wang and G. Liu, Organometallics, 2017, 36, 1027 CrossRef CAS.
- Selected examples: (a) D. H. Kim, S. U. Son and Y. K. Chung, Org. Lett., 2003, 5, 3151 CrossRef CAS PubMed; (b) W. Yu, W. Zhang, Z. Liu and Y. Zhang, Chem. Commun., 2016, 52, 6837 RSC; (c) M. Ueda, T. Ueno, Y. Suyama and I. Ryu, Chem. Commun., 2016, 52, 13237 RSC; (d) L. Kong, X. Yang, X. Zhou, S. Yu and X. Li, Org. Chem. Front., 2016, 3, 813 RSC.
- Selected examples: (a) R. C. Larock and M. J. Doty, J. Org. Chem., 1993, 58, 4579 CrossRef CAS; (b) J. Vicente, J.-A. Abad and J. Gil-Rubio, Organometallics, 1996, 15, 3509 CrossRef CAS; (c) J. Vicente, J.-A. Abad, B. López-Peláez and E. Martínez-Viviente, Organometallics, 2002, 21, 58 CrossRef CAS; (d) A. A. Pletnev, Q. Tian and R. C. Larock, J. Org. Chem., 2002, 67, 9276 CrossRef CAS PubMed; (e) H. Tsukamoto and Y. Kondo, Org. Lett., 2007, 9, 4227 CrossRef CAS PubMed; (f) X. Chen, Q. He, Y. Xie and C. Yang, Org. Biomol. Chem., 2013, 11, 2582 RSC; (g) A. N. Butkevich, B. Ranieri, L. Meerpoel, I. Stansfield, P. Angibaud, A. Corbua and J. Cossy, Org. Biomol. Chem., 2014, 12, 728 RSC; (h) B. Suchand and G. Satyanarayana, J. Org. Chem., 2017, 82, 372 CrossRef CAS PubMed.
- Selected examples: (a) T. Fukuyama, N. Chatani, F. Kakiuchi and S. Murai, J. Org. Chem., 1997, 62, 5647 CrossRef CAS; (b) Y. Kuninobu, T. Matsuki and K. Takai, Org. Lett., 2010, 12, 2948 CrossRef CAS PubMed; (c) X.-Q. Pan, J.-P. Zou, G.-L. Zhang and W. Zhang, Chem. Commun., 2010, 46, 1721 RSC; (d) J. Zhou, G.-L. Zhang, J.-P. Zou and W. Zhang, Eur. J. Org. Chem., 2011, 2011, 3412 CrossRef CAS; (e) J. Zhang, D. Wu, X. Chen, Y. Liu and Z. Xu, J. Org. Chem., 2014, 79, 4799 CrossRef CAS PubMed; (f) M. E. Domaradzki, Y. Long, Z. She, X. Liu, G. Zhang and Y. Chen, J. Org. Chem., 2015, 80, 11360 CrossRef CAS PubMed; (g) D. H. Dethe and G. M. Murhade, Chem. Commun., 2015, 51, 10891 RSC.
- (a) X. Yan, S. Zou, P. Zhao and C. Xi, Chem. Commun., 2014, 50, 2775 RSC; (b) P. Zhao, Y. Liu and C. Xi, Org. Lett., 2015, 17, 4388 CrossRef CAS PubMed.
- K. R. Babu and F. A. Khan, Org. Biomol. Chem., 2015, 13, 299 Search PubMed.
- (a) A. V. Vasilyev, S. Walspurger, P. Pale and J. Sommer, Tetrahedron Lett., 2004, 45, 3379 CrossRef CAS; (b) A. V. Vasilyev, S. Walspurger, M. Haouas, J. Sommer, P. Pale and A. P. Rudenkoa, Org. Biomol. Chem., 2004, 2, 3483 RSC; (c) A. V. Vasil’ev, S. Walspurger, P. Pale, J. Sommer, M. Haouas and A. P. Rudenko, Russ. J. Org. Chem., 2004, 40, 1769 CrossRef.
- C. Wang, J. Yang, X. Cheng, E. Li and Y. Li, Tetrahedron Lett., 2012, 53, 4402 CrossRef CAS.
- (a) C. Pan, B. Huang, W. Hu, X. Feng and J.-T. Yu, J. Org. Chem., 2016, 81, 2087 CrossRef CAS PubMed; (b) X.-S. Zhang, J.-Y. Jiao, X.-H. Zhang, B.-L. Hu and X.-G. Zhang, J. Org. Chem., 2016, 81, 5710 CrossRef CAS PubMed.
- (a) E.-I. Negishi and L. Anastasia, Chem. Rev., 2003, 103, 1979 CrossRef CAS PubMed; (b) R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874 CrossRef CAS PubMed.
- (a) N. Chernyak, S. I. Gorelsky and V. Gevorgyan, Angew. Chem., 2011, 123, 2390 (Angew. Chem. Int. Ed., 2011, 50, 2342) CrossRef; (b) Z.-Q. Wang, W.-W. Zhang, L.-B. Gong, R.-Y. Tang, X.-H. Yang, Y. Liu and J.-H. Li, Angew. Chem., 2011, 123, 2390 (Angew. Chem. Int. Ed., 2011, 50, 8968) CrossRef; (c) D. Zheng, S. Li and J. Wu, Org. Lett., 2012, 14, 2655 CrossRef CAS PubMed; (d) S.-Y. Yu, H. Zhang, Y. Gao, L. Mo, S. Wang and Z.-J. Yao, J. Am. Chem. Soc., 2013, 135, 11402 CrossRef CAS PubMed; (e) C. Dong, Z. Liao, X. Xu and H. Zhou, J. Heterocycl. Chem., 2014, 51, 1282 CrossRef CAS.
- (a) N. Asao, T. Nogami, S. Lee and Y. Yamamoto, J. Am. Chem. Soc., 2003, 125, 10921 CrossRef CAS PubMed; (b) N. Asao, T. Kasahara and Y. Yamamoto, Angew. Chem., 2003, 115, 3628 (Angew. Chem. Int. Ed., 2003, 42, 3504) CrossRef; (c) J.-L. Zhu, A.-R. Germain and J. A. Porco Jr, Angew. Chem., 2004, 116, 1259 (Angew. Chem. Int. Ed., 2004, 43, 1239) CrossRef; (d) N. Asao, K. Sato and Y. Yamamoto, J. Org. Chem., 2005, 70, 3682 CrossRef CAS PubMed; (e) J.-L. Zhu, N. P. Grigoriadis, J.-P. Lee and J. A. Porco Jr, J. Am. Chem. Soc., 2005, 127, 9342 CrossRef CAS PubMed; (f) H. Kusama, H. Funami, M. Shido, Y. Hara, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2005, 127, 2709 CrossRef CAS PubMed; (g) S. Shin, A.-K. Gupta, C.-Y. Rhim and C.-H. Oh, Chem. Commun., 2005, 41, 4429 RSC; (h) D. Yue, N. D. Cá and R. C. Larock, J. Org. Chem., 2006, 71, 3381 CrossRef CAS PubMed; (i) A.-B. Beeler, S. Su, C.-A. Singleton and J. A. Porco Jr, J. Am. Chem. Soc., 2007, 129, 1413 CrossRef CAS PubMed; (j) Y.-C. Hsu, C.-M. Ting and R.-S. Liu, J. Am. Chem. Soc., 2009, 131, 2090 CrossRef CAS PubMed; (k) L.-P. Liu and G. B. Hammond, Org. Lett., 2010, 12, 4640 CrossRef CAS PubMed; (l) M. Terada, F. Li and Y. Toda, Angew. Chem., 2014, 126, 239 (Angew. Chem. Int. Ed., 2014, 53, 235) CrossRef; (m) B. Guo, L. Zheng, L. Yang and R. Hua, J. Org. Chem., 2014, 79, 4352 CrossRef CAS PubMed; (n) S. Zhu, H. Huang, Z. Zhang, T. Ma and H. Jiang, J. Org. Chem., 2014, 79, 6113 CrossRef CAS PubMed; (o) Q. Xu, P. Gu, F. Wang and M. Shi, Org. Chem. Front., 2015, 2, 1475 RSC; (p) T. Miao, Z.-Y. Tian, Y.-M. He, F. Chen, Y. Chen, Z.-X. Yu and Q.-H. Fan, Angew. Chem., 2017, 129, 4199 (Angew. Chem. Int. Ed., 2017, 56, 4135) CrossRef; (q) J. Sun, J.-K. Qiu, Y.-N. Wu, W.-J. Hao, C. Guo, G. Li, S.-J. Tu and B. Jiang, Org. Lett., 2017, 19, 754 CrossRef CAS PubMed.
- (a) D. Jiang and J. W. Herndon, Org. Lett., 2000, 2, 1267 CrossRef CAS PubMed; (b) Y. Luo, J. W. Herndon and F. Cervantes-Lee, J. Am. Chem. Soc., 2003, 125, 12720 CrossRef CAS PubMed; (c) T. Godet, C. Vaxelaire, C. Michel, A. Milet and P. Belmont, Chem.–Eur. J., 2007, 13, 5632 CrossRef CAS PubMed; (d) K. Sekine, A. Takayanagi, S. Kikuchi and T. Yamada, Chem. Commun., 2013, 49, 11320 RSC; (e) W.-Z. Zhang, L.-L. Shi, C. Liu, X.-T. Yang, Y.-B. Wang, Y. Luo and X.-B. Lu, Org. Chem. Front., 2014, 1, 275 RSC.
- (a) M. Dell'Acqua, G. Abbiati, A. Arcadi and E. Rossi, Org. Biomol. Chem., 2011, 9, 7836 RSC; (b) S. Manojveer and R. Balamurugan, Org. Lett., 2014, 16, 1712 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data for compound 2n. Copies of 1H NMR and 13C NMR of products. ESI-HRMS spectrum for detecting the reaction system. CCDC 1510707. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra05487d |
| This journal is © The Royal Society of Chemistry 2017 |