Open Access Article
Open Access ArticleDirect writing of a conducting polymer pattern in aqueous solution by using an ultrashort laser pulse†
Neha Agarwala,
Hyobong Ryu a,
Melanie Mangangb,
Wilhelm Pflegingb and
Jungtae Kim*a
a,
Melanie Mangangb,
Wilhelm Pflegingb and
Jungtae Kim*a
aKorea Institute of Science and Technology Europe GmbH, Campus E7 1, 66123 Saarbrücken, Germany. E-mail: tais@kist-europe.de
bApplied Material Physics, Karlsruhe Institute of Technology, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 7th August 2017
Abstract
A new method for the polymerization and patterning of a conducting polymer by using a femtosecond laser is presented. In this research, pyrrole-3-carboxylic acid (PCA), an acid derivative of pyrrole, was used because of its higher water solubility and favorable adhesion to the glass surface. The PCA pattern was fabricated in a microfluidic channel between gold electrodes and comprised micro-/nano structures. The optimized parameters of the femtosecond laser were investigated for obtaining sharp and defined conducting polymer patterns. The aqueous state of the polymerization enhanced the possibility of applying additional dopants for extending the functionality of the pattern, including embedding of bio molecules. The generated patterns of the conducting polymer were observed and evaluated by atomic force microscopy and conductivity measurements.
Introduction
Conducting polymers are widely used in many applications, such as organic light-emitting diodes, solar cells, memory devices, and field-effect transistors (FETs), because of their good conductivity and processability.1,2 Various conducting polymers such as polypyrrole,3 polythiophene,4 polyaniline5 or poly(3,4-ethylenedioxythiophen) (PEDOT)6–8 provide not only good conductivity but also biocompatibility for electrochemical sensing, and incorporation of biological molecules such as proteins or cells. They can be also prepared in aqueous solution which avoids denaturation and conformational changes of biomolecules compared to organic solvents.9 Among the various conducting polymers, polypyrrole is one of the most widely used materials due to its excellent stability under severe environmental conditions.10Different approaches have been utilized in order to fabricate polypyrrole patterns onto a surface of the substrate such as chemical, electrochemical polymerization and photo-polymerization.11 However, lithography and other chemical polymerization processes may include either high temperature or high acidic or basic environments which would not be applicable for biological molecules as well as many processing steps and photomasks.12
In comparison to the conventional methods like lithography or electrochemical polymerization, direct laser writing (DLW) is utilized as the advancement in the field of polymerization and structuring due to the following advantages;13,14 DLW has several advantages of high precision structures, easy fabrication of structures, fast and relatively reproducible patterns without a photomask. With the utilization of the ultrashort pulses, nonlinear optical processes such as two-photon absorption can take place. The structure formations are three dimensional in micro/nano scale.
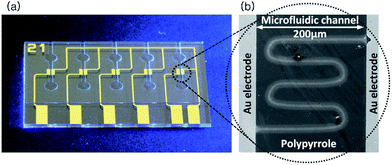
Furthermore, the DLW can be adequately utilized in aqueous condition, which is the most favorable for biological materials. The conventional chemical and electrochemical polymerization techniques in aqueous solution is slow and this long polymerization time leads to irregular and unstable solidification generating inaccurate structures.15 Whereas DLW of the aqueous solution to fabricate sub-micron structures on the substrate is advantageous as the electron flow for polymerization can be maintained local only in the interested area. Therefore, the writing area is only the focal area and the structures are formed in the focal plane.12 Also, the adhesion of the polymer layer to the substrate is one of the advantages of the DLW process due to the varied emission wavelengths depending on the mechanism used on the photon generation.16 Since, the monomers can be prepared in different concentrations to form the aqueous solution; there is a possibility of applying additional dopants in order to enhance the functionalities of the material.17 In this work, we have performed pyrrole polymerization in aqueous solution by using direct fs laser writing technique. In order to conduct the process, a microfluidic chip has been fabricated which has gold electrodes in a microfluidic channel on a quartz glass substrate (Fig. 1(a)). The polymerization was done in the gap between the gold electrodes. The aqueous mixture solution was injected into the microfluidic channel and the laser beam was focused onto the glass surface adjacent to the gold electrode edge for the polymerization to take place.
The pattern has been drawn using the laser to form micro-/nano structures of the conductive polymer as shown in Fig. 1(b). After the polymerization, conductivity and surface topology of the structure were measured and analyzed.
Materials and methods
Materials
Pyrrole-3-carboxylic acid (PCA) of analytical grade was purchased from Sigma-Aldrich. (3-Aminopropyl) triethoxysilane and N-(3-triethoxysilypropyl)-4-hydroxybutyramide, i.e., both the different types of silanes were used for the silanization of the surfaces and were bought from Sigma-Aldrich. The polyamidoamine (PAMAM) G4.0 dendrimers were obtained via Sigma-Aldrich Chemie GmBH from Dendritech Inc. (USA). A 100 mM aqueous solution of PCA was prepared from dissolving of 22 μg of PCA in 2 mL of distilled water. A dendrimer mixed solution was prepared with 50% (vol%) mixture of PAMAM G4.0 dendrimers and PCA.Microfluidic chip
The quartz glass, 700 nm thick, was cleaned by piranha solution. After the rinsing step with using de-ionized (DI) water, 10 nm Cr as adhesion layer, and 20 nm Au layer were deposited by magnetron sputtering. The electrode pattern was defined by optical lithography using positive photoresist and subsequent wet etching process with a mixture of KI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I2
I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI water (4
DI water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to remove the Au, and a dedicated chromium etchant. Finally, micro channel structures have been fabricated by using 100 μm thick SU-8 dry film and photolithography processes on the quartz glass wafer.
40) to remove the Au, and a dedicated chromium etchant. Finally, micro channel structures have been fabricated by using 100 μm thick SU-8 dry film and photolithography processes on the quartz glass wafer.
Methods
The microfluidic chips were cleaned in an ultrasonic bath for 5 minutes using isopropanol and then dried with nitrogen. Oxygen plasma treatment was applied for 3 minutes with the power of 70 W. In order to get –OH or –NH2 terminated groups on the quartz glass surface, 2% N-(3-triethoxysilypropyl)-4-hydroxybutyramide or (3-aminopropyl) triethoxysilane was prepared in toluene and the chips were dip-casted for 30 minutes in an oven at 80 °C respectively. After rinsing with toluene and drying with nitrogen, the chips were cured at 100 °C in an oven for an hour.Conducting polymer patterns were fabricated by using a micromachining workstation (PS450-TO, Optec, Belgium) equipped with an ultrafast fiber laser (Tangerine, Amplitude Systems, France) in Karlsruhe Nano Micro Facility. It is operated with an average power of 20 W and maximum pulse energy of 100 μJ at 1030 nm (TEMOO with M2 < 1.3). The pulse duration can be tuned in the range from 350 fs up to 10 ps and the wavelength can be switched from fundamental (1030 nm) to 2nd (515 nm) or 3rd (343 nm) harmonics. For the polymerization study, the 343 nm wavelength was chosen with a laser repetition rate of 200 kHz and a spot size of about 20 μm (see ESI: Fig. S2†). The pulse duration was set to 400 fs. The laser beam was scanned over the sample surface using a RhothorTM Laser Deflection Systems scan head (Newson Engineering BV, Belgium). The process was performed by scanning the laser beam using a f-theta lens with a focal length of 100 mm. The chip was flipped over and mounted on the XYZ piezo stage. The laser beam was focused through the 700 μm quartz glass substrate into the aqueous solution which was injected in the microfluidic channel. The laser movement and the focusing on the sample were controlled by the software of the laser system. Several laser and process parameters such as laser power, writing speed, number of writing repetitions and focal position were tested.
A phase contrast microscope was employed in order to take images of the polymerized pattern. Atomic force microscopy was also done with a Park Systems NX10 AFM equipped with a non-contact mode probe (PPP-NCLR-10, Nanosensors). The conductivity of the patterns was measured using Keysight B1500A Semiconductor Device Analyzer.
Results and discussion, experimental
The results of laser polymerization were dependent on several factors comprising of surface type of the substrate, different aqueous mixture solutions including concentration variations and the laser parameters. A number of tests have been performed to fabricate fine and sharp pattern from polymerized PCA. The adhesion between a deposited polymer and a substrate is mainly determined by the interfacial properties of the substrate. In this work, the adhesion property has been tested by two different surface modifications. (3-Aminopropyl)triethoxysilane (APTES) and N-(3-triethoxysilypropyl)-4-hydroxybutyramide were used introducing –NH2 and –OH groups on the quartz glass surface respectively for enhancement of the adhesion. Several preliminary tests were performed for the optimization of the surface properties suitable for the polymerization. It was found that the –OH terminated groups showed relatively uniform adhesion along the laser writing path whereas the –NH2 terminated groups led to strong but scattered and radical adhesion (see ESI: Fig. S3†).During the laser polymerization process, various parameters of the laser were tested whereas the speed of the laser beam was always maintained at 100 mm s−1. We selected low power that could derive polymerization with maximum 1000 repetitions suppressing possible damage of biological molecules in future applications. The laser power was varied between 0.3–7 mW (34–40% of the laser power).
However, maximum number of repetitions was kept low during the tests with laser power of 2 mW or higher because, for those laser power and high number of repetitions, the surface between the gold electrodes showed no pattern along the laser path whereas the gold electrodes were obviously damaged by the laser.
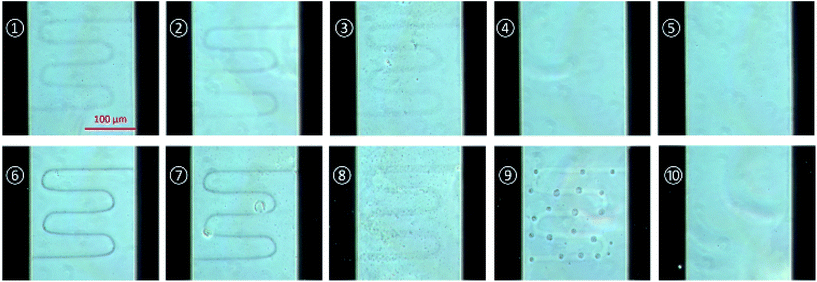
For the comparison of the results, writing by the laser power of 0.3 mW and 0.6 mW were tested with several writing repetitions such as 1000, 750, 500, 250 and 100 and the microscopic images were taken as follows.
Fig. 2 shows the polymerized structures between the gold electrodes from a laser power of 0.3 mW and 0.6 mW with various writing repetitions. The image was taken by phase contrast microscope after isopropanol washing and drying. As shown in Fig. 2, the conducting polymer structure with 0.6 mW laser power shows more precisely defined structure than 0.3 mW and can be formed well as repetitions number increase.
However, the conductivity results show that polymerization induced by a laser power of 0.3 mW leads to a higher conductivity in comparison to the results of 0.6 mW power. A comparative data of the conductivity measurements are shown in Fig. 3. Patterns on hydroxyl group terminated surface showed higher conductivity in overall. The conductivity of max. 17 S cm−1 has been obtained from PCA polymer pattern with 0.3 mW laser power and 750 laser writing repetition. The patterns with a higher laser power (0.6 mW) showed low conductivity although the pattern appearance was finer.
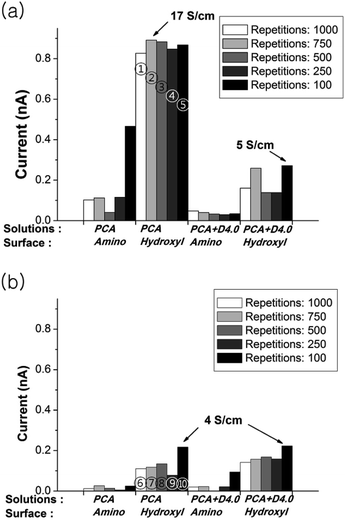 | ||
| Fig. 3 Conductivity comparisons of fabricated patterns by various parameters. The current is measured at 2 V and laser power is (a) 0.3 mW and (b) 0.6 mW. ①–⑩ are denoted to the samples of Fig. 2. | ||
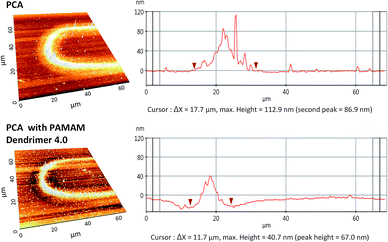
Fig. 4 shows AFM analysis results from one of the curved part of the pattern. The polymerized pattern shows that the width of the structure is around 18 μm in accordance with diameter of the laser spot. However, only the area of 7–8 μm width along the center line shows higher density and height of 113 μm by 0.3 mW laser power with 1000 writing repetitions from the PCA solution. This type of profile has been obtained due to the Gaussian nature of the femtosecond laser intensity.
A mixture of PAMAM G4.0 dendrimer and PCA has been used to demonstrate direct functionalization of the conductive pattern by applying dopants. The dendrimer can be incorporated with PCA polymer and embedded with specific moiety for active biological targets.
Comparing results from the PCA solution and the PAMAM G4.0 dendrimer mixture, the width of the pattern shows considerably reduced height of 40 nm as well as the reduced width of around 12 μm. The addition of dendrimer led to increased absorption of the laser power and showed the possibility to control the dimensions of the structure. Several different laser and process parameters were investigated for different types of aqueous mixtures of PCA such as phosphate buffer (pH 7.0) in order to optimize the parameters suitable for the application as a bio-sensor. Phosphate buffer is a medium in which biological molecules stay stable and does not denaturate from its original form.
Conclusions
A direct fs laser writing technique has been employed for the patterning process rather than the conventional lithography or fused deposition techniques. Conducting polymer patterns have been obtained from the aqueous mixture solution in the middle of the microfluidic channels. Several processing parameters such as laser power, repetitions of writing as well as surface modification of the glass substrate have been tested. Using an aqueous solution and a microfluidic chip, the presented method showed the possibility for direct fabrication of a sensor element with biological molecules. Although further studies and evaluations are required, the results from the fs laser method showed the possibility of having a biological molecule integrated into the pattern using a PCA solution.Acknowledgements
We acknowledge KIST Europe Basic fund project 11512 for the financial support. The laser structuring work has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 644971. Finally, the support for laser processing by the Karlsruhe Nano Micro Facility (KNMF, http://www.knmf.kit.edu/) a Helmholtz research infra-structure at the Karlsruhe Institute of Technology (KIT) is gratefully acknowledged.References
- B. Adhikari and S. Majumdar, Prog. Polym. Sci., 2004, 29(7), 699–766 CrossRef CAS.
- S. Roy and Z. Gao, Nano Today, 2009, 4, 318–334 CrossRef CAS.
- A. Mulchandani and N. V. Myung, Conducting polymer nanowires-based label-free biosensors, Curr. Opin. Biotechnol., 2011, 22, 502–508, DOI:10.1016/j.copbio.2011.05.508.
- A. Gelmi, M. J. Higgins and G. G. Wallace, Physical surface and electromechanical properties of doped polypyrrole biomaterials, Biomaterials, 2010, 31, 1974–1983, DOI:10.1016/j.biomaterials.2009.11.040.
- Z. Niu, M. a. Bruckman, B. Harp, C. M. Mello and Q. Wang, Bacteriophage M13 as a scaffold for preparing conductive polymeric composite fibers, Nano Res., 2008, 1, 235–241, DOI:10.1007/s12274-008-8027-2.
- K. C. Donavan, J. A. Arter, G. A. Weiss and R. M. Penner, Virus-Poly(3,4-ethylenedioxythiophene) biocomposite films, Langmuir, 2012, 28, 12581–12587, DOI:10.1021/la302473j.
- J. A. Arter, D. K. Taggart, T. M. McIntire, R. M. Penner and G. A. Weiss, Virus-PEDOT nanowires for biosensing, Nano Lett., 2010, 10, 4858–4862, DOI:10.1021/nl1025826.
- J. a. Arter, J. E. Diaz, K. C. Donavan, T. Yuan, R. M. Penner and G. a. Weiss, Virus-polymer hybrid nanowires tailored to detect prostate-specific membrane antigen, Anal. Chem., 2012, 84, 2776–2783, DOI:10.1021/ac203143y.
- A. Malinauskas, J. Malinauskiene and A. Ramanavičius, Conducting polymer-based nanostructurized materials: electrochemical aspects, Nanotechnology, 2005, 16, R51–R62, DOI:10.1088/0957-4484/16/10/R01.
- A. Ramanavicius, A. Ramanaviciene and A. Malinauskas, Electrochemical sensors based on conducting polymer-polypyrrole, Electrochim. Acta, 2006, 51, 6025–6037, DOI:10.1016/j.electacta.2005.11.052.
- M. F. Attia, T. Azib, Z. Salmi, A. Singh, P. Decorse, N. Battaglini, H. Lecoq, M. Omastová, A. A. Higazy, A. M. Elshafei, M. M. Hashem and M. M. Chehimi, One-step UV-induced modification of cellulose fabrics by polypyrrole/silver nanocomposite films, J. Colloid Interface Sci., 2013, 393, 130–137, DOI:10.1016/j.jcis.2012.11.008.
- S. D. Gittard and R. J. Narayan, Laser direct writing of micro- and nano-scale medical devices, Expert Rev. Med. Devices, 2010, 7, 343–356, DOI:10.1586/erd.10.14.
- C. Breck Hitz, J. J. Ewing and J. Hecht, Introduction to Laser Technology, Wiley-IEEE Press, 4th edn, 2014, ISBN: 978-0-470-91620-9. Search PubMed.
- K. Ke, E. F. Hasselbrink and A. J. Hunt, Rapidly prototyped three-dimensional nanofluidic channel networks in glass substrates, Anal. Chem., 2005, 77, 5083–5088, DOI:10.1021/ac0505167.
- T. N. Kim, K. Campbell, A. Groisman, D. Kleinfeld and C. B. Schaffer, Femtosecond laser-drilled capillary integrated into a microfluidic device, Appl. Phys. Lett., 2005, 86, 1–3, DOI:10.1063/1.1926423.
- J. Sone, K. Yamada, A. Asami and J. Chen, Sub-Micrometer Size Structure Fabrication Using a Conductive Polymer, Micromachines, 2014, 6, 96–109, DOI:10.3390/mi6010096.
- Y. H. Lee, J. Y. Lee and D. S. Lee, A novel conducting soluble polypyrrole composite with a polymeric co-dopant, Synth. Met., 2000, 114, 347–353, DOI:10.1016/S0379-6779(00)00268-X.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05195f |
| This journal is © The Royal Society of Chemistry 2017 |