Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Precursor preparation of Zn–Al layered double hydroxide by ball milling for enhancing adsorption and photocatalytic decoloration of methyl orange
Jun Qu*a,
Xiaoman Hea,
Xuewei Lia,
Ziqiang Aia,
Yujie Lia,
Qiwu Zhang *a and
Xinzhong Liub
*a and
Xinzhong Liub
aSchool of Resources and Environmental Engineering, Wuhan University of Technology, Luoshi Road 122, Wuhan, Hubei 430070, China. E-mail: zhangqw@whut.edu.cn; forsjun@whut.edu.cn; Tel: +86 17771490085
bCollege of Ecological Environment and Urban Construction, Fujian University of Technology, Fuzhou 350118, China
First published on 21st June 2017
Abstract
An amorphous photocatalyst was prepared via simple dry milling of Zn basic carbonate (Zn4CO3(OH)6·H2O) and Al hydroxide (Al(OH)3) as a precursor of Zn–Al layered double hydroxide (LDH) and the precursor was agitated in water to simply synthesize the Zn–Al LDH for comparison. The adsorption and photocatalytic activity of the Zn–Al LDH and the precursor were studied via the removal of methyl orange (MO) and decoloration of MO under ultraviolet light irradiation in aqueous solution, respectively. The precursor exhibits much higher decoloration efficiency towards MO than the Zn–Al LDH product because of the synergistic effect of the intercalation reaction and its photocatalytic activity. The amorphous precursor could easily incorporate MO molecules to form MO intercalated LDH, allowing easy access of the organic pollutant to the photocatalyst sample, resulting in an obvious improvement in photocatalytic performance. The novel idea to use the precursor sample instead of the synthesized final product may be applied in other fields to increase the required performances.
1 Introduction
Layered double hydroxides (LDH), also known as anion clays or hydrotalcite-like compounds, with zinc or titanium located in their sheets have been widely used as photocatalysts1 for the degradation of organic pollutants or water-splitting.2,3 No photocatalytic properties were reported with Mg–Al LDHs, whereas the ternary Mg–Zn–Al LDH formed with zinc was found to be able to degrade organic pollutants under light irradiation,4 and high photocatalytic performance was obtained when Mg was entirely replaced by Zn, forming the binary Zn–Al LDH. There has been much work on the synthesis and modification of Zn–Al to enhance its photocatalytic activity. Dutta et al.5 synthesized a composite with Zn–Al LDH and ZnO and reported high efficiency for the decomposition of Congo Red. Lin et al.6 proposed a method to manufacture titanate-intercalated Zn–Al LDH and demonstrated its superior photocatalytic activity. Carriazo et al.7 studied the photocatalytic activity of Zn–Al LDH calcined at different temperatures. In these reports, significant attention was paid to control the morphology of LDH or develop composite catalysts with precious metals. These elaborate efforts will surely increase the cost of production and thus limit the actual value of these materials in practical applications.8,9Tongamp et al.10,11 first reported a solvent-free mechanochemical method (two-step milling: dry milling followed by wet milling) to synthesize Mg–Al LDH. Subsequently, other types of LDH were fabricated.12 Qu et al.13–15 synthesized Ca–Al LDH, Li–Al LDH and tetraborate pillared Li–Al via the two-step milling method and Ferencz et al.16,17 prepared Ca–Fe and Ca–Sn LDH in the same manner. The reported mechanochemical approach based on solid state reaction exhibits some obvious advantages such as no need for heating treatment and no discharge of waste water as in the traditional hydrothermal process. Recently improvements in the adsorption of target pollutants were observed with the mechanochemically prepared LDH products. The dry milling sample, which is known as the precursor, was found to be an excellent anion adsorbent toward anion organic or inorganic pollutants in our recently published works. Wang et al.18 compared the MO adsorption performance of the precursor of Mg–Al LDH, which was prepared by the dry milling of magnesium hydroxide and aluminum hydroxide, with that of well-crystallized Mg–Al LDH products and found that the precursor showed an obviously higher adsorption capacity toward MO. The precursor of ettringite, which was prepared by the dry milling of dihydrate calcium sulfate, calcium hydroxide and aluminum hydroxide, also possessed a much higher adsorption capacity toward phosphate than that of ettringite.19 However, the abovementioned work is limited to the area of anion adsorption. Thus, more work is needed to broaden the application of mechanochemically prepared precursors.
Close contact between the photocatalyst and the organic pollutant is essential for the photocatalytic reaction, which is believed to have a massive impact on the photocatalytic efficiency. Extensive research has been conducted to enhance the access of photocatalysts to target pollutants, whether by increasing the specific surface area of the photocatalyst or dispersing the photocatalyst in porous supporting materials such as diatomite.3,20 However, such elaborate efforts usually increase the production cost, therefore it is difficult to use these materials to purify contaminated water.
Our recent work18 proved that the precursor of Mg–Al LDH prepared by ball-milling exhibits a much higher adsorption capacity than that of the synthesized adsorbent which was mainly realized by surface adsorption. Herein, based on our recently reported phenomenon that the precursor exhibits much higher adsorption capacity than the synthesized adsorbent, we prepare an amorphous photocatalyst (the precursor of Zn–Al LDH) by simply dry-milling Zn4CO3(OH)6·H2O and Al(OH)3 to introduce further photocatalytic activity besides the enhanced adsorption performance. The precursor is found to show much better adsorption and photocatalytic performance toward MO than the well-crystallized Zn–Al LDH, which indicates that the precursor may achieve both easy access to the target by adsorption and decoloration by catalysis. It is believed that such easily prepared precursor meets both the requirements of low cost and high performance and would contribute to the purification of waste water. As a reference experiment, the cationic organic molecule rhodamine B (RHB), which cannot be adsorbed by the LDH, is also used to compare the photocatalytic actions between the precursor and Zn–Al LDH samples.
2 Experimental
2.1 Materials and methods
Zn4CO3(OH)6·H2O and Al(OH)3 (Sinopharm Group Co Ltd., China, analytical reagent) were used as raw materials with no further purification. A planetary ball mill (Pulverisette-7, Fritsch, Germany), which has two mill pots (45 cm3 inner volume each) made of zirconium dioxide with 7 zirconium dioxide balls of 15 mm in diameter, was used to conduct the ball-milling operation. Methyl orange (C14H14N3SO3Na, Aladdin®, China, content 96%) and rhodamine B (C28H31ClN2O3, Aladdin®, China, analytical reagent) were applied to simulate organic contaminated waste water.A two-step operation was used for the synthesis of the precursor and Zn–Al LDH. In the first dry grinding process, 2 g of basic zinc carbonate and Al(OH)3 (Zn/Al molar ratio of 2/1) mixture was ground on a planetary ball mill for two hours at 600 rpm to obtain the precursor. In the second agitation step, 1 g of the precursor was put into a capped Erlenmeyer flask filled with 100 mL of distilled water and stirred for 4 hours on a magnetic stirring apparatus (524G, Meiyingpu, China) at 1200 rpm to fabricate Zn–Al LDH.
2.2 Characterization
XRD analysis was used to identify the phases and phase changes of the samples on a powder diffractometer (MAX-RB RU-200B, Rigaku, Japan) using CuKα radiation (λ = 1.5403 Å) in the 2θ range of 5° and 70°. The microstructures of the prepared samples were observed on a scanning electron microscope (JSM-5610LVJEOL, Jeol, Japan). FT-IR spectra of the samples were obtained on Nicolet 6700, Thermo, America, using KBr as a diluent over 4000–500 cm−1. A UV/VIS/NIR spectrometer (Lambda 750 S, PerkinElmer, America) was used to record the UV-vis diffusive reflectance spectra of the prepared samples.2.3 Adsorption and photocatalytic activity
3 Results and discussion
3.1 Results
Our previous study18 demonstrated the feasibility of manufacturing LDH via a mechanochemical route in which Mg(OH)2 and Al(OH)3 are first activated by dry ball milling to obtain the precursor and then stirred in water to prepare Mg–Al LDH. Thus, a similar mechanochemical approach was applied to fabricate Zn–Al LDH and its precursor using Zn4CO3(OH)6·H2O and Al(OH)3 as raw materials. Fig. 1 depicts the XRD patterns of the raw materials mixture, the precursor (prepared by dry milling of raw materials at 600 rpm with Zn/Al molar ratio at 2/1) and Zn–Al LDH (fabricated by agitating the precursor in water). The diffraction peaks of Zn4CO3(OH)6·H2O and Al(OH)3 were observed in the XRD patterns of the raw materials. After the dry milling operation, an amorphous sample, namely the precursor, was obtained. Agitating the precursor in water at room temperature resulted in the formation of Zn–Al LDH (JCPDS card 48-1022) with the d003 value of 0.77 nm.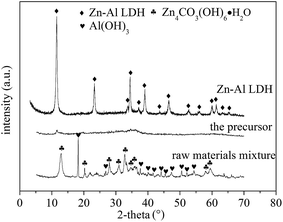 | ||
| Fig. 1 XRD patterns of the raw materials mixture, precursor (prepared by dry milling of raw materials at 600 rpm) and Zn–Al LDH (fabricated by agitating the precursor in water). | ||
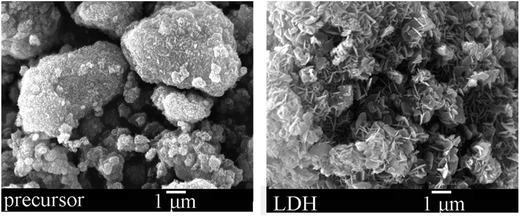
To show the microstructure of the precursor and LDH, the SEM photographs of the precursor and Zn–Al LDH are displayed in Fig. 2. The particles in the precursor sample formed large thick-massive agglomerates without an evident layered structure. When the precursor was agitated in water to prepare the LDH sample, a sheet structure was observed. The agitation operation helped the grain growth of crystalline Zn–Al LDH. In this work, we evaluated the adsorption as well as the photocatalytic performance of the Zn–Al LDH precursor toward MO. The Zn–Al LDH prepared by grinding and agitation operation here was also studied under the same conditions for comparison.
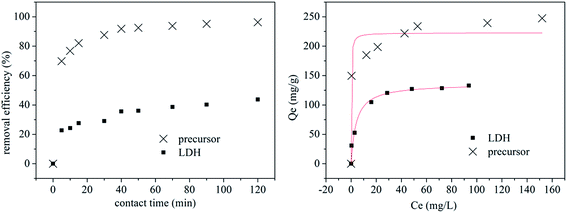
The excellent adsorption performances of the Mg–Al LDH precursor18 and ettringite precursor19 were reported in our previous papers. In this work, the precursor of Zn–Al LDH also shows outstanding adsorption efficiency toward MO. Fig. 3 exhibits the adsorption kinetic experiment (left) and adsorption isotherm experiment (right) results of the precursor and Zn–Al LDH toward MO. The Zn–Al LDH displays a lower removal rate (43.69%) than that of the precursor (96.25%) toward MO after 120 min stirring. The adsorption isotherm experiment results show that the adsorption capacity of the precursor could reach about 250 mg g−1, which is nearly the double that of the Zn–Al LDH (132 mg g−1) toward MO. The Langmuir isotherm, which is assumed to undergo monolayer type coverage of the sorbent on an adsorbent surface,21 was used to describe the obtained data of adsorption isotherm experiments as shown by the red line in Fig. 3. Eqn (1) represents the Langmuir isotherm model.
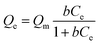 | (1) |
The adsorption kinetic experiment data were processed as pseudo-first order and pseudo-second order and the results are presented in Table 1. The rate constant of the precursor was higher than that of the LDH which indicates a higher adsorption rate for the removal of MO by the precursor. Furthermore, the pseudo-second order model was found to fit better with the correlation coefficient R2 of 0.9961 and 0.9441 for the precursor and LDH, respectively, which are higher than that of the pseudo-first order model. The adsorption rate-determining steps for MO removal by the precursor and LDH were believed to be chemical absorption.
| Model | Pseudo-first order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|
| Parameters | k1 | R2 | qe (mg g−1) | k2 | R2 | qe (mg g−1) |
| a Where, k1 and k2 are the rate constants, qt is the amount of solute adsorbed on the adsorbent at time t, and qe is the amount at equilibrium. | ||||||
| Precursor | 0.2406 | 0.9729 | 114.3019 | 0.0038 | 0.9961 | 120.4404 |
| LDH | 0.1102 | 0.8779 | 46.9974 | 0.0029 | 0.9441 | 52.4855 |
Zn–Al has been demonstrated to be an excellent photocatalyst for the degradation of organic pollutants.7,23 In this work, more attention is paid not only to the adsorption property but also the photocatalytic performance of the precursor toward MO.
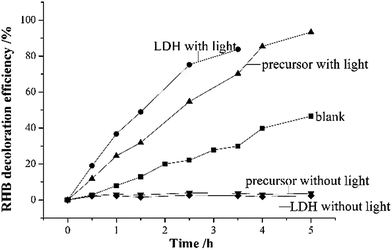
The contact area between a photocatalyst and organic pollutants is believed to have a massive impact on its photocatalytic efficiency. A higher contact area often results in a better photocatalytic performance.3,20 The adsorption experiments demonstrate that the precursor could adsorb more MO molecules into its structure, or in other words, the precursor provides a higher contact area for the MO molecules which might promote the photocatalytic decoloration efficiency. To confirm this assumption, the photocatalytic performance of the precursor and LDH for the decoloration of MO solution was determined and the results are displayed in Fig. 5.
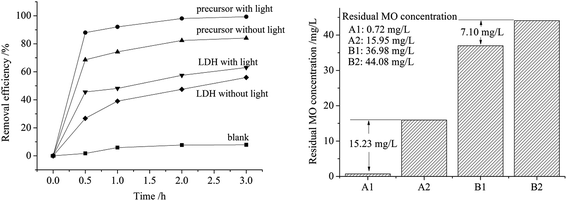
Fig. 4 exhibits the experimental data of the photocatalytic decoloration of MO (initial concentration 100 ppm) in aqueous solution by the precursor and as-prepared Zn–Al LDH and the comparison in the remaining MO concentrations by the precursor (A1 with irradiation and A2 without irradiation) and the LDH (B1 with irradiation and B2 without irradiation). The precursor could remove MO up to almost 100% under irradiation, whereas the LDH sample reached a removal rate of only 63% under the same conditions. A better adsorption performance of the precursor toward MO without light irradiation was also observed with an 84% removal rate for the precursor and 56% for the LDH, where a similar improvement in adsorption capacity by the precursor to that of the synthesized LDH was reported for the Mg–Al LDH sample.18
Although a further decrease in the remaining MO concentrations after the treatment of light irradiation to the adsorption operation was observed on both the precursor and LDH samples, a larger decrease by 15.23 mg L−1 was observed with the precursor than that of the LDH sample by 7.10 mg L−1, which indicates higher photocatalytic activity by the precursor sample. It is understood that it is more difficult for the photocatalyst to degrade pollutants at low concentrations. The precursor realized enhanced photocatalytic performance in a relatively low concentration of MO which also proves the improvement in photochemical catalytic ability. For a clear description of the photocatalytic decoloration results, a pseudo-first order model was used to fit the kinetic curve of the MO photocatalytic decoloration, as shown in Table 2. High correlation coefficient R2 values were obtained, which indicates that the pseudo-first-order model well explains the kinetic curve of MO photocatalytic decoloration. The highest rate constant was observed for the photocatalytic decoloration of MO by the precursor, which indicates the highest reaction rate. The simulated qe was 121.33 mg L−1 and 102.05 mg L−1 with a gap of 18.83 mg L−1 for the precursor with or without irradiation, respectively, whereas the gap for LDH with or without light was 4.88 mg L−1 which is much less than that of the precursor. Both the experimental and simulated data prove the enhanced photocatalytic performance of the precursor for the photocatalytic decoloration of MO. We believe that the improvement in photocatalytic activity might result from the physically closer contact of the MO to the adsorbent, as confirmed by the higher removal efficiency in the adsorption section. This simply prepared precursor in a state of massive agglomeration demonstrates better adsorption for MO molecules when dispersed in MO solution, and decomposes the adsorbed MO compositions much more easily when irradiated under ultraviolet light. Thus, the precursor sample exhibits good performances for both adsorption and catalysis.
| Parameter | Pseudo-first order qt = qe(1 − e−kt) | ||
|---|---|---|---|
| k | R2 | qe | |
| a Where, k1 is the rate constant, qt is the amount of solute adsorbed on the adsorbent at time t, and qe is the amount at equilibrium. | |||
| Precursor with light | 4.5620 | 0.9959 | 121.3376 |
| Precursor without light | 3.4225 | 0.9914 | 102.0477 |
| LDH with light | 2.5042 | 0.9647 | 73.7035 |
| LDH without light | 1.2396 | 0.9893 | 68.8201 |
3.2 Discussion
Fig. 5 shows the XRD patterns of LDH, L-MO-LDH (LDH sample with MO adsorption) and P-MO-LDH (the precursor sample with MO adsorption). New peaks at d003 = 2.5 nm and d006 = 1.24 nm were observed in both L-MO-LDH and P-MO-LDH which could be attributed to the phase of MO intercalated LDH.26 The peak intensity represents the content of the corresponding material. To compare the relative amount of MO intercalated LDH in the L-MO-LDH and P-MO-LDH samples, eqn (2) was applied.
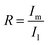 | (2) |
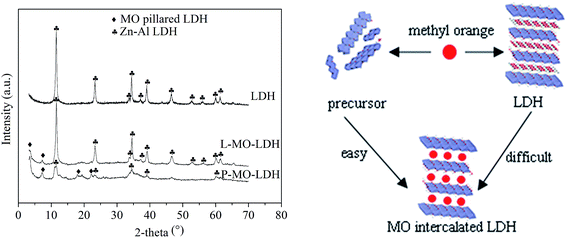 | ||
| Fig. 5 XRD patterns (left) of LDH, L-MO-LDH (LDH sample with MO adsorption) and P-MO-LDH (the precursor sample with MO adsorption) and the adsorption model (right) of the precursor and LDH. | ||
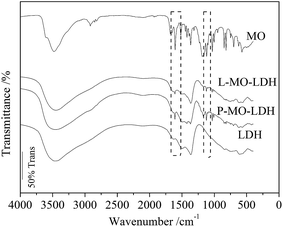
Besides the XRD characterization with the typical appearances of MO intercalated LDH, IR analysis is another effective way to describe the MO intercalation reaction of Zn–Al LDH. Fig. 6 displays the FT-IR spectra of LDH, P-MO-LDH, L-MO-LDH and MO. The LDH sample shows the typical FT-IR spectrum of Zn–Al–CO3 LDH,27 in which the broad absorption band at 3461 cm−1 is due to the O–H stretching of the hydroxyl groups. The peaks below 1000 cm−1 are attributed to the lattice vibration bands of M–O (M = Zn and Al). The peaks at 1364 cm−1, 1492 cm−1 and 1620 cm−1 result from the asymmetric stretching vibrations of the interlayer carbonate anions, the ν3 vibrations of the carbonate and the infrared bands of free water, respectively. After MO adsorption, the typical infrared bands of the MO molecule such as 1606 cm−1 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching) and 1119 cm−1 (the vibrations of –SO3) are clearly observed in the spectra of P-MO-LDH (1609 cm−1 and 1122 cm−1, respectively) and L-MO-LDH (1609 cm−1 and 1123 cm−1, respectively) as shown by the dashed box in Fig. 7. The transmittance of the bands is a reflection of the corresponding material content in the sample. It is observed that the transmittance of the peaks at around 1609 cm−1 (N
N stretching) and 1119 cm−1 (the vibrations of –SO3) are clearly observed in the spectra of P-MO-LDH (1609 cm−1 and 1122 cm−1, respectively) and L-MO-LDH (1609 cm−1 and 1123 cm−1, respectively) as shown by the dashed box in Fig. 7. The transmittance of the bands is a reflection of the corresponding material content in the sample. It is observed that the transmittance of the peaks at around 1609 cm−1 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching) and 1122 cm−1 (the vibrations of –SO3) is lower for P-MO-LDH than that of L-MO-LDH, which indicates more MO molecules exist in P-MO-LDH and is consistent with the results in Fig. 5.
N stretching) and 1122 cm−1 (the vibrations of –SO3) is lower for P-MO-LDH than that of L-MO-LDH, which indicates more MO molecules exist in P-MO-LDH and is consistent with the results in Fig. 5.
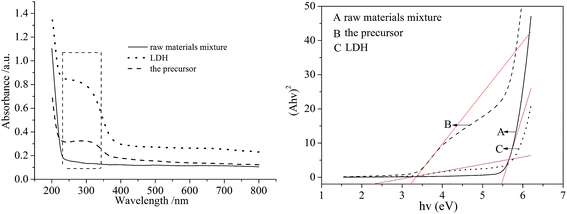 | ||
| Fig. 7 UV-vis adsorption spectra (left) and plots of (Ahν)2 vs. hν (right) for the raw materials mixture, precursor and LDH samples. | ||
From the results in Fig. 5 and 6, it can be concluded that two mechanisms contributed to the enhanced adsorption activity of the precursor: the disorderly precursor could provide more adsorption sites than the closed structure of Zn–Al LDH for MO removal and the precursor could intercalate more MO molecules into the gallery space of LDH than that of the Zn–Al LDH.
| Ahν = K(hν − Eg)n/2 | (3) |
We reported that the co-grinding of raw materials to produce an amorphous precursor is essential for its adsorption properties in our previously published work on the precursor of Ca–Al for Cr(VI) removal.30 The co-existence of the raw materials or simply mixed separately milled materials showed no obvious adsorption behavior for anionic pollutants. Combining the results of the UV-vis adsorption spectra and band gaps of the prepared samples, it can be concluded that dry milling induced a chemical reaction between the raw materials, forming the basic structure of Zn–Al LDH which enhanced the photocatalytic action for MO molecules.
Close contact between the photocatalyst and the organic pollutant is essential for the photocatalytic reaction. Increasing the specific surface area of the photocatalyst or dispersing the photocatalyst in porous supporting materials provides a higher contact area for the photocatalyst to decompose organic pollutants.20 In this precursor system, the enhanced photocatalytic activity of the precursor also from the high contact area of the photocatalyst. It has been proven that the precursor could provide more adsorption sites for MO molecules and intercalate more MO molecules into its gallery space. In other words, the contact area between the precursor and MO molecules was definitely higher than that of the LDH.
Fig. 8 displays the XRD patterns of the precursor and LDH samples dispersed in MO solution with or without irradiation. Without light irradiation, in the patterns of both samples, besides the peaks of Zn–Al LDH phases, new peaks at d003 = 2.5 nm and d006 = 1.24 nm are observed which could be attributed to the phase of MO intercalated LDH.23 The peak intensity of MO intercalated LDH from the precursor sample is much higher than that from the LDH sample, which confirms the higher adsorption of MO. With light irradiation, the peaks of MO intercalated LDH from the LDH sample disappeared completely and became much lower for the precursor sample. As is well known, the photocatalytic reaction degrades organic molecules on the surface of photocatalysts such as TiO2.31 In the case of LDH, the photocatalytic activity sites came from the layered sheets of the LDH. The catalytic sites in the internal space might not work well if the organic pollutant is just adsorbed on the surface, which is quite a long distance from the activity sites. MO intercalation makes it possible for the efficient use of catalytic sites in the internal space as well as that on the surface. In other words, the contact area between the photocatalyst and organic pollutants could be increased when a intercalation reaction takes place. The results in Fig. 8 demonstrate that the photocatalytic properties of LDH came from the sheets of the Zn–Al LDH and intercalated MO molecules were decomposed in the gallery space, as the photocatalytic model shows in Fig. 8. The precursor could intercalate more MO molecules into the layered space of LDH to contact with the sheets of the LDH, which means a higher contact area between MO and the precursor, resulting in the higher photocatalytic efficiency of the precursor.
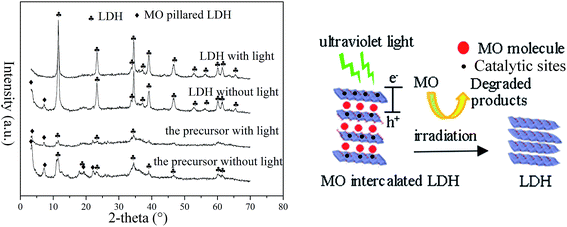 | ||
| Fig. 8 XRD patterns (left) of the used samples for the removal of MO with or without irradiation and the photocatalytic model (right) of the precursor and LDH. | ||
Many previous works have established and discussed the photocatalytic activities of Zn type LDH.32,33 Zn–Al LDH could produce two main intermediate active species (superoxide O2˙− and hydroxyl radicals OH˙) under irradiation for the photocatalytic decoloration of MO. Clear evidence29,33 has proven that O2˙− plays the main role in the initiation of the photocatalytic process in LDH photocatalytic systems.
The LDH samples are well known anionic absorbents, which exhibit high capacity toward anionic organic samples such as MO in this work. On the other hand, very low adsorption capacity was observed with the cationic reagent RHB. To verify our assumption that the excellent adsorption performance of the precursor contributes to the enhanced photocatalytic activity, the photocatalytic decoloration of RHB (initial concentration 4 ppm) by the precursor and as-prepared Zn–Al LDH, in which no adsorption process was involved, was conducted as a reference experiment, and the results are shown in Fig. 9. Without light irradiation, no obvious changes in the remaining RHB concentration were observed on both the precursor and Zn–Al LDH samples, which confirm that they have no adsorption ability toward RHB. Under irradiation, both samples exhibited photocatalytic activity toward RHB and higher efficiency was obtained by the LDH sample than the precursor. Without the adsorption action in this case, it was reasonable to obtain higher photocatalytic efficiency by using the Zn–Al LDH than the precursor sample due to the much better crystallinity, dispersion state and light adsorption of the Zn–Al LDH, as shown in Fig. 1, 2 and 4. These results from RHB reflect that in the case of the precursor to the MO, there exists a synergistic effect of both adsorption and photocatalytic action to enhance the removal efficiency, which is as high as close to complete decoloration.
The data from both MO and RHB confirm the photocatalytic action of the precursor and Zn–Al LDH samples prepared via a simple milling operation. The precursor sample shows enhanced adsorption and photocatalytic activity compared to that of the Zn–Al LDH sample toward MO. The disordered precursor provides more adsorption sites and intercalates more MO molecules into the gallery space of LDH than the closed structure of Zn–Al LDH for MO removal. Thus, a high contact area between the precursor and MO was obtained which resulted in enhanced adsorption and photocatalytic activity. The efficient MO removal almost to complete decoloration particularly on the precursor sample at a relatively high concentration confirms that this method could produce photocatalysts with a high performance at a low cost. Another obvious advantage is that hydroxides and carbonates, which are stable, cheap and easily available compared with the soluble nitrate or sulfate salts of zinc and aluminium used by the co-precipitation method, are directly used as starting samples to prepare the products without emission of other impurities and wastewater. We believe that the precursor prepared by this method could be applied as photocatalyst and would contribute to real wastewater treatment due its overall super performance as a result of the synergistic effect of its adsorption and catalysis capacity.
4 Conclusions
An amorphous precursor of the Zn–Al LDH photocatalyst was simply prepared by dry milling Zn4CO3(OH)6·H2O and Al(OH)3. The precursor intercalates more MO molecules into the layered space of LDH to contact with the sheets of the LDH and exhibits super a photocatalytic performance toward MO decoloration based on the synergistic effects of intercalation reaction and catalysis. This useful sample could be easily obtained without elaborate efforts to prepare fine particles with high specific surface areas or composites to disperse the catalyst in a supporting material to increase both adsorption and catalysis performances, thus offering a cheap alternative to contribute to wastewater treatment at a reasonably low cost. The idea of using the precursor instead of the synthesized final product may serve other purposes where the close access of the target sample by adsorption is required.Acknowledgements
This work was supported by “the Fundamental Research Funds for the Central Universities” (grant no. 2016-YB-027).References
- S. J. Xia, F. X. Liu, Z. M. Ni, J. L. Xue and P. P. Qian, Layered double hydroxides as efficient photocatalysts for visible-light degradation of rhodamine B, J. Colloid Interface Sci., 2013, 405, 195–200 CrossRef CAS PubMed.
- Z. Yang, F. Wang, C. Zhang, G. Zeng, X. Tan, Z. Yu, Y. Zhong, H. Wang and F. Cui, Utilization of LDH-based materials as potential adsorbents and photocatalysts for the decontamination of dyes wastewater: a review, RSC Adv., 2016, 6, 79415–79436 RSC.
- K. M. Parida and L. Mohapatra, Carbonate intercalated Zn/Fe layered double hydroxide: a novel photocatalyst for the enhanced photo degradation of azo dyes, Chem. Eng. J., 2012, 179, 131–139 CrossRef CAS.
- J. S. Valente, F. Tzompantzi, J. Prince, J. G. H. Cortez and R. Gomez, Adsorption and photocatalytic degradation of phenol and 2,4-dichlorophenoxiacetic acid by Mg–Zn–Al layered double hydroxides, Appl. Catal., B, 2009, 90, 330–338 CrossRef CAS.
- K. Dutta, S. Das and A. Pramanik, Concomitant synthesis of highly crystalline Zn–Al layered double hydroxide and ZnO: phase interconversion and enhanced photocatalytic activity, J. Colloid Interface Sci., 2012, 366, 28–36 CrossRef CAS PubMed.
- B. Lin, P. Sun, Y. Zhou, S. Jiang, B. Gao and Y. Chen, Interstratified nanohybrid assembled by alternating cationic layered double hydroxide nanosheets and anionic layered titanate nanosheets with superior photocatalytic activity, J. Hazard. Mater., 2014, 280, 156–163 CrossRef CAS PubMed.
- D. Carriazo, M. D. Arco, E. García-López, G. Marcì, C. Martín, L. Palmisano and V. Rives, Zn, Al hydrotalcites calcined at different temperatures: preparation, characterization and photocatalytic activity in gas–solid regime, J. Mol. Catal. A: Chem., 2011, 342–343, 83–90 CrossRef CAS.
- D. Basu, A. Das, D. Wang, J. J. George, K. W. Stöckelhuber, R. Boldt, A. Leuteritz and G. Heinrich, Fire-safe and environmentally friendly nanocomposites based on layered double hydroxides and ethylene propylene diene elastomer, RSC Adv., 2016, 6, 26425–26436 RSC.
- Á. Deák, L. Janovák, E. Csapó, D. Ungor, I. Pálinkó, S. Puskás, T. Ördög, T. Ricza and I. Dékány, Layered double oxide (LDO) particle containing photoreactive hybrid layers with tunable superhydrophobic and photocatalytic properties, Appl. Surf. Sci., 2016, 389, 294–302 CrossRef.
- W. Tongamp, Q. Zhang and F. Saito, Preparation of meixnerite (Mg–Al–OH) type layered double hydroxide by a mechanochemical route, J. Mater. Sci., 2007, 42, 9210–9215 CrossRef CAS.
- W. Tongamp, Q. Zhang and F. Saito, Mechanochemical route for synthesizing nitrate form of layered double hydroxide, Powder Technol., 2008, 185, 43–48 CrossRef CAS.
- J. Qu, Q. Zhang, X. He and S. Song, Mechanochemical approaches to synthesize layered double hydroxides: a review, Appl. Clay Sci., 2016, 119, 185–219 CrossRef CAS.
- J. Qu, X. He, B. Wang, L. Zhong, L. Wan, X. Li, S. Song and Q. Zhang, Synthesis of Li–Al layered double hydroxides via a mechanochemical route, Appl. Clay Sci., 2016, 120, 24–27 CrossRef CAS.
- J. Qu, L. Zhong, Z. Li, M. Chen, Q. Zhang and X. Liu, Effect of anion addition on the syntheses of Ca–Al layered double hydroxide via a two-step mechanochemical process, Appl. Clay Sci., 2016, 124–125, 267–327 CrossRef CAS.
- J. Qu, X. Li, Z. Lei, Z. Li, M. Chen and Q. Zhang, Mechano-hydrothermal synthesis of tetraborate pillared Li–Al layered double hydroxides, J. Am. Ceram. Soc., 2016, 99, 151–1154 CrossRef.
- Z. Ferencz, M. Szabados, M. Ádok-Sipiczki, K. Ákos, Z. Kónya, P. Sipos and I. Pálinkó, , Mechanochemically assisted synthesis of pristine Ca(II)Sn(IV)-layered double hydroxides and their amino acid intercalated nanocomposites, J. Mater. Sci., 2014, 49, 8478–8486 CrossRef CAS.
- Zs. Ferencz, M. Szabados, G. Varga, Z. Csendes, Á. Kukovecz, Z. Kónya, S. Carlson, P. Sipos and I. Pálinkó, Mechanochemical synthesis and intercalation of Ca(II)Fe(III)-layered double hydroxides, J. Solid State Chem., 2016, 233, 236–243 CrossRef CAS.
- B. Wang, J. Qu, X. Li, X. He and Q. Zhang, Precursor preparation to promote the adsorption of Mg–Al layered double hydroxide, J. Am. Ceram. Soc., 2016, 99, 2882–2885 CrossRef CAS.
- L. Zhong, J. Qu, X. Li, X. He and Q. Zhang, Simultaneous synthesis of ettringite and absorbate incorporation by aqueous agitation of a mechanochemically prepared precursor, RSC Adv., 2016, 6, 35203–35209 RSC.
- Z. Sun, Z. Hu, Y. Yan and S. Zheng, Effect of preparation conditions on the characteristics and photocatalytic activity of TiO2/purified diatomite composite photocatalysts, Appl. Surf. Sci., 2014, 314, 251–259 CrossRef CAS.
- L. Ma, Q. Wang, S. M. Islam, Y. Liu, S. Ma and M. G. Kanatzidis, Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42− ion, J. Am. Chem. Soc., 2016, 138, 2858–2866 CrossRef CAS PubMed.
- A. Farrukh, A. Akram, A. Ghaffar, S. Hanif, A. Hamid, H. Duran and B. Yameen, Design of polymer-brush-grafted magnetic nanoparticles for highly efficient water remediation, ACS Appl. Mater. Interfaces, 2013, 5, 3784–3793 CAS.
- E. M. Seftel, E. Popovici, M. Mertens, K. D. Witte, G. V. Tendeloo, P. Cool and E. F. Vansant, Zn-Al layered double hydroxides: synthesis, characterization and photocatalytic application, Microporous Mesoporous Mater., 2008, 113, 296–304 CrossRef CAS.
- L. Mohapatra, D. Patra, K. Parida and S. J. Zaidi, Enhanced Photocatalytic Activity of a Molybdate-Intercalated Iron-Based Layered Double Hydroxide, Eur. J. Inorg. Chem., 2017, 3, 723–733 CrossRef.
- L. Mohapatra and K. Parida, A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts, J. Mater. Chem. A, 2016, 4, 10744–10766 CAS.
- P. Zhang, G. Qian, H. Shi, X. Ruan, J. Yang and R. L. Frost, Mechanism of interaction of hydrocalumites (Ca/Al-LDH) with methyl orange and acidic scarlet GR, J. Colloid Interface Sci., 2012, 365, 110–116 CrossRef CAS PubMed.
- K. Yang, L. G. Yan, Y. M. Yang, S. J. Yu, R. R. Shan, H. Q. Yu, B. C. Zhu and B. Du, Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: kinetics, isotherms and mechanisms, Sep. Purif. Technol., 2014, 124, 36–42 CrossRef CAS.
- S.-J. Xia, F.-X. Liu, Z.-M. Ni, W. Shi, J.-L. Xue and P.-P. Qian, Ti-based layered double hydroxides: Efficient photocatalysts for azo dyes degradation under visible light, Appl. Catal., B, 2014, 144, 570–579 CrossRef CAS.
- H. Li, Q. Deng, J. Liu, W. Hou, N. Du, R. Zhang and X. Tao, Synthesis, characterization and enhanced visible light photocatalytic activity of Bi2MoO6/Zn–Al layered double hydroxide hierarchical heterostructures, Catal. Sci. Technol., 2014, 4, 1028–1037 CAS.
- L. Zhong, X. He, J. Qu, X. Li, Z. Lei, Q. Zhang and X. Liu, Precursor preparation for Ca-Al layered double hydroxide to remove hexavalent chromium coexisting with calcium and magnesium chloride, J. Solid State Chem., 2017, 245, 200–206 CrossRef CAS.
- D. P. Kumar, N. L. Reddy, M. Karthikeyan, N. Chinnaiah, V. Bramhaiah, V. D. Kumari and M. V. Shankar, Synergistic effect of nanocavities in anatase TiO2, nanobelts for photocatalytic degradation of methyl orange dye in aqueous solution, J. Colloid Interface Sci., 2016, 477, 201–208 CrossRef PubMed.
- K. Parida, L. Mohapatra and N. Baliarsingh, Effect of Co2+ substitution in the framework of carbonate intercalated Cu/Cr LDH on structural, electronic, optical, and photocatalytic properties, J. Phys. Chem. C, 2012, 116, 22417–22424 CAS.
- L. Mohapatra, K. Parida and M. Satpathy, Molybdate/tungstate intercalated oxo-bridged Zn/Y LDH for solar light induced photodegradation of organic pollutants, J. Phys. Chem. C, 2012, 116, 13063–13070 CAS.
| This journal is © The Royal Society of Chemistry 2017 |