DOI:
10.1039/C7RA05339H
(Paper)
RSC Adv., 2017,
7, 32478-32487
Convenient preparation of thioglycomimetics: S-glycosyl sulfenamides, sulfinamides and sulphonamides†
Received
11th May 2017
, Accepted 19th June 2017
First published on 27th June 2017
Abstract
A series of S-glycosyl sulfenamide derivatives has been prepared in good yield from glycosyl thiols using N-bromosuccinimide (NBS) or N-chlorosuccinimide (NCS) as activator under significantly fast reaction conditions avoiding the use of hazardous reagents. Controlled and complete oxidation of the sulfenamide derivatives under mild reaction conditions led to the formation of the corresponding sulfinamides and sulfonamides in excellent yield.
Introduction
1-Thiosugar derivatives are useful intermediates for the preparation of a variety of glycomimetics and pharmaceutically important compounds.1,2 Because of the extra stability of the anomeric carbon–sulfur bond they are resistant towards enzymatic hydrolysis and thereby considered as useful intermediates in the design of novel enzyme inhibitors.3,4 Over the years, a plethora of reports have appeared in the literature for the construction of glycomimetics and neoglycoconjugates in which 1-thiosugars have been used extensively.5–7 Among several glycomimetics developed so far, glycosyl sulphonamides, sulfinamides and sulfenamides are noteworthy. Although sulphonamide functionality can be found in several compounds having therapeutic potential, most of them are aromatic in nature.8 Till date, only a few reports are available on the preparation of S-glycosyl sulphonamides. Poulsen et al.,9,10 and von Itzstein et al.11 prepared S-glycosyl sulfenamide derivative by the treatment of glycosylthioacetate with diethyl bromomalonate in the presence of an amine. In another report, Knapp et al.12 described the preparation of S-glycosyl sulfenamide from glycosyl thiol via the formation of glycosyl sulfenyl chloride generated in situ by the treatment with sulfuryl chloride followed by addition of an appropriate amine. In addition, S-glycosyl sulfenamide derivatives have also been prepared by the treatment of diglycosyl disulfide with amine in the presence of silver salts.13 Earlier, Kahne et al. demonstrated the formation of glycosyl sulfenate intermediates during the glycosylation reaction using glycosyl sulfoxides.14 In an ongoing program towards the preparation of glycomimetics, we were in need to prepare S-glycosyl sulfenamide derivatives and their oxidized products starting from glycosyl thiols. Following earlier reported reaction conditions, treatment of glycosyl thiols with diethyl bromomalonate or sulfuryl chloride followed by reaction with amine did not furnish satisfactory yield of glycosyl sulfenamide instead diglycosylated disulfide derivative was obtained as predominant product. Therefore, it is pertinent to develop novel reaction condition to overcome these shortcomings. In searching for a better alternative, we envisioned that treatment of glycosyl thiol with a halonium ion (X+) generating agent such as N-bromosuccinimide (NBS) or N-chlorosuccinimide (NCS) or N-iodosuccinimide (NIS) or carbon tetrabromide (CBr4) etc. could lead to the in situ formation of glycosyl sulfenyl halide intermediate, which on treatment with appropriate amine could furnish glycosyl sulfenamide derivative and further oxidation of the product could provide glycosyl sulfinamide and sulphonamide derivatives. Reaction of simple alkyl and aryl thiols with N-halosuccinimide was reported earlier by Abe et al.15 for the preparation of N-alkyl or N-arylthio succinimides derivatives via in situ generation of sulfenyl chloride (Scheme 1). We report herein our findings on the treatment of glycosyl thiols with halonium ion followed by reaction with different amines to furnish glycosyl sulfenamides and their oxidized products (Scheme 2).
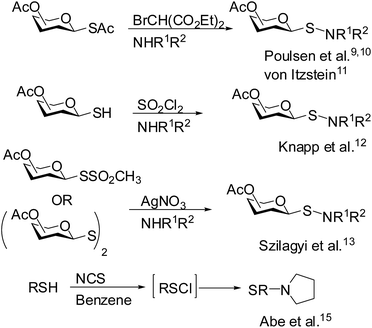 |
| | Scheme 1 Previously reported reaction methodologies for the preparation of S-glycosyl sulfenamide derivatives. | |
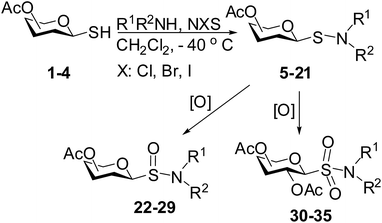 |
| | Scheme 2 Preparation of glycosyl sulfenamide derivatives using N-halosuccinimides in the presence of an amine and their oxidized products. | |
Results and discussion
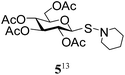
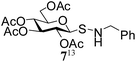
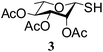
In a set of initial experiments, it was decided to screen a set of halonium ion source, such as NIS, NBS and NCS for the generation of stable glycosyl sulfenium halide intermediate for its reaction with appropriate amines. In order to do so, compound 1 was treated with a varied quantity of N-halosuccinimide in CH2Cl2 at low temperature and room temperature. After a series of experimentation it was observed that treatment of compound 1 with NBS (1 equiv.) or NCS (1 equiv.) in CH2Cl2 at −40 °C in the presence of piperidine resulted in the formation of expected glycosyl sulfenamide derivative (5) in 60% yield together with diglucosyldisulfide (5a) in 25% yield within 2 min. Changing the reaction condition by the variation of temperature, time and quantity of reagents did not reflect any further improvement in the yield of the product. Satisfactory yields of the corresponding glycosyl sulfenamide derivatives were also obtained using primary amines and aromatic amines under similar reaction conditions. In contrast, treatment of compound 1 with NIS (1 equiv.) in the presence of secondary amines furnished poor yield of the corresponding sulfenamide derivatives and use of primary amines or aromatic amines led to the formation of disulfide derivative only. In another experiment, treatment of compound 1 with a combination of carbon tetrabromide (CBr4) (1 equiv.) and triethylamine (Et3N) in the presence of piperidine in CH2Cl2 at room temperature instantly led to the exclusive formation of disulfide derivative instead of expected sulfenamide derivative. There was no improvement in the formation of required product by carrying out the reaction at low temperature (−10 to −30 °C). A number of commonly used solvents such as CH2Cl2, CHCl3, THF, DMF, toluene, CH3CN etc. were screened and CH2Cl2 was found as the best solvent to furnish highest yield of the products. Detailed observation on the optimization of the reaction conditions is presented in Table 1. A comparative study has been carried out for the formation of glycosyl sulfenamide from glycosyl thiols using the present reaction condition together with earlier reported conditions, which is presented in Table 2. It is noteworthy to mention that the present reaction condition has several advantages such as, significantly fast, simple reaction condition, good yielding, involves non-hazardous reagents without requirement of any special reaction condition. Following the optimized reaction condition a series of S-glycosyl sulfenamide derivatives (5–21) have been synthesized in good yield (Table 3). The reaction condition is significantly fast and better yield of the sulfenamide derivatives were obtained using aliphatic amines in comparison to the aromatic amines. The reaction condition is compatible to the various functional groups used for the functionalization of carbohydrates. In every case minor quantities of diglycosyl disulfide was obtained as by product. All synthesized products were characterized with their NMR and mass spectral analysis.
Table 1 Halonium ion (X+) mediated preparation of glucosyl piperidinyl sulfenamide derivative from compound 1 in the presence of piperidine in a variety of solvents
|
| Sl. no. |
Thiol |
Activator |
Solvent |
Temp (°C) |
Time (min) |
5 (%) |
5a (%) |
| 1 |
1 |
NBS |
CH2Cl2 |
25 |
>2 |
0 |
92 |
| 2 |
1 |
NBS |
CH2Cl2 |
−40 |
2 |
75 |
20 |
| 3 |
1 |
NBS |
CH2Cl2 |
−60 |
5 |
75 |
20 |
| 4 |
1 |
NCS |
CH2Cl2 |
25 |
2 |
0 |
94 |
| 5 |
1 |
NCS |
CH2Cl2 |
−40 |
2 |
75 |
20 |
| 6 |
1 |
NIS |
CH2Cl2 |
−40 |
2 |
30 |
60 |
| 7 |
1 |
NIS |
CH2Cl2 |
−60 |
2 |
40 |
50 |
| 8 |
1 |
CBr4 |
CH2Cl2 |
−40 |
2 |
0 |
95 |
| 9 |
1 |
NCS |
(CH2Cl)2 |
−40 |
2 |
74 |
15 |
| 10 |
1 |
NCS |
CHCl3 |
−40 |
2 |
72 |
20 |
| 11 |
1 |
NCS |
THF |
−40 |
30 |
40 |
50 |
| 12 |
1 |
NCS |
DMF |
−40 |
15 |
15 |
70 |
| 13 |
1 |
NCS |
CH3CN |
−40 |
20 |
20 |
60 |
| 14 |
1 |
NCS |
Toluene |
−40 |
120 |
25 |
30 |
Table 2 Comparative studies for the preparation of glycosyl sulfenamide 4 using different activators in CH2Cl2
| Sl. no. |
Thiol |
Activator |
Solvent |
Temp (°C) |
Timea (min) |
Timeb (h) |
5 (%) |
| Time required for the formation of sulfenyl chloride. Time allowed at room temperature after the formation of sulfenyl chloride. |
| 1 |
1 |
SO2Cl2 |
CH2Cl2 |
−40 |
30a |
2 |
55 |
| 2 |
1 |
BrCH(CO2Et)2 |
CH2Cl2 |
20 |
20a |
12 |
65 |
| 3 |
1 |
NBS |
CH2Cl2 |
−40 |
2 |
— |
75 |
| 4 |
1 |
NCS |
CH2Cl2 |
−40 |
2 |
— |
75 |
| 5 |
1 |
AgNO3 |
CH3CN |
20 |
— |
24 |
35 |
Table 3 Preparation of glycosyl sulfenamide derivatives using NBS or NCS in CH2Cl2 at −40 °Ca
| Sl. no. |
Thiol |
Sulfenamide |
Time (min) |
Yield (%) |
| NH4OH used as amine. |
| 1 |
 |
 |
2 |
75 |
| 2 |
1 |
 |
2 |
70 |
| 3 |
1 |
 |
2 |
68 |
| 4 |
1 |
 |
2 |
66 |
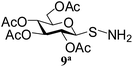
| 5 |
1 |
 |
5 |
60 |
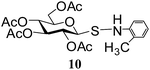
| 6 |
1 |
 |
5 |
68 |
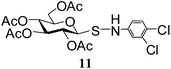
| 7 |
1 |
 |
5 |
66 |
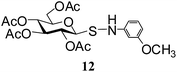
| 8 |
1 |
 |
5 |
68 |
| 9 |
 |
 |
2 |
68 |
| 10 |
2 |
 |
2 |
74 |
| 11 |
2 |
 |
2 |
67 |
| 12 |
2 |
 |
5 |
66 |
| 13 |
 |
 |
5 |
65 |
| 14 |
3 |
 |
5 |
65 |
| 15 |
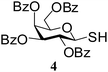 |
 |
5 |
82 |
| 16 |
4 |
 |
5 |
85 |
| 17 |
4 |
 |
5 |
72 |
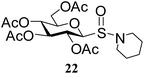
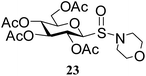
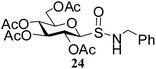
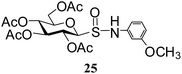
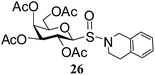
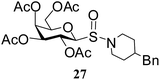
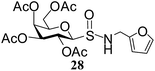
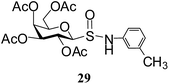
After preparing a series of glycosyl sulfenamide derivatives it was sought to achieve glycosyl sulfinamide and sulphonamide derivatives applying suitable oxidizing conditions. Following the earlier findings reported by Knapp et al.,12 controlled treatment of compound 5 with 1.0 equiv. of 3-chloroperbenzoic acid (mCPBA) in CH2Cl2 at −20 °C furnished corresponding sulfinamide 22 in 72% yield without formation of overoxidized product (e.g. sulphonamide). Applying similar reaction conditions, a series of glycosyl sulfinamide derivatives (22–29) have been synthesized (Table 4). It is noteworthy that glycosylsulfoxides were obtained as a mixture of regioisomers, which were inseparable by column chromatography. The ratio of the isomers was calculated from the integration values in the 1H NMR spectra of compounds.
Table 4 Preparation of glycosyl sulfinamides from the corresponding sulfenamides using mCPBA at −20 °Ca
| Sl. no. |
Compound |
Sulfinamides |
Isomeric ratio |
Yield (%) |
| All reactions took 3 h for completion. |
| 1 |
5 |
 |
3 |
78 |
| 2 |
6 |
 |
3 |
75 |
| 3 |
7 |
 |
3 |
75 |
| 4 |
12 |
 |
3 |
76 |
| 5 |
13 |
 |
3 |
75 |
| 6 |
14 |
 |
3 |
76 |
| 7 |
15 |
 |
3 |
74 |
| 8 |
16 |
 |
3 |
76 |
Having achieved the successful transformation of glycosyl sulfenamides into sulfinamides, we turned our attention towards the preparation of glycosyl sulphonamide derivatives. For this purpose, we have applied a rapid, neutral oxidation condition using a combination of KMnO4 and CuSO4·5H2O, which have been used earlier13 for the oxidation of sulphides into sulfone derivatives in our laboratory. A series of glycosyl sulfenamide derivatives have been treated with a mixture of KMnO4/CuSO4·5H2O (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CH3CN–H2O (5
1) in CH3CN–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature to furnish excellent yield of corresponding sulphonamide derivatives (30–35) in short period of time (Table 5).
1) at room temperature to furnish excellent yield of corresponding sulphonamide derivatives (30–35) in short period of time (Table 5).
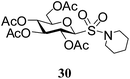
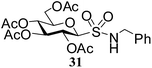
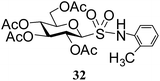
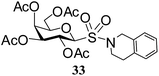
Table 5 Preparation of glycosyl sulfonamides from the corresponding sulfenamides using KMnO4/CuSO4·5H2O at room temperature
| Sl. no. |
Compound |
Sulfonamides |
Time (min) |
Yield (%) |
| 1 |
5 |
 |
30 |
90 |
| 2 |
7 |
 |
30 |
85 |
| 3 |
10 |
 |
45 |
84 |
| 4 |
13 |
 |
45 |
90 |
| 5 |
14 |
 |
30 |
90 |
| 6 |
16 |
 |
45 |
86 |
Conclusions
In summary, a series of glycosyl sulfenamides, sulfinamides and sulfonamides have been synthesized from glycosyl thiols under mild reaction conditions using easily accessible reagents. These compounds could be useful as precursors for the development of pharmaceutically important glycomimetics. Noteworthy to mention that the formation of sulfenamide derivatives is significantly fast and should be considered as better alternative for the preparation of a wide range of glycosyl sulfenamides, sulfinamides and sulphonamides because of their operational simplicity, use of mild reaction conditions avoiding hazardous reagents, selectivity for the product formation, reasonably high yield, easy to scale up.
Experimental
General methods
All reactions were monitored by thin layer chromatography over silica gel coated TLC plates. The spots on TLC were visualized by warming ceric sulphate (2% Ce(SO4)2 in 2 N H2SO4) sprayed plates on a hot plate. Silica gel 230–400 mesh was used for column chromatography. 1H and 13C NMR, 2D COSY, HSQC spectra were recorded on Bruker Avance 500 MHz spectrometer using CDCl3 as solvent and TMS as internal reference unless stated otherwise. Chemical shift values are expressed in δ ppm. ESI-MS were recorded on a Micromass mass spectrometer. Elementary analysis was carried out on Carlo Erba analyzer.
Typical experimental condition for the preparation of glycosyl sulfenamide (5–21)
A solution of per-O-acetylated glycosyl thiol (1 mmol) and amine (1 mmol) in anhydrous CH2Cl2 (10 mL) was cooled to −40 °C. To the cooled reaction mixture was added a solution of NBS or NCS (1 mmol) in CH2Cl2 (5 mL) drop wise and the reaction stirred for appropriate time (Table 2). The reaction takes place instantaneously. The reaction mixture was diluted with CH2Cl2 (50 mL) and successively washed with 5% Na2S2O3 (50 mL) and H2O (50 mL). The organic layer was dried (Na2SO4) and concentrated under reduced pressure. The crude product was purified over SiO2 using hexane–EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give pure compound 5–21 (Table 2). All products were characterized using their spectral analysis. Analytical data of synthesized compounds those are not reported earlier:
1) to give pure compound 5–21 (Table 2). All products were characterized using their spectral analysis. Analytical data of synthesized compounds those are not reported earlier:
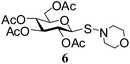
N-Morpholinyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfenamide (6). 1H NMR (500 MHz, CDCl3): δ 5.19 (m, 2H, H-2, H-3), 5.04 (t, J = 8.5 Hz, 1H, H-4), 4.55 (d, J = 9.5 Hz, 1H, H-1), 4.20 (dd, J = 12.0, 4.5 Hz, 1H, H-6a), 4.11 (dd, J = 12.0, 1.2 Hz, 1H, H-6b), 3.72–3.60 (m, 5H, H-5, 2 OCH2), 3.06–2.92 (m, 4H, 2 NCH2), 2.06, 2.03, 2.02, 2.01 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7, 168.8, 168.7 (4C, 4 CH3CO), 85.5 (C-1), 75.7 (C-5), 74.1 (C-3), 68.0 (C-4), 67.5 (C-2), 67.3 (OCH2), 61.9 (C-6), 57.4 (NCH2), 20.5, 20.4 (2C), 20.3 (4 CH3CO); ESI-MS: 472.1 [M + Na]+; anal. calcd for C18H27NO10S (449.47): C, 48.10; H, 6.05; found: C, 47.95; H, 6.25.
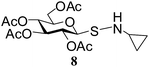
N-Cyclopropyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfenamide (8). 1H NMR (500 MHz, CDCl3): δ 5.27 (t, J = 9.5 Hz, 1H, H-2), 5.15 (t, J = 10 Hz, 1H, H-3), 5.06 (t, J = 9.5 Hz, 1H, H-4), 4.24 (dd, J = 12.0, 4.0 Hz, 1H, H-6a), 4.20–4.13 (m, 1H, H-6b, H-1), 3.74–3.68 (m, 1H, H-5), 3.31 (br s, 1H, NH), 2.63–2.59 (m, 1H, NCH), 2.06, 2.05, 2.02, 2.00 (4 s, 12H, 4 COCH3), 0.58–0.51 (m, 4H, 2 CH2); 13C NMR (125 MHz, CDCl3): δ 170.1, 169.7, 169.6, 169.1 (4C, 4 CH3CO), 87.4 (C-1), 75.7 (C-5), 73.7 (C-3), 68.1 (C-4), 67.4 (C-2), 61.7 (C-6), 33.5 (CH), 20.6, 20.5 (2C), 20.4 (4 CH3CO), 8.8 (CH2), 8.4 (CH2); ESI-MS: 442.1 [M + Na]+; anal. calcd for C17H25NO9S (419.45): C, 48.68; H, 6.01; found: C, 48.50; H, 6.20.
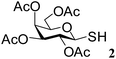
1-S-(2,3,4,6-Tetra-O-acetyl)-β-D-glucopyranosyl sulfenamide (9). 1H NMR (500 MHz, CDCl3): δ 5.30 (t, J = 9.5 Hz, 1H, H-2), 5.19 (t, J = 9.5 Hz, 1H, H-3), 5.03 (t, J = 9.5 Hz, 1H, H-4), 4.30 (dd, J = 12.5, 5.0 Hz, 1H, H-6a), 4.24 (d, J = 11 Hz, 1H, H 6b), 4.0 (d, J = 9.5 Hz, 1H, H-1), 3.80–3.75 (m, 1H, H-5), 2.53 (br s, 2H, NH2), 2.10, 2.07, 2.02, 2.01 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.2, 169.8, 169.6, 169.1 (4C, 4 CH3CO), 86.4 (C-1), 75.9 (C-5), 73.4 (C-3), 68.1 (C-4), 66.5 (C-2), 61.9 (C-6), 20.5, 20.4 (2C), 20.3 (4 CH3CO); ESI-MS: 402.1 [M + Na]+; anal. calcd for C14H21NO9S (379.38): C, 44.32; H, 5.58; found: C, 44.15; H, 5.74.
N-(2-Methylphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfenamide (10). 1H NMR (500 MHz, CDCl3): δ 7.42–6.72 (m, 4H, Ar-H), 5.26 (t, J = 9.5 Hz, 1H, H-2), 5.12 (t, J = 9.5 Hz, 1H, H-3), 5.05 (s, 1H, NH), 4.95 (t, J = 9.5 Hz, 1H, H-4), 4.27–4.24 (m, 1H, H-6a), 4.21 (d, J = 9.5 Hz, 1H, H-1), 4.05 (dd, J = 12.5, 4.0 Hz, 1H, H-6b), 3.67–3.60 (m, 1H, H-5), 2.25 (s, 3H, CH3), 2.14, 2.00, 1.99, 1.91 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.0, 169.9, 169.6, 168.9 (4C, 4 COCH3), 144.7–113.6 (Ar-C), 87.9 (C-1), 75.9 (C-5), 73.5 (C-3), 68.0 (C-4), 67.6 (C-2), 61.4 (C-6), 20.8, 20.6, 20.4, 20.3 (4 CH3CO), 17.2 (CH3); ESI-MS: 492.1 [M + Na]+; anal. calcd for C21H27NO9S (469.51): C, 53.72; H, 5.80; found: C, 53.56; H, 6.00.
N-(3,4-Dichlorophenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfenamide (11). 1H NMR (500 MHz, CDCl3): δ 7.28–6.87 (m, 3H, Ar-H), 5.27 (t, J = 9.5 Hz, 1H, H-2), 5.15 (br s, 1H, NH), 5.1 (t, J = 9.5 Hz, 1H, H-3), 4.92 (t, J = 9.5 Hz, 1H, H-4), 4.19–4.16 (m, 1H, H-6a), 4.15 (d, J = 9.5 Hz, 1H, H-1), 4.08 (dd, J = 12.5, 4.0 Hz, 1H, H-6b), 3.68–3.64 (m, 1H, H-5), 2.13, 2.00, 1.99, 1.92 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.1, 170, 169.6, 168.8 (4 COCH3), 146.9–114.2 (Ar-C), 87.9 (C-1), 75.7 (C-5), 73.2 (C-3), 67.7 (C-4), 67.4 (C-2), 61.3 (C-6), 20.6, 20.4, 20.3, 20.2 (4 CH3CO); ESI-MS: 546.0 [M + Na]+; anal. calcd for C20H23Cl2NO9S (524.37): C, 45.81; H, 4.42; found: C, 45.66; H, 4.58.
N-(3-Methoxyphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfenamide (12). 1H NMR (500 MHz, CDCl3): δ 7.03–6.36 (m, 4H, Ar-H), 5.28 (t, J = 9.5 Hz, 1H, H-2), 5.16–5.10 (m, 2H, NH, H-3), 4.95 (t, J = 9.5 Hz, 1H, H-4), 4.24 (d, J = 10.0 Hz, 1H, H-1), 4.19 (dd, J = 12.0, 1.5 Hz, 1H, H-6a), 4.14–4.05 (m, 1H, H-6b), 3.75 (s, 3H, OCH3), 3.68–3.65 (m, 1H, H-5), 2.12, 2.01, 1.99, 1.92 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.0, 169.7, 169.6, 169.0 (4 COCH3), 160.3–107.9 (Ar-C), 87.9 (C-1), 75.6 (C-5), 73.5 (C-3), 67.9 (C-4), 67.6 (C-2), 61.5 (C-6), 54.6 (OCH3), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 508.1 [M + Na]+; anal. calcd for C21H27NO10S (485.50): C, 51.95; H, 5.61; found: C, 51.80; H, 6.80.
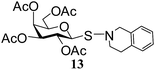
N-(3,4-Dihydroisoquinolinyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfenamide (13). 1H NMR (500 MHz, CDCl3): δ 7.26–6.95 (m, 4H, Ar-H), 5.37 (d, J = 3.0 Hz, 1H, H-4), 5.32 (t, J = 10.0 Hz, 1H, H-2), 5.06 (dd, J = 10.0, 3.5 Hz, 1H, H-3), 4.68 (d, J = 10.0 Hz, 1H, H-1), 4.23 (s, 2H, NCH2), 4.16 (dd, J = 11.5, 6.5 Hz, 1H, H-6a), 4.09 (dd, J = 11.5, 6.5 Hz, 1H, H-6b), 3.38–3.26 (m, 2H, NCH2), 3.06–2.90 (m, 2H, CH2), 2.09, 2.02, 2.01, 1.97 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7, 169.0 (4 COCH3), 134.9–125.6 (Ar-C), 86.9 (C-1), 74.0 (C-5), 72.0 (C-4), 67.0 (C-3), 65.0 (C-2), 61.4 (C-6), 58.8 (NCH2), 55.2 (NCH2), 30.0 (CH2), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 518.1 [M + Na]+; anal. calcd for C23H29NO9S (495.54): C, 55.75; H, 5.90; found: C, 55.60; H, 5.72.
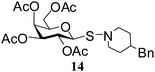
N-(4-Benzylpiperidinyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosylsulfenamide (14). 1H NMR (500 MHz, CDCl3): δ 7.25–7.08 (m, 5H, Ar-H), 5.39 (d, J = 3.0 Hz, 1H, H-4), 5.26 (t, J = 10.0 Hz, 1H, H-2), 5.06 (dd, J = 9.5, 3.5, Hz, 1H, H-3), 4.67 (d, J = 10.0 Hz, 1H, H-1), 4.14–4.06 (m, 2H, H-6ab), 3.96–3.89 (m, 1H, H-5), 3.24–3.03 (m, 2H, NCH2), 2.88–2.74 (m, 2H, NCH2), 2.5 (d, J = 6.5 Hz, 2H, PhCH2), 2.15, 2.05, 2.01, 1.98 (4 s, 12H, 4 COCH3), 1.60 (m, 2H, CH2), 1.40 (br s, 1H, CH); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7 (2C), 169.0 (4 COCH3), 140–125.7 (Ar-C), 85.9 (C-1), 73.9 (C-5), 72.0 (C-4), 67.2 (C-3), 65.1 (C-2), 61.3 (C-6), 58.6 (NCH2), 57.2 (NCH2), 42.9 (PhCH2), 36.8 (CH), 33.3 (CH2), 33.0 (CH2), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 560.2 [M + Na]+; anal. calcd for C26H35NO9S (537.62): C, 58.09; H, 6.56; found: C, 57.93; H, 6.75.
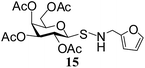
N-(2-Furanylmethyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfenamide (15). 1H NMR (500 MHz, CDCl3): δ 7.35–6.22 (m, 3H, Ar-H), 5.42 (d, J = 2.5 Hz, 1H, H-4), 5.38 (t, J = 10.0 Hz, 1H, H-2), 5.12 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 4.26 (dd, J = 14.5, 2.5 Hz, 1H, H-6a), 4.18–4.05 (m, 2H, H-1, H-6b), 3.92–3.88 (m, 1H, H-5), 3.18 (s, 1H, NH), 2.16, 2.07, 2.05, 1.87 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.0, 169.9, 169.8, 169.7 (4 COCH3), 153–107.2 (Ar-C), 88.6 (C-1), 74.3 (C-5), 71.7 (C-4), 67.1 (C-3), 64.7 (C-2), 61.3 (C-6), 50.2 (NCH2), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 482.1 [M + Na]+; anal. calcd for C19H25NO10S (459.47): C, 49.67; H, 5.48; found: C, 49.50; H, 5.65.
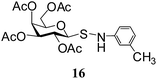
N-(3-Methylphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosylsulfenamide (16). 1H NMR (500 MHz, CDCl3): δ 7.07–6.64 (m, 4H, Ar-H), 5.31 (br s, 1H, NH), 5.29 (t, J = 10.0 Hz, 1H, H-2), 5.17 (d, J = 5.5 Hz, 1H, H-4), 5.09 (d, J = 10.0 Hz, 1H, H-3), 4.29 (d, J = 10.0 Hz, 1H, H-1), 4.12–4.10 (m, 1H, H-6a), 4.05–4.03 (m, 1H, H-6b), 3.88 (m, 1H, H-5), 2.29 (s, 3H, CH3), 2.17, 2.07, 2.02, 1.95 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.1, 170, 169.9, 169.7 (4 COCH3), 147.0–112.6 (Ar-C), 88.6 (C-1), 73.9 (C-5), 71.5 (C-4), 66.8 (C-3), 64.9 (C-2), 61.2 (C-6), 21.4 (CH3), 20.8, 20.5 (2C), 20.3 (4 COCH3); ESI-MS: 492.1 [M + Na]+; anal. calcd for C21H27NO9S (469.51): C, 53.72; H, 5.80; found: C, 53.56; H, 6.00.
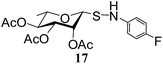
N-(4-Fluorophenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-L-rhamnopyranosylsulfenamide (17). 1H NMR (500 MHz, CDCl3): δ 6.81–6.54 (m, 4H, Ar-H), 5.37 (dd, J = 1.3 Hz, 1H, H-2), 5.03 (t, J = 8.5 Hz, 1H, H-4), 4.94 (dd, J = 10.5, 3.5 Hz, 1H, H-3), 4.60 (d, J = 1.2 Hz, 1H, H-1), 3.60–3.55 (m, 1H, H-5), 3.44 (s, 1H, NH), 2.17, 2.02, 1.95 (3 s, 9H, 3 COCH3), 1.29 (d, J = 6.5 Hz, 3H, CCH3); 13C NMR (125 MHz, CDCl3): δ 169.8, 169.4 (2C) (3COCH3), 142.2–115.5 (Ar-C), 84.8 (C-1), 72.9 (C-3), 70.9 (C-5), 69.9 (C-4), 69.6 (C-2), 20.7, 20.5 (2C) (3 COCH3), 17.4 (CCH3); ESI-MS: 438.1 [M + Na]+; anal. calcd for C18H22FNO7S (415.43): C, 52.04; H, 5.34; found: C, 51.87; H, 5.53.
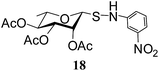
N-(3-Nitrophenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-L-rhamnopyranosylsulfenamide (18). 1H NMR (500 MHz, CDCl3): δ 7.56–6.90 (m, 4H, Ar-H), 5.41 (dd, J = 3.5, 1.5 Hz, 1H, H-2), 5.07 (t, J = 10.0 Hz, 1H, H-4), 4.97 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 4.64 (br s, 1H, H-1), 3.97 (br s, 1H, NH), 3.63–3.60 (m, 1H, H-5), 2.21, 2.05, 1.98 (3 s, 9H, 3 COCH3), 1.33 (d, J = 6.5 Hz, 3H, CCH3); 13C NMR (125 MHz, CDCl3): δ 169.8, 169.4 (2C) (3 COCH3), 129.8–119.0 (Ar-C), 84.8 (C-1), 72.9 (C-3), 70.9 (C-5), 69.9 (C-4), 69.6 (C-2), 20.7, 20.5 (2C) (3 CH3CO), 17.4 (CCH3); ESI-MS: 465.1 [M + Na]+; anal. calcd for C18H22N2O9S (442.44): C, 48.86; H, 5.01; found: C, 48.70; H, 5.20.
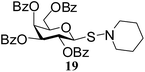
N-Piperidinyl-1-S-(2,3,4,6-tetra-O-benzoyl)-β-D-galactopyranosyl sulfenamide (19). 1H NMR (500 MHz, CDCl3): δ 8.12–7.23 (m, 20H, Ar-H), 6.10 (d, J = 3.0 Hz, 1H, H-4), 5.90 (t, J = 10.0 Hz, 1H, H-2), 5.16 (dd, J = 10.0, 3.5 Hz, 1H, H-3), 5.02 (d, J = 10.0 Hz, 1H, H-1), 4.62–4.60 (m, 1H, H-5), 4.41–4.37 (m, 2H, H-6ab), 3.07–3.04 (m, 2H, NCH2), 2.94–2.92 (m, 2H, NCH2), 1.58–1.50 (m, 4H, CH2), 1.38–1.36 (m, 2H, CH2); 13C NMR (125 MHz, CDCl3): δ 165.4 (2C), 165.1 (2C) (4 PhCO), 133.5–128.2 (Ar-C), 86.2 (C-1), 74.8 (C-3), 73.2 (C-4), 68.5 (C-2), 66.0 (C-5), 62.5 (C-6), 59.0 (2C, NCH2), 27.0 (2C, CH2); ESI-MS: 718.2 [M + Na]+; anal. calcd for C39H37NO9S (695.21): C, 67.32; H, 5.36; found: C, 67.20; H, 5.50.
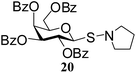
N-Pyrrolidinyl-1-S-(2,3,4,6-tetra-O-benzoyl)-β-D-galactopyranosyl sulfenamide (20). 1H NMR (500 MHz, CDCl3): δ 8.08–7.23 (m, 20H, Ar-H), 6.01 (d, 2.5 Hz, 1H, H-4), 5.99 (t, J = 10.0 Hz, 1H, H-2), 5.63 (dd, J = 10.0, 3.5 Hz, 1H, H-3), 4.89 (d, J = 10.0 Hz, 1H, H-1), 4.68–4.62 (m, 1H, H-5), 4.39–4.35 (m, 2H, H-6ab), 3.13–3.10 (m, 4H, NCH2), 1.82–1.78 (m, 4H, CH2); 13C NMR (125 MHz, CDCl3): δ 165.9, 165.5, 165.3, 165.2 (4 PhCO), 133.5–128.2 (Ar-C), 87.7 (C-1), 74.8 (C-3), 73.3 (C-4), 68.4 (C-2), 66.0 (C-5), 62.4 (C-6), 57.0 (2C, NCH2), 25.6 (2C, CH2); ESI-MS: 704.2 [M + Na]+; anal. calcd for C38H35NO9S (681.20): C, 66.95; H, 5.17; found: C, 66.80; H, 5.30.
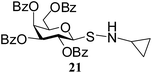
N-Cyclopropyl-1-S-(2,3,4,6-tetra-O-benzoyl)-β-D-galactopyranosyl sulfenamide (21). 1H NMR (500 MHz, CDCl3): δ 8.07–7.23 (m, 20H, Ar-H), 6.04 (d, J = 3.0 Hz, 1H, H-4), 5.98 (t, J = 10.0 Hz, 1H, H-2), 5.75 (dd, J = 10.0, 3.5 Hz, 1H, H-3), 4.70–4.65 (m, 1H, H-5), 4.50 (d, J = 10.0 Hz, 1H, H-1), 4.40–4.30 (m, 2H, H-6ab), 3.50 (br s, 1H, NH), 2.88–2.82 (m, 1H, NCH), 1.51–1.49 (m, 4H, CH2); 13C NMR (125 MHz, CDCl3): δ 165.4 (2C), 165.2 (2C) (4 PhCO), 133.5–128.2 (Ar-C), 88.4 (C-1), 75.0 (C-3), 72.6 (C-4), 68.4 (C-2), 66.0 (C-5), 62.1 (C-6), 33.6 (NCH), 9.0, 8.7 (2C, CH2); ESI-MS: 690.1 [M + Na]+; anal. calcd for C37H33NO9S (667.18): C, 66.55; H, 4.98; found: C, 66.40; H, 5.20.
Typical experimental condition for the preparation of glycosyl sulfinamide (22–29)
A solution of glycosyl sulfenamide (1.0 mmol) in CH2Cl2 (15 mL) was cooled to −20 °C. To the cooled reaction mixture was added mCPBA (1.0 mmol) and it was allowed to stir at same temperature for 3 h. The reaction was quenched by addition of satd. Na2SO3 (15 mL) and extracted with CH2Cl2 (50 mL). The organic layer was successively washed with satd. NaHCO3 (50 mL), H2O (50 mL), dried (Na2SO4) and concentrated. The crude product was purified over SiO2 using hexane–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give pure sulfinamide derivatives (22–29) as a mixture of regioisomers (Table 3). Analytical data of synthesized compounds those are not reported earlier:
2) to give pure sulfinamide derivatives (22–29) as a mixture of regioisomers (Table 3). Analytical data of synthesized compounds those are not reported earlier:
N-Piperidinyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfinamide (22) (major isomer). 1H NMR (500 MHz, CDCl3): δ 5.33 (t, J = 9.0 Hz, 1H, H-3), 5.25 (t, J = 9.5 Hz, 1H, H-2), 5.05 (t, J = 8.5 Hz, 1H, H-4), 4.25–4.19 (m, 2H, H-6ab), 4.11 (d, J = 10.0 Hz, 1H, H-1), 3.71–3.69 (m, 1H, H-5), 3.22–3.17 (m, 4H, 2 OCH2), 2.06, 2.03, 2.02, 2.01 (4 s, 12H, 4 COCH3), 1.70–1.54 (m, 6H, 3 CH2); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7, 168.8, 168.7 (4C, 4 CH3CO), 90.1 (C-1), 76.4 (C-5), 73.5 (C-3), 68.3 (C-4), 67.7 (C-2), 61.9 (C-6), 48.4 (NCH2), 26.2 (2C), 24.3 (3 CH2), 20.5, 20.4 (2C), 20.3 (4 CH3CO); ESI-MS: 486.1 [M + Na]+; anal. calcd for C19H29NO10S (463.50): C, 49.23; H, 6.31; found: C, 49.10; H, 6.47.
N-Morpholinyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfinamide (23) (major isomer). 1H NMR (500 MHz, CDCl3): δ 5.32 (t, J = 9.0 Hz, 1H, H-3), 5.24 (t, J = 9.5 Hz, 1H, H-2), 5.01 (t, J = 8.5 Hz, 1H, H-4), 4.25–4.19 (m, 1H, H-6a), 4.17–4.12 (m, 2H, H-1, H-6b), 3.75–3.69 (m, 5H, H-5, 2 OCH2), 3.03–3.20 (m, 4H, 2 NCH2), 2.06, 2.03, 2.02, 2.01 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.0, 169.7, 168.8, 168.7 (4C, 4 CH3CO), 90.5 (C-1), 77.2 (C-5), 73.2 (C-3), 68.0 (C-4), 67.8 (C-2), 67.0 (OCH2), 61.9 (C-6), 47.4 (NCH2), 20.5, 20.4 (2C), 20.3 (4 CH3CO); ESI-MS: 488.1 [M + Na]+; anal. calcd for C18H27NO11S (465.47): C, 46.45; H, 5.85; found: C, 46.30; H, 6.00.
N-Benzyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfinamide (24) (major isomer). 1H NMR (500 MHz, CDCl3): δ 7.37–7.25 (m, 5H, Ar-H), 5.22–5.17 (m, 2H, H-2, H-3), 5.07 (t, J = 9.5 Hz, 1H, H-4), 4.68–4.66 (m, 1H, NH), 4.35–4.15 (m, 5H, H-1, H-6ab, NCH2), 4.17–4.15 (m, 1H, H-5), 2.10, 2.07, 2.02, 2.01 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7 (2C), 169.0 (4 COCH3), 138.6–124.1 (Ar-C), 91.7 (C-1), 76.9 (C-5), 73.7 (C-3), 68.4 (C-4), 67.4 (C-2), 61.1 (C-6), 48.2 (CNH), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 508.1 [M + Na]+; anal. calcd for C21H27NO10S (485.50): C, 51.95; H, 5.61; found: C, 51.80; H, 5.75.
N-(3-Methoxyphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfinamide (25): (major isomer). 1H NMR (500 MHz, CDCl3): δ 7.03–6.36 (m, 4H, Ar-H), 5.43 (t, J = 9.5 Hz, 1H, H-2), 5.21–5.06 (m, 3H, NH, H-3, H-4), 4.41 (d, J = 10.0 Hz, 1H, H-1), 4.29–4.06 (m, 2H, H-6ab), 3.80 (s, 3H, OCH3), 3.72–3.68 (m, 1H, H-5), 2.12, 2.01, 1.99, 1.92 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.0, 169.7, 169.6, 169.0 (4 COCH3), 160.3–107.9 (Ar-C), 89.0 (C-1), 69.9 (C-5), 69.8 (C-3), 69.2 (C-4), 67.8 (C-2), 61.3 (C-6), 55.2 (OCH3), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 524.1 [M + Na]+; anal. calcd for C21H27NO11S (501.13): C, 50.29; H, 5.43; found: C, 50.12; H, 5.58.
N-(3,4-Dihydroisoquinolinyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfinamide (26) (major isomer). 1H NMR (500 MHz, CDCl3): δ 7.26–6.95 (m, 4H, Ar-H), 5.51 (t, J = 10.0 Hz, 1H, H-2), 5.36 (d, J = 3.0 Hz, 1H, H-4), 5.06 (dd, J = 10.0, 3.5 Hz, 1H, H-3), 4.48–4.40 (s, 2H, NCH2), 4.20 (d, J = 10.0 Hz, 1H, H-1), 4.16–4.00 (m, 2H, H-6ab), 3.38–3.26 (m, 2H, NCH2), 3.85–3.79 (m, 1H, H-5), 3.55–3.50 (m, 2H, CH2), 2.09, 2.02, 2.01, 1.97 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7, 169.0 (4 COCH3), 138.9–114.0 (Ar-C), 90.9 (C-1), 75.2 (C-5), 71.4 (C-4), 66.8 (C-3), 65.5 (C-2), 61.4 (C-6), 46.8 (NCH2), 44.6 (NCH2), 33.8 (CH2), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 534.1 [M + Na]+; anal. calcd for C23H29NO10S (511.54): C, 54.00; H, 5.71; found: C, 53.82; H, 5.85.
N-(4-Benzylpiperidinyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfinamide (27) (major isomer). 1H NMR (500 MHz, CDCl3): δ 7.27–7.08 (m, 5H, Ar-H), 5.52 (t, J = 10.0 Hz, 1H, H-2), 5.42 (d, J = 3.0 Hz, 1H, H-4), 5.06 (dd, J = 9.5, 3.5, Hz, 1H, H-3), 4.10 (d, J = 10.0 Hz, 1H, H-1), 4.09–4.03 (m, 2H, H-6ab), 3.96–3.89 (m, 1H, H-5), 3.62–3.48 (m, 2H, NCH2), 2.88–2.75 (m, 2H, NCH2), 2.54 (d, J = 6.5 Hz, 2H, PhCH2), 2.15, 2.05, 2.01, 1.98 (4 s, 12H, 4 COCH3), 1.80–1.60 (m, 2H, CH, CH2); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7 (2C), 169.0 (4 COCH3), 139.6–125.7 (Ar-C), 90.6 (C-1), 75.1 (C-5), 71.4 (C-4), 65.3 (C-3), 65.2 (C-2), 61.2 (C-6), 47.2 (NCH2), 43.0 (NCH2), 36.3 (CH), 32.5 (PhCH2), 32.3 (CH2), 29.8 (CH2), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 576.2 [M + Na]+; anal. calcd for C26H35NO10S (553.62): C, 56.41; H, 6.37; found: C, 56.30; H, 6.55.
N-(2-Furanylmethyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfinamide (28) (major isomer). 1H NMR (500 MHz, CDCl3): δ 7.38–6.27 (m, 3H, Ar-H), 5.48 (d, J = 2.5 Hz, 1H, H-4), 5.36 (t, J = 10.0 Hz, 1H, H-2), 5.06 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 4.77–4.70 (m, 1H, NH), 4.40–4.26 (m, 3H, H-1, CH2), 4.24–4.16 (m, 2H, H-6ab), 4.06–4.02 (m, 1H, H-5), 2.16, 2.07, 2.05, 1.87 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.0, 169.9, 169.8, 169.7 (4 COCH3), 153–107.2 (Ar-C), 89.6 (C-1), 75.3 (C-5), 71.6 (C-4), 66.7 (C-3), 65.7 (C-2), 60.5 (C-6), 40.5 (NCH2), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 498.1 [M + Na]+; anal. calcd for C19H25NO11S (475.47): C, 48.00; H, 5.30; found: C, 47.86; H, 5.45.
N-(3-Methylphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfinamide (29) (major isomer). 1H NMR (500 MHz, CDCl3): δ 7.07–6.64 (m, 4H, Ar-H), 5.58 (t, J = 10.0 Hz, 1H, H-2), 5.46 (br s, 2H, H-4, NH), 5.21 (dd, J = 9.5, 3.0 Hz, 1H, H-3), 4.40 (d, J = 10.0 Hz, 1H, H-1), 4.38–4.18 (m, 2H, H-6ab), 4.15–4.10 (m, 3H, H-5), 2.34 (s, 3H, CH3), 2.17, 2.07, 2.02, 1.95 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 170.1, 170, 169.9, 169.7 (4 COCH3), 140.0–116.6 (Ar-C), 89.6 (C-1), 76.9 (C-5), 71.5 (C-4), 67.8 (C-3), 65.3 (C-2), 61.4 (C-6), 22.4 (CH3), 20.8, 20.5 (2C), 20.3 (4 COCH3); ESI-MS: 508.1 [M + Na]+; anal. calcd for C21H27NO10S (485.50): C, 51.95; H, 5.61; found: C, 51.80; H, 5.75.
Typical experimental condition for the preparation of glycosyl sulfonamide (30–35)
To a solution of glycosyl sulfenamide (1.0 mmol) in CH3CN–H2O (15 mL; 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was added a mixture of solid KMnO4/CuSO4·5H2O (500 mg; 1.5
1 v/v) was added a mixture of solid KMnO4/CuSO4·5H2O (500 mg; 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and it was allowed to stir at room temperature for appropriate time (Table 4). After completion of the reaction (TLC; hexane
1 molar ratio) and it was allowed to stir at room temperature for appropriate time (Table 4). After completion of the reaction (TLC; hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 1
EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the reaction mixture concentrated under reduced pressure and the crude mass was extracted with CH2Cl2 (50 mL). The organic layer was washed with water (50 mL), dried (Na2SO4) and concentrated under reduced pressure. The crude product was purified over SiO2 using hexane–EtOAc (3
1), the reaction mixture concentrated under reduced pressure and the crude mass was extracted with CH2Cl2 (50 mL). The organic layer was washed with water (50 mL), dried (Na2SO4) and concentrated under reduced pressure. The crude product was purified over SiO2 using hexane–EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give pure sulfonamide derivatives (30–35) (Table 4). Analytical data of synthesized compounds those are not reported earlier:
1) to give pure sulfonamide derivatives (30–35) (Table 4). Analytical data of synthesized compounds those are not reported earlier:
N-Piperidinyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfonamide (30). 1H NMR (500 MHz, CDCl3): δ 5.34 (t, J = 9.5 Hz, 1H, H-2), 5.24 (t, J = 9.5 Hz, 1H, H-3), 5.09 (t, J = 9.5 Hz, 1H, H-4), 4.50 (d, J = 10.0 Hz, 1H, H-1), 4.26 (dd, J = 12.5, 5.0 Hz, 1H, H-6a), 4.20 (dd, J = 12.5, 6.5 Hz, 1H, H-6b), 3.80–3.77 (m, 1H, H-5), 3.40–3.30 (br s, 4H, 4 NCH), 2.08, 2.05, 2.04, 2.01 (4 s, 12H, 4 COCH3), 1.70–1.55 (m, 6H, 6 CH); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7 (2C), 169 (4 COCH3), 87.9 (C-1), 76.1 (C-5), 73.3 (C-3), 67.5 (C-4), 67.4 (C-2), 61.5 (C-6), 47.5 (NCH2), 25.9 (CH2), 23.8 (CH2), 20.5, 20.4 (2C), 20.3 (4 CH3CO); ESI-MS: 502.1 [M + Na]+; anal. calcd for C19H29NO11S (479.50): C, 47.59; H, 6.10; found: C, 47.42; H, 6.25.
N-Benzyl-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfonamide (31). 1H NMR (500 MHz, CDCl3): δ 7.57–7.22 (m, 6H, Ar-H, NH), 5.22 (t, J = 10.0 Hz, 1H, H-2), 5.15–5.10 (m, 1H, H-3), 4.99 (t, J = 9.5 Hz, 1H, H-4), 4.45 (dd, J = 12.5, 6.0 Hz, 1H, H-6a), 4.36 (dd, J = 11.0, 5.5 Hz, 1H, H-6b), 4.29–4.20 (m, 2H, NCH2), 4.11 (d, J = 12.5 Hz, 1H, H-1), 3.67–3.62 (m, 1H, H-5), 2.10, 2.07, 2.02, 2.01 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7 (2C), 169.0 (4 COCH3), 140.6–124.1 (Ar-C), 87.7 (C-1), 76.2 (C-5), 72.7 (C-3), 67.7 (C-4), 67.4 (C-2), 61.0 (C-6), 53.0 (CNH), 20.6, 20.5 (2C), 20.4 (4 CH3CO); ESI-MS: 524.1 [M + Na]+; anal. calcd for C21H27NO11S (501.50): C, 50.29; H, 5.43; found: C, 50.15; H, 5.60.
N-(2-Methylphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-glucopyranosyl sulfonamide (32). 1H NMR (500 MHz, CDCl3): δ 7.44–7.11 (m, 4H, Ar-H), 5.44 (t, J = 10.0 Hz, 1H, H-2), 5.29 (br s, 1H, NH), 5.26 (t, J = 9.5 Hz, 1H, H-3), 5.10 (t, J = 9.5 Hz, 1H, H-4), 4.46 (d, J = 10.0 Hz, 1H, H-1), 4.23 (dd, J = 9.0, 3.5 Hz, 1H, H-6a), 4.12–4.06 (m, 1H, H-6b), 3.82–3.79 (m, 1H, H-5), 2.39 (s, 3H, CH3), 2.07, 2.05, 2.02, 1.97 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.9, 169.7, 169.5, 168.8 (4 COCH3), 134.1–119 (6C, Ar-C), 86.1 (C-1), 76.3 (C-5), 72.8 (C-3), 67.5 (C-4), 67.3 (C-2), 60.0 (C-6), 20.8 (CH3), 20.8, 20.4 (2C), 20.3 (4 CH3CO); ESI-MS: 524.1 [M + Na]+; anal. calcd. for C21H27NO11S (501.50): C, 50.29; H, 5.43; found: C, 50.15; H, 5.62.
N-(3,4-Dihydroisoquinolinyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfonamide (33). 1H NMR (500 MHz, CDCl3): δ 7.25–7.07 (m, 4H, Ar-H), 5.50 (t, J = 10.0 Hz, 1H, H-2), 5.34 (d, J = 3.0 Hz, 1H, H-4), 5.03 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 4.66–4.54 (m, 2H, H-6ab), 4.52 (d, J = 10.0 Hz, 1H, H-1), 3.96–3.94 (m, 3H, NCH2, H-5), 3.76–3.66 (m, 2H, NCH2), 2.99–2.97 (m, 2H, CH2), 2.07, 2.00, 1.97, 1.96 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.6, 169.5 (2C), 169 (4 COCH3), 133.1–125.9 (Ar-C), 88.8 (C-1), 74.7 (C-5), 71.2 (C-4), 66.5 (C-3), 64.4 (C-2), 60.6 (C-6), 47.9 (NCH2), 44.1 (NCH2), 29.2 (CH2), 20.6, 20.3 (2C), 20.2 (4 CH3CO); ESI-MS: 550.1 [M + Na]+; anal. calcd for C23H29NO11S (527.54): C, 52.36; H, 5.54; found: C, 52.20; H, 5.67.
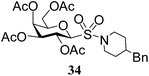
N-(4-Benzylpiperidinyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfonamide (34). 1H NMR (500 MHz, CDCl3): δ 7.27–7.08 (m, 5H, Ar-H), 5.50 (t, J = 10.0 Hz, 1H, H-2), 5.41 (br s, 1H, H-4), 5.05 (d, J = 10.0 Hz, 1H, H-3), 4.47 (d, J = 10.0 Hz, 1H, H-1), 4.19–4.10 (m, 2H, H-6ab), 4.02–3.99 (m, 1H, H-5), 3.91–3.82 (m, 2H, NCH2), 2.92–2.80 (m, 2H, NCH2), 2.56 (d, J = 7.0 Hz, 2H, PhCH2), 2.19, 2.06, 2.04, 1.99 (4 s, 12H, 4 COCH3), 1.78–1.70 (m, 2H, CH2), 1.67–1.63 (m, 1H, CH); 13C NMR (125 MHz, CDCl3): δ 169.7, 169.5, 168.9 (2C) (4 COCH3), 139.1–123.8 (Ar-C), 88.6 (C-1), 74.6 (C-5), 71.2 (C-4), 66.7 (C-3), 64.3 (C-2), 60.9 (C-6), 47.4 (CNH), 46.5 (CNH), 42.7 (PhCH2), 37.7 (CH), 32.1 (CH2), 32.0 (CH2), 20.6, 20.5, 20.4, 20.3 (4 CH3CO); MALDI-MS: 592.1 [M + Na]+; anal. calcd for C26H35NO11S (569.62): C, 54.82; H, 6.19; found: C, 54.70; H, 6.35.
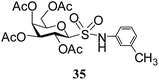
N-(3-Methylphenyl)-1-S-(2,3,4,6-tetra-O-acetyl)-β-D-galactopyranosyl sulfonamide (35). 1H NMR (500 MHz, CDCl3): δ 7.20–6.99 (m, 4H, Ar-H), 5.59 (t, J = 10.0 Hz, 1H, H-2), 5.31 (s, 1H, NH), 5.29 (d, J = 1.2 Hz, 1H, H-4), 5.05 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 4.41 (d, J = 10.0 Hz, 1H, H-1), 4.15–4.10 (m, 2H, H-6ab), 3.99–3.95 (m, 1H, H-5), 2.36 (s, 3H, CH3), 2.12, 2.05, 2.00, 1.97 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3): δ 169.6, 169.5 (2C), 169.4 (4 COCH3), 139.0–119.1 (Ar-C), 86.7 (C-1), 74.9 (C-5), 70.9 (C-4), 66.6 (C-3), 64.5 (C-2), 61.1 (C-6), 50.2 (NCH), 21.3 (CH3), 20.7, 20.5, 20.4, 20.3 (4 CH3CO); ESI-MS: 524.1 [M + Na]+; anal. calcd for C21H27NO11S (501.50): C, 50.29; H, 5.43; found: C, 50.16; H, 5.61.
Acknowledgements
A. G. thanks CSIR, New Delhi for providing junior research fellowship. This work was supported by SERB, New Delhi (Project No. EMR/2015/000282) (AKM).
Notes and references
-
(a) E. J. Grayson, S. J. Ward, A. L. Hall, P. M. Rendle, D. P. Gamblin, A. S. Batsanov and B. G. Davis, J. Org. Chem., 2005, 70, 9740–9754 CrossRef CAS PubMed;
(b) K. Pachamuthu and R. R. Schmidt, Chem. Rev., 2006, 106, 160–187 CrossRef CAS PubMed;
(c) M. L. Uhrig, V. E. Manzano and O. Varela, Eur. J. Org. Chem., 2006, 162–168 CrossRef CAS;
(d) M. von Itzstein, Curr. Opin. Struct. Biol., 2008, 18, 558–566 CrossRef CAS PubMed.
-
(a) L. Lázár, M. Csávás, M. Tóth, L. Somsak and A. Borbás, Chem. Pap., 2015, 69, 889–895 Search PubMed;
(b) Y. Ding, M. O. Contour-Galcera, J. Ebel, C. Ortiz-Mellet and J. Defaye, Eur. J. Org. Chem., 1999, 1143–1152 CrossRef CAS;
(c) M. Yoshikawa, T. Murakami, K. Yashiro and H. Matsuda, Chem. Pharm. Bull., 1998, 46, 1339–1340 CrossRef CAS PubMed;
(d) Z. J. Witczak and J. M. Culhane, Appl. Microbiol. Biotechnol., 2005, 69, 237–244 CrossRef CAS PubMed.
-
(a) B. Ernst and J. L. Magnani, Nat. Rev. Drug Discovery, 2009, 8, 661–677 CrossRef CAS PubMed;
(b) Z. J. Witczak and D. Boryczewski, Bioorg. Med. Chem. Lett., 1998, 8, 3265–3268 CrossRef CAS PubMed.
- F. Schweizer and O. Hindsgaul, Curr. Opin. Chem. Biol., 1999, 3, 291–298 CrossRef CAS PubMed.
- K. Dzierzba, M. Grec, G. Pastuch-Gawolek, T. Lipinski, J. Pietkiewicz and A. Gamian, Acta Pol. Pharm., 2012, 69, 1224–1238 CAS.
-
(a) D. P. Galonic and D. Y. Gin, Nature, 2007, 446, 1000–1007 CrossRef CAS PubMed;
(b) R. Adamo, A. Nilo, B. Castagner, O. Boutureira, F. Berti and G. J. L. Bernardes, Chem. Sci., 2013, 4, 2995–3008 RSC.
- E. M. Scanlan, V. Corcé and A. Malone, Molecules, 2014, 19, 19137–19151 CrossRef PubMed.
- C. T. Supuran, Nat. Rev. Drug Discovery, 2008, 7, 168–181 CrossRef CAS PubMed.
- M. Lopez, N. Drillaud, L. F. Bornaghi and S.-A. Poulsen, J. Org. Chem., 2009, 74, 2811–2816 CrossRef CAS PubMed.
- M. Lopez, L. F. Bornaghi, H. Driguez and S.-A. Poulsen, J. Org. Chem., 2011, 76, 2965–2975 CrossRef CAS PubMed.
-
(a) D. J. Owen and M. von Itzstein, Carbohydr. Res., 2000, 328, 287–292 CrossRef CAS PubMed;
(b) D. J. Owen, C. B. Davis, R. D. Hartnell, P. D. Madge, R. J. Thomson, A. K. J. Chong, R. L. Coppel and M. von Itzstein, Bioorg. Med. Chem. Lett., 2007, 17, 2274–2277 CrossRef CAS PubMed.
- S. Knapp, E. Darout and B. Amorelli, J. Org. Chem., 2006, 71, 1380–1389 CrossRef CAS PubMed.
-
(a) T.-Z. Illyes, D. Molnar-Gabor and L. Szilagyi, Carbohydr. Res., 2004, 339, 1561–1564 CrossRef CAS PubMed;
(b) M. Hürzeler, B. Bernet and A. Vasella, Helv. Chim. Acta, 1992, 75, 557–588 CrossRef.
- J. Gildersleeve, R. A. Pascal Jr and D. Kahne, J. Am. Chem. Soc., 1998, 120, 5961–5969 CrossRef CAS.
- Y. Abe, T. Nakabayashi and J. Tsurugi, Bull. Chem. Soc. Jpn., 1973, 46, 1898–1899 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of NMR spectra of compounds 4–31. See DOI: 10.1039/c7ra05339h |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*































![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CH3CN–H2O (5
1) in CH3CN–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature to furnish excellent yield of corresponding sulphonamide derivatives (30–35) in short period of time (Table 5).
1) at room temperature to furnish excellent yield of corresponding sulphonamide derivatives (30–35) in short period of time (Table 5).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give pure compound 5–21 (Table 2). All products were characterized using their spectral analysis. Analytical data of synthesized compounds those are not reported earlier:
1) to give pure compound 5–21 (Table 2). All products were characterized using their spectral analysis. Analytical data of synthesized compounds those are not reported earlier:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give pure sulfinamide derivatives (22–29) as a mixture of regioisomers (Table 3). Analytical data of synthesized compounds those are not reported earlier:
2) to give pure sulfinamide derivatives (22–29) as a mixture of regioisomers (Table 3). Analytical data of synthesized compounds those are not reported earlier:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was added a mixture of solid KMnO4/CuSO4·5H2O (500 mg; 1.5
1 v/v) was added a mixture of solid KMnO4/CuSO4·5H2O (500 mg; 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and it was allowed to stir at room temperature for appropriate time (Table 4). After completion of the reaction (TLC; hexane
1 molar ratio) and it was allowed to stir at room temperature for appropriate time (Table 4). After completion of the reaction (TLC; hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 1
EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the reaction mixture concentrated under reduced pressure and the crude mass was extracted with CH2Cl2 (50 mL). The organic layer was washed with water (50 mL), dried (Na2SO4) and concentrated under reduced pressure. The crude product was purified over SiO2 using hexane–EtOAc (3
1), the reaction mixture concentrated under reduced pressure and the crude mass was extracted with CH2Cl2 (50 mL). The organic layer was washed with water (50 mL), dried (Na2SO4) and concentrated under reduced pressure. The crude product was purified over SiO2 using hexane–EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give pure sulfonamide derivatives (30–35) (Table 4). Analytical data of synthesized compounds those are not reported earlier:
1) to give pure sulfonamide derivatives (30–35) (Table 4). Analytical data of synthesized compounds those are not reported earlier:







