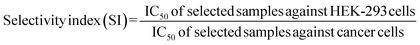Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and in vitro biological evaluation of novel diaminothiophene scaffolds as antitumor and anti-influenza virus agents. Part 2†
Khurshed Bozorov ab,
Jiang yu Zhaoa,
Li Fei Nieac,
Hai-Rong Maa,
Khayrulla Bobakulovab,
Rui Huac,
Nigora Rustamovaac,
Guozheng Huang
ab,
Jiang yu Zhaoa,
Li Fei Nieac,
Hai-Rong Maa,
Khayrulla Bobakulovab,
Rui Huac,
Nigora Rustamovaac,
Guozheng Huang a,
Thomas Efferthd and
Haji A. Aisa
a,
Thomas Efferthd and
Haji A. Aisa *a
*a
aKey Laboratory of Plant Resources and Chemistry in Arid Regions, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, 40-1 South Beijing Rd, Urumqi, Xinjiang 830011, PR China. E-mail: haji@ms.xjb.ac.cn
bInstitute of the Chemistry of Plant Substances, Academy of Sciences of Uzbekistan, 77, Mirzo Ulugbek str., 100170, Tashkent, Uzbekistan
cUniversity of Chinese Academy of Sciences, 19 A Yuquan Rd, Beijing, 100049, PR China
dDepartment of Pharmaceutical Biology, Institute of Pharmacy and Biochemistry, University of Mainz, 555128, Mainz, Germany
First published on 19th June 2017
Abstract
On the basis of high-throughput screening, fragment-based drug discovery, structure–activity relationships and building block analysis methods, herein we report the synthesis and biological evaluation of a novel series of diethyl 2,5-diaminothiophene-3,4-dicarboxylate derivatives. All of the prepared Schiff bases (with mono-, di- and poly-substituents at the aromatic portion), mono- and bis-amides of diethyl 2,5-diaminothiophene-3,4-dicarboxylate, were evaluated against various human cancer and non-cancerous (only for active compounds) cell lines, as well as influenza A (subtypes FM/1/47/H1N1, hanfang/359/95/H3N2) and B (subtype jifang/13/97) viruses. The obtained results suggest that some selected compounds revealed promising antitumor and antiviral activities and may serve as lead compounds for further drug discovery and development.
Introduction
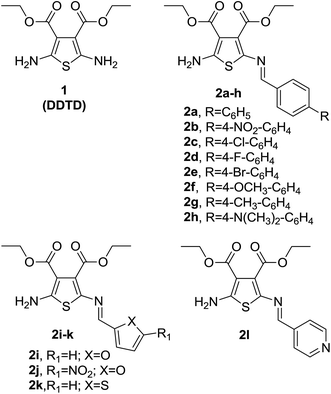
Advances in high-throughput screening (HTS), fragment-based drug discovery and building block analysis methods are powerful tools for the discovery and optimization of new drug leads in medicinal chemistry.1–4 Of particular importance are building blocks, because the drug likeness of a compound can be characterized by its building blocks.5 Likewise, this approach represents a rapid way to prepare drug candidates in medicinal chemistry.6 On the other hand, it is worth designing effective small molecules with low IC50 values at this first step of investigations. BBs containing various pharmacophore ring systems, linkers or fragments,7 play a key role for HTS-based drug discovery.8–10Discoveries of new anticancer drug candidates are still of tremendous challenging due to the complexity of cancer biology and also of great emergency, since cancer is still the leading cause of human death in all over the world.11 Natural products remain a good arsenal for providing new entities to treat cancerous cells, however wide chemical modification of the synthetic small molecules, as well as, their easy availability attracts more attention from pharmacists. In this way, small molecules are priority candidates for treatment of diseases in pharmacy. Among the prospectively using small molecule family in medicinal chemistry is 2-aminothiophene (2-ATs) scaffolds. Aside from their diverse biological properties,12–15 these classes of building blocks were reported as potential anticancer16–18 and antiviral agents.19–21 Besides, cyclic 2-AT derivatives, such as thieno[2,3-d]pyrimidines (TPs) also provide anticancer properties,22 as well as, act as PI3K,23 FGFR1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and EGFR25,26 inhibitors, indicating many more perspectives of 2-AT libraries as initially estimated.
24 and EGFR25,26 inhibitors, indicating many more perspectives of 2-AT libraries as initially estimated.
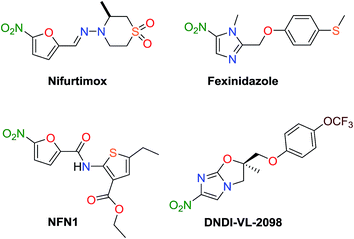
On the basis of these facts, we selected diethyl 2,5-diaminothiophene-3,4-dicarboxylate (DDTD) building block for the present investigation containing symmetrical NH2 at C-2 and C-5 and ester groups at C-3 and C-4 positions. Recently, we described Schiff bases of DDTD with only 4-substituents at the benzene ring (Chart 1) as potential anticancer and antimicrobial compounds and discussed the role of various substituents to DDTD under the aspect of structure activity relationships (SARs).27 Among them, some promising nitroaromatic compounds were discovered which need further modifications. On the other hand, although nitroaromatic drugs (Fig. 1) possess hepatotoxicity effects, latest reports have asserted that the ability SARs to mechanism of actions for drugs related nitro group indicates importance and perspectives to continue this area in medicinal chemistry.28–32
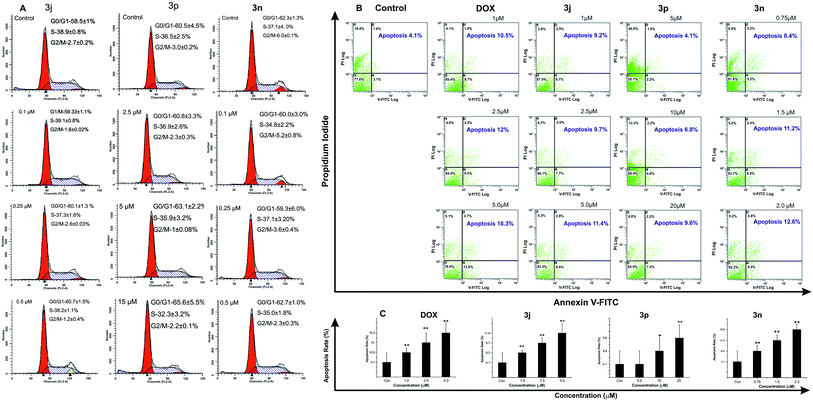
Thus, continuing our previous research on DDTD, we now investigated novel synthesized Schiff base (mainly were selected aldehydes with di- and poly-substituents at the aromatic portion), mono- and bis-amides of DDTD for their antiproliferative properties against cell lines of tumoral and normal (here selected most active samples) types, and influenza A and B viruses. Additionally, we also investigated the effects of compound 3j, 3n and 3p on cell apoptosis compared with doxorubicin (DOX) which was used as reference drug in these class derivatives.33,34
Results and discussion
Chemistry
The target Schiff bases (3a–p) were prepared by a previously reported synthetic route27,35 with minor changes (Scheme 1). In the present study, toluene was used as reaction solvent instead of n-butanol, enabling fast and clear workup procedure. The mixture of DDTD and appropriate aryl- and heteroaryl-aldehydes was heated at reflux in toluene for half an hour, and then evaporated in vacuo. Recrystallization of the residue from ethanol provided the target bases in excellent yield (79–94%).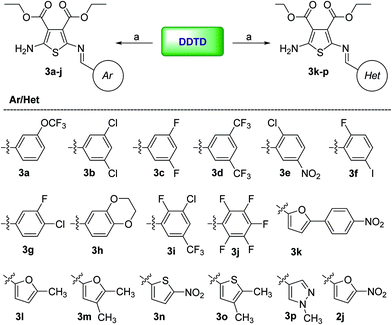 | ||
| Scheme 1 Synthesis of DDTD Schiff bases 3a–p. Reagents and conditions: (a) aromatic aldehydes, toluene, reflux 30 min. | ||
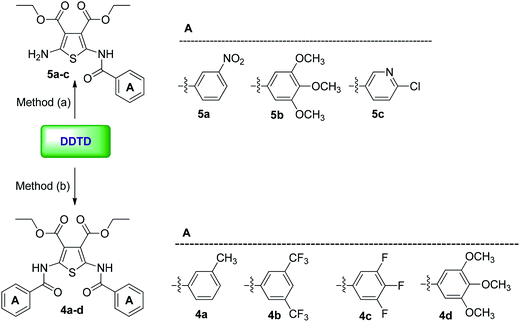
If DDTD interacted with different aromatic acid chlorides under basic conditions (methods a and b), only symmetrical bis-amides of DDTD (compounds 4a–d) were obtained as final products. Particularly, this was observed with aromatic acid chlorides related to methyl group at position C-3 (4a), trifluoromethyl in positions C-3 and C-5 (4b) as well as fluoro group in positions C-3, C-4, and C-5 (4c) in the benzene portion of aromatic system. Because of planar structure of the compounds 4a–d, it was found that by the enthalpy the optimal configuration is Z forms for these compounds. Mono-amides of DDTD (5a and 5c) were obtained in only two cases. It was also possible to yield mono-(5b) and symmetrical bis-(4d) amides of DDTD with 3,4,5-trimethoxybenzoyl chloride. For compound 5b, the product yield was 53% using method A. Applying method B resulted in the symmetrical bis product (4d) in excellent yield (92%) (Scheme 2).
Biological evaluation
| Histotype | Prostate | Lung | Colon | Breast | |
|---|---|---|---|---|---|
| Cell lines | PC-3 | A549 | HCT-15 | T47D | |
| No. | Compounds | IC50 (±SD, μM) | |||
| 1 | 2j | n.d. | 22.8 ± 0.6 | 10.2 ± 0.6 | 4.6 ± 0.8 |
| 2 | 3a | 33.3 ± 0.3 | n.d. | 34.0 ± 0.3 | n.d. |
| 3 | 3b | n.d. | n.d. | n.d. | n.d. |
| 4 | 3c | 69.8 ± 5.9 | 17.9 ± 2.1 | 40.9 ± 4.8 | 20.6 ± 2.2 |
| 5 | 3d | 65.0 ± 4.8 | 33.0 ± 3.5 | 40.2 ± 4.2 | 51.0 ± 5.2 |
| 6 | 3e | 55.0 ± 0.5 | n.d. | n.d. | n.d. |
| 7 | 3f | 45.4 ± 4.9 | 28.0 ± 2.6 | 57.7 ± 5.8 | 28.8 ± 3.0 |
| 8 | 3g | 42.1 ± 5.4 | 11.0 ± 1.0 | 37.3 ± 3.6 | 17.7 ± 2.1 |
| 9 | 3h | 29.6 ± 0.3 | 60.1 ± 0.9 | 22.3 ± 0.4 | 4.50 ± 0.3 |
| 10 | 3i | n.d. | n.d. | n.d. | n.d. |
| 11 | 3j | 12.0 ± 0.7 | 4.60 ± 0.7 | 2.59 ± 0.3 | 6.40 ± 0.4 |
| 12 | 3k | 25.2 ± 2.6 | 28.3 ± 2.9 | 31.9 ± 4.1 | 20.6 ± 2.3 |
| 13 | 3l | 17.3 ± 1.8 | 31.3 ± 3.4 | 25.9 ± 2.7 | 22.9 ± 2.4 |
| 14 | 3m | 20.9 ± 2.6 | 16.1 ± 2.0 | 30.9 ± 3.3 | 23.6 ± 3.2 |
| 15 | 3n | 10.9 ± 1.3 | 1.30 ± 0.2 | 6.10 ± 0.3 | 21.4 ± 2.2 |
| 16 | 3o | 36.1 ± 4.6 | 14.8 ± 1.5 | 101.6 ± 8.7 | n.d. |
| 17 | 3p | 15.7 ± 1.7 | 14.9 ± 1.6 | 23.3 ± 2.5 | 16.8 ± 1.8 |
| 18 | 4a | n.d. | 66.1 ± 0.6 | 12.0 ± 0.1 | n.d. |
| 19 | 4b | 11.6 ± 0.8 | n.d. | 12.3 ± 0.1 | n.d. |
| 20 | 4c | 9.38 ± 0.2 | n.d. | 14.2 ± 0.1 | 62.9 ± 0.3 |
| 21 | 4d | n.d. | 71.6 ± 0.6 | n.d. | 12.1 ± 0.4 |
| 22 | 5a | 11.5 ± 0.6 | n.d. | 20.8 ± 1.1 | n.d. |
| 23 | 5b | 128.6 ± 1.4 | n.d. | n.d. | 6.0 ± 0.6 |
| 24 | 5c | 6.93 ± 0.3 | 67.0 ± 1.1 | 7.22 ± 0.3 | 22.3 ± 0.8 |
| 25 | Doxorubicin | 7.40 ± 1.6 | 6.50 ± 1.4 | 7.92 ± 0.3 | 25.5 ± 0.5 |
In general, Table 1 shows that among the all synthesized Schiff bases, only 5-nitro-2-thienyl derivative 3n demonstrated antiproliferative activity on all tumoral cell lines. Perhaps, herein nitrothienyl group played a key role for increasing activity. In corresponding reports photochemical and acceptor behavior of the 5-nitro-2-thienyl group were well explained.40,41 Moreover, nitrothienyl prodrugs may provide effective and selective delivery of the cytotoxic or cytostatic compound to a solid tumor. It was reported that in oxic liver homogenates, nitrothienyl related compounds was metabolically stable, as well as, when incubated in aerobic A549 whole-cell suspensions also was observe stability, which suggest that such substitution has also successfully inhibited undesired aerobic metabolism.42 If bearing in mind that nitrothienyl containing scaffolds has been shown to demonstrate modest activity in human xenograft multiple myeloma and B-cell leukemia in vivo models,43 further developments with these class derivatives seem promising.
6-Chloronicotinamido-DDTD monoamide (5c) showed high antiproliferative activity, than DOX towards PC-3, HCT-15, and on T47D cancer cell lines, among the synthesized mono-(5a–c) and symmetrical (4a–d) amide derivatives (Table 1), which confirming anticancer drugs properties of discovered that nicotinamide containing compositions.44 Moreover, nicotinamide is a member of vitamin B family, which has no serious side effects in human organism.
Both symmetrical and mono-DDTD amides 4d and 5b, which contained with 3,4,5-trimethoxy phenyl fragments, increased antiproliferative activity towards T47D cells. This finding once more confirmed the importance of the 3,4,5-trimethoxy substituent for drug discovery.45 Another symmetrical amides, which inserted substituents such as methyl at C-3 (4a), ditrifluoro methyl at C-3, C-5 (4b) and trifluoro at C-3, C-4, C-5-(4c) also resulted in considerable cytotoxicity towards HCT-15 cells.
The results revealed that SI of these potentials was not high for compare with known anticancer drugs (Table 2). However, when SI between A549 and HEK-293 cells were calculated for derivative 3n, satisfactory SI (50) have been obtained, which deserves further investigation. In addition, 6-chloronicotinamido-DDTD monoamide (5c) demonstrated good selectivity when SI of PC-3 and HCT-25 cells were calculated between HEK-293 cells, while compound 2j showed similar SI value between T47D and HEK-293 cells. It was found that penta-fluoro-derivative 3j which was most potent on cancer cell lines, did not provide any selectivity.
| Compounds | IC50 (±SD, μM) | SIPC−3 | SIA549 | SIHCT−15 | SIT47D |
|---|---|---|---|---|---|
| 2j | 111.3 ± 2.8 | nd | 4.9 ± 4.7 | 11 ± 4.7 | 24.1 ± 3.5 |
| 3j | 31.4 ± 1.7 | 2.6 ± 2.4 | 6.8 ± 2.4 | 12.1 ± 5.6 | 4.9 ± 4.2 |
| 3n | 65.0 ± 2.2 | 5.9 ± 1.7 | 50 ± 11 | 10.6 ± 7.3 | 3.0 ± 0.01 |
| 5c | 160.0 ± 2.3 | 23 ± 7.7 | 2.4 ± 2.0 | 22 ± 7.7 | 7.1 ± 2.9 |
| Compounds | TC50a (μM) | Anti-influenza H1N1 virus | Anti-influenza H3N2 virus | Anti-influenza B virus | |||
|---|---|---|---|---|---|---|---|
| IC50b | SIc | IC50 | SI | IC50 | SI | ||
| a 50% cytotoxic concentration.b 50% virus-inhibitory concentration, determined by the CPE inhibition assay.c Selectivity index (TC50/IC50).d Results are expressed as mean values (±SEM) derived from two replicates.e Not determined. | |||||||
| 2j | 8.90 | 0.94 ± 0.35d | 10.2 | 2.40 ± 1.28 | 3.7 | 0.79 ± 0.13 | 11.5 |
| 3a | 10.69 | 6.28 ± 0.08 | 1.7 | 2.07 ± 0.91 | 5.7 | 9.54 ± 0.90 | 1.1 |
| 3b | 32.08 | >166.67 | >3.0 | 11.40 ± 1.19 | 2.8 | 7.37 ± 1.10 | 4.3 |
| 3c | >500 | >166.67 | nde | 111.12 ± 78.57 | 6.0 | 131.45 ± 49.81 | 4.1 |
| 3d | 96.23 | 19.50 ± 2.80 | 5.0 | 7.54 ± 1.93 | 13.2 | 10.69 ± 0.01 | 9.0 |
| 3e | >500 | >166.67 | nd | 45.12 ± 9.37 | >11.4 | 75.90 ± 28.76 | >7.1 |
| 3f | 129.34 | 21.32 ± 3.21 | 6.0 | 24.57 ± 3.15 | 5.3 | 42.17 ± 6.35 | 3.1 |
| 3g | 73.12 | 7.41 ± 1.94 | 9.9 | 1.81 ± 0.35 | 41.2 | 18.26 ± 3.10 | 4.0 |
| 3h | 129.34 | 18.75 ± 2.45 | 6.9 | 11.25 ± 4.42 | 12.5 | 39.17 ± 8.90 | 3.3 |
| 3i | >500 | >166.67 | >3.0 | >166.67 | >3.0 | >166.67 | >3.0 |
| 3j | 32.08 | 10.69 ± 0.01 | 3.0 | 26.73 ± 3.73 | 1.2 | 14.40 ± 0.04 | 2.2 |
| 3k | 38.52 | 10.69 ± 0.06 | 3.6 | 2.63 ± 0.01 | 14.6 | 14.30 ± 0.01 | 2.7 |
| 3l | 96.23 | 30.82 ± 17.39 | 3.7 | 14.07 ± 0.01 | 6.8 | 18.50 ± 0.03 | 5.2 |
| 3m | 388.03 | 60.10 ± 6.75 | 6.5 | 97.00 ± 9.58 | 4.0 | 14.61 ± 5.54 | 16.2 |
| 3n | 32.08 | 3.56 ± 0.01 | 9.0 | 3.72 ± 1.51 | 9.4 | 10.52 ± 2.80 | 3.0 |
| 3o | 96.23 | 9.79 ± 2.35 | 10.2 | 28.30 ± 2.20 | 3.4 | 24.82 ± 1.90 | 3.9 |
| 3p | 10.69 | 4.54 ± 0.36 | 2.2 | 9.81 ± 1.31 | 1.1 | 8.29 ± 0.90 | 1.3 |
| 4a | 166.67 | 37.60 ± 7.81 | 4.6 | 79.60 ± 8.62 | 2.1 | 55.52 ± 5.75 | 2.9 |
| 4b | 240.37 | 52.50 ± 9.54 | 4.6 | 67.34 ± 7.45 | 3.6 | 59.11 ± 3.50 | 4.0 |
| 4c | 288.68 | 145.18 ± 21.90 | 2.0 | >166.67 | >3.0 | >166.67 | nd |
| 4d | 166.67 | 32.08 ± 0.02 | 5.2 | 62.26 ± 5.66 | 2.7 | 55.60 ± 4.63 | 3.0 |
| 5a | 240.37 | 18.52 ± 0.05 | 13 | >166.67 | >3.0 | 9.32 ± 1.94 | 10.6 |
| 5b | 240.37 | 32.08 ± 0.01 | 7.5 | 49.34 ± 8.80 | 5.0 | 43.12 ± 0.03 | 5.6 |
| 5c | 346.68 | 42.21 ± 0.02 | 8.2 | 56.90 ± 20.77 | 6.4 | 100.47 ± 40.84 | 3.8 |
| Oseltamivir | 1260 | 3.56 ± 0.53 | 343.4 | 1.39 ± 0.37 | 915.3 | 119.58 ± 5.81 | 10.6 |
| RBV | 1164.1 | 2.62 ± 0.79 | 465.6 | 1.77 ± 1.00 | 784.8 | 1.13 ± 0.43 | 1116.9 |
Conclusions
Recently, we had discovered Schiff bases of DDTD (with only 4-substituents at the benzene ring) as potential anticancer and antimicrobial agents,27 now in this investigation we have developed our previous work with additional assays, such as anti-influenza virus activities, cell cycle and apoptotic analysis, also have been calculated SI of promising compounds by using human normal cell (HEK-293) as well as have synthesized novel mono- and bis-amides and Schiff bases (in this time were selected aldehydes with di- and poly-substituents at the aromatic portion) of DDTD on the basis SARs. It was observed that, DDTD derivatives (3j, 3n, 3p, and 5c) showed significant cytotoxicity against different human tumor cells. In particular, 2-amino-5-[(E)-[(5-nitrothiophen-2-yl)methylidene]amino]thiophene-derivative (3n) showed strong cytotoxic activity and selectivity. It significantly induced apoptosis in A549 cells at low micromolar concentrations. Regarding DDTD amides, 2-(6-chloronicotinamido-) containing amide 5c displayed consistent antiproliferative activity showing lower IC50 values than DOX in PC-3 prostate, HCT-15 colon and T47D breast cancer cell lines. Moreover, evaluation of target compounds against influenza A and B viruses showed moderate activity, in particular potential hit compound 2j and 3g were more or similar active than or with control drugs oseltamivir and RBV. Thus, we concluded that our results are promising for the future design of antitumor and antiviral activity of DDTD derivatives.Experimental section
Chemistry
General procedure for the synthesis of DDTD (1)
A mixture of elemental sulfur 10 g (312 mmol), ethylcyanoacetate (71.2 g; 623 mmol), DMF (50 mL) and TEA (10 mL; 71 mmol) was allowed to react for 6 days with continuous stirring at 15 °C. The obtained mixture was added to 3 L cool water and stirred over 4 h. The precipitate was filtered and dried giving a yellow solid (59 g; 73%).General procedure for the synthesis of compounds 3a–p
Aromatic aldehydes (0.3 mmol) were added to a 0.2 mmol solution of the DDTD (1) in toluene (10 mL). The mixture was refluxed for 0.5 h. Further, the solution was evaporated at low pressure and the residue was purified and recrystallized from ethanol.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.62 (m, 2H, Ar-H), 7.41 (t, J = 7.9 Hz, 1H, Ar-H), 7.23 (br.d, J = 8.0 Hz, 1H, Ar-H), 6.41 (br.s, 2H, NH2), 4.43 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.26 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.44 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.46 (C
CH), 7.62 (m, 2H, Ar-H), 7.41 (t, J = 7.9 Hz, 1H, Ar-H), 7.23 (br.d, J = 8.0 Hz, 1H, Ar-H), 6.41 (br.s, 2H, NH2), 4.43 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.26 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.44 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.46 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.49 (C
O), 164.49 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.26 (NH2CS), 150.62 (N
O), 160.26 (NH2CS), 150.62 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 149.79 (Ar-C), 138.04 (Ar-C), 133.43 (C), 131.28 (C), 130.18 (Ar-CH), 127.00 (Ar-CH), 123.09 (Ar-CH), 120.59 (q, J = 257.7 Hz, CF3), 119.86 (Ar-CH), 103.34 (C), 61.78 (CH2), 60.52 (CH2), 14.45 (CH3), 14.31 (CH3). MS (ESI): m/z 431 [M + H]+.
CH), 149.79 (Ar-C), 138.04 (Ar-C), 133.43 (C), 131.28 (C), 130.18 (Ar-CH), 127.00 (Ar-CH), 123.09 (Ar-CH), 120.59 (q, J = 257.7 Hz, CF3), 119.86 (Ar-CH), 103.34 (C), 61.78 (CH2), 60.52 (CH2), 14.45 (CH3), 14.31 (CH3). MS (ESI): m/z 431 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.58 (d, J = 1.9 Hz, 2H, Ar-H), 7.35 (t, J = 1.9 Hz, 1H, Ar-H), 6.44 (br.s, 2H, NH2), 4.44 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.26 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.45 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.34 (C
CH), 7.58 (d, J = 1.9 Hz, 2H, Ar-H), 7.35 (t, J = 1.9 Hz, 1H, Ar-H), 6.44 (br.s, 2H, NH2), 4.44 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.26 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.45 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.34 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.41 (C
O), 164.41 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.52 (NH2CS), 149.04 (N
O), 160.52 (NH2CS), 149.04 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 138.77 (C), 135.50 (2Ar-C), 132.99 (Ar-C), 131.94 (C), 130.35 (Ar-CH), 126.46 (2Ar-CH), 103.43 (C), 61.86 (CH2), 60.57 (CH2), 14.50 (CH3), 14.32 (CH3). MS (ESI): m/z 416 [M + H]+.
CH), 138.77 (C), 135.50 (2Ar-C), 132.99 (Ar-C), 131.94 (C), 130.35 (Ar-CH), 126.46 (2Ar-CH), 103.43 (C), 61.86 (CH2), 60.57 (CH2), 14.50 (CH3), 14.32 (CH3). MS (ESI): m/z 416 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.98 (td, J = 8.5; 6.6 Hz, 1H, Ar-H), 6.91 (td, J = 8.5, 2.5 Hz, 1H, Ar-H), 6.81 (ddd, J = 11.0, 8.5, 2.5 Hz, 1H, Ar-H), 6.50 (br.s, 2H, NH2), 4.41 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.42 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.31 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.66 (C
CH), 7.98 (td, J = 8.5; 6.6 Hz, 1H, Ar-H), 6.91 (td, J = 8.5, 2.5 Hz, 1H, Ar-H), 6.81 (ddd, J = 11.0, 8.5, 2.5 Hz, 1H, Ar-H), 6.50 (br.s, 2H, NH2), 4.41 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.42 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.31 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.66 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.60 (dd, J = 254.5, 12.2 Hz, Ar-CF), 164.44 (C
O), 164.60 (dd, J = 254.5, 12.2 Hz, Ar-CF), 164.44 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 162.50 (dd, J = 256.0, 11.9 Hz, Ar-CF), 160.36 (NH2CS), 144.40 (dd, J = 4.6, 2.3 Hz, N
O), 162.50 (dd, J = 256.0, 11.9 Hz, Ar-CF), 160.36 (NH2CS), 144.40 (dd, J = 4.6, 2.3 Hz, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 134.11 (C), 130.69 (C), 129.12 (dd, J = 9.8, 4.2 Hz, Ar-C), 120.39 (dd, J = 9.4, 3.9 Hz, Ar-C), 112.38 (dd, J = 21.9, 3.5 Hz, Ar-C), 104.08 (t, J = 25.31 Hz, Ar-C), 103.00 (C), 61.70 (CH2), 60.41 (CH2), 14.43 (CH3), 14.31 (CH3). MS (ESI): m/z 383 [M + H]+.
CH), 134.11 (C), 130.69 (C), 129.12 (dd, J = 9.8, 4.2 Hz, Ar-C), 120.39 (dd, J = 9.4, 3.9 Hz, Ar-C), 112.38 (dd, J = 21.9, 3.5 Hz, Ar-C), 104.08 (t, J = 25.31 Hz, Ar-C), 103.00 (C), 61.70 (CH2), 60.41 (CH2), 14.43 (CH3), 14.31 (CH3). MS (ESI): m/z 383 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.86 (s, 1H, Ar-H), 6.45 (br.s, 2H, NH2), 4.45 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.27 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.46 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.20 (C
CH), 7.86 (s, 1H, Ar-H), 6.45 (br.s, 2H, NH2), 4.45 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.27 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.46 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.20 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.38 (C
O), 164.38 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.77 (NH2CS), 148.07 (N
O), 160.77 (NH2CS), 148.07 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 137.92 (Ar-C), 132.97 (C), 132.43 (C), 132.16 (q, J = 32.4 Hz, 2Ar-C), 127.87 (2Ar-CH), 123.65 (Ar-CH), 123.23 (q, J = 272.8 Hz, 2CF3), 103.61 (C), 61.93 (CH2), 60.67 (CH2), 14.45 (CH3), 14.28 (CH3). MS (ESI): m/z 483 [M + H]+.
CH), 137.92 (Ar-C), 132.97 (C), 132.43 (C), 132.16 (q, J = 32.4 Hz, 2Ar-C), 127.87 (2Ar-CH), 123.65 (Ar-CH), 123.23 (q, J = 272.8 Hz, 2CF3), 103.61 (C), 61.93 (CH2), 60.67 (CH2), 14.45 (CH3), 14.28 (CH3). MS (ESI): m/z 483 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.13 (dd, J = 8.8; 2.7 Hz, 1H, Ar-H), 7.54 (d, J = 8.8 Hz, 1H, Ar-H), 6.50 (br.s, 2H, NH2), 4.48 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.27 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.50 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.33 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.12 (C
CH), 8.13 (dd, J = 8.8; 2.7 Hz, 1H, Ar-H), 7.54 (d, J = 8.8 Hz, 1H, Ar-H), 6.50 (br.s, 2H, NH2), 4.48 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.27 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.50 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.33 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.12 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.40 (C
O), 164.40 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.08 (NH2CS),147.10 (Ar-C), 145.36 (N
O), 161.08 (NH2CS),147.10 (Ar-C), 145.36 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 140.61 (Ar-C), 134.62 (Ar-C), 133.66 (C), 132.75 (C), 131.11 (Ar-CH), 125.09 (Ar-CH), 123.44 (Ar-CH), 103.78 (C), 62.11 (CH2), 60.70 (CH2), 14.59 (CH3), 14.28 (CH3). MS (ESI): m/z 426.5 [M + H]+.
CH), 140.61 (Ar-C), 134.62 (Ar-C), 133.66 (C), 132.75 (C), 131.11 (Ar-CH), 125.09 (Ar-CH), 123.44 (Ar-CH), 103.78 (C), 62.11 (CH2), 60.70 (CH2), 14.59 (CH3), 14.28 (CH3). MS (ESI): m/z 426.5 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.61 (ddd, J = 8.6; 4.9; 2.3 Hz, 1H, Ar-H), 6.83 (dd, J = 10.2; 8.6 Hz, 1H, Ar-H), 6.50 (br.s, 2H, NH2), 4.45 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.47 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.46 (C
CH), 7.61 (ddd, J = 8.6; 4.9; 2.3 Hz, 1H, Ar-H), 6.83 (dd, J = 10.2; 8.6 Hz, 1H, Ar-H), 6.50 (br.s, 2H, NH2), 4.45 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.47 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.46 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.39 (C
O), 164.39 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.98 (d, J = 255.0 Hz, Ar-C), 160.69 (NH2CS), 143.29 (d, J = 4.6 Hz, N
O), 161.98 (d, J = 255.0 Hz, Ar-C), 160.69 (NH2CS), 143.29 (d, J = 4.6 Hz, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 140.66 (d, J = 8.0 Hz, Ar-CH), 136.44 (d, J = 2.4 Hz, Ar-CH), 133.62 (C), 131.81 (C), 125.94 (d, J = 10.2 Hz, Ar-C), 118.01 (d, J = 21.9 Hz, Ar-CH), 103.23 (C), 87.83 (d, J = 3.6 Hz, Ar-C), 61.84 (CH2), 60.50 (CH2), 14.56 (CH3), 14.29 (CH3). MS (ESI): m/z 491 [M + H]+.
CH), 140.66 (d, J = 8.0 Hz, Ar-CH), 136.44 (d, J = 2.4 Hz, Ar-CH), 133.62 (C), 131.81 (C), 125.94 (d, J = 10.2 Hz, Ar-C), 118.01 (d, J = 21.9 Hz, Ar-CH), 103.23 (C), 87.83 (d, J = 3.6 Hz, Ar-C), 61.84 (CH2), 60.50 (CH2), 14.56 (CH3), 14.29 (CH3). MS (ESI): m/z 491 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.54 (m, 1H, Ar-H), 7.40 (m, 2H, Ar-H), 6.45 (br.s, 2H, NH2), 4.42 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.43 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.45 (C
CH), 7.54 (m, 1H, Ar-H), 7.40 (m, 2H, Ar-H), 6.45 (br.s, 2H, NH2), 4.42 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.43 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.45 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.44 (C
O), 164.44 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.35 (NH2CS), 158.40 (d, J = 249.4 Hz, Ar-C), 149.81 (d, J = 3.1 Hz, N
O), 160.35 (NH2CS), 158.40 (d, J = 249.4 Hz, Ar-C), 149.81 (d, J = 3.1 Hz, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 136.59 (d, J = 6.7 Hz, Ar-C), 133.30 (C), 131.27 (C), 130.95 (Ar-CH), 124.97 (d, J = 3.3 Hz, Ar-CH), 123.55 (d, J = 18.0 Hz, Ar-C), 115.23 (d, J = 22.4 Hz, Ar-CH), 103.30 (C), 61.79 (CH2), 60.52 (CH2), 14.49 (CH3), 14.32 (CH3). MS (ESI): m/z 499.5 [M + H]+.
CH), 136.59 (d, J = 6.7 Hz, Ar-C), 133.30 (C), 131.27 (C), 130.95 (Ar-CH), 124.97 (d, J = 3.3 Hz, Ar-CH), 123.55 (d, J = 18.0 Hz, Ar-C), 115.23 (d, J = 22.4 Hz, Ar-CH), 103.30 (C), 61.79 (CH2), 60.52 (CH2), 14.49 (CH3), 14.32 (CH3). MS (ESI): m/z 499.5 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.29 (d, J = 2.0 Hz, 1H, Ar-H), 7.20 (dd, J = 8.2; 2.0 Hz, 1H, Ar-H), 6.85 (d, J = 8.2 Hz, 1H, Ar-H), 6.44 (br.s, 2H, NH2), 4.41 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (m, 6H, OCH2CH3, OCH2CH2O), 1.42 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.86 (C
CH), 7.29 (d, J = 2.0 Hz, 1H, Ar-H), 7.20 (dd, J = 8.2; 2.0 Hz, 1H, Ar-H), 6.85 (d, J = 8.2 Hz, 1H, Ar-H), 6.44 (br.s, 2H, NH2), 4.41 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (m, 6H, OCH2CH3, OCH2CH2O), 1.42 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.86 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.53 (C
O), 164.53 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.72 (NH2CS), 152.39 (N
O), 159.72 (NH2CS), 152.39 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 146.35 (Ar-C), 143.73 (Ar-C), 134.65 (C), 129.70 (Ar-C), 128.69 (C), 122.68 (Ar-CH), 117.52 (Ar-CH), 116.86 (Ar-CH), 102.67 (C), 64.65 (OCH2), 64.20 (OCH2), 61.60 (OCH2CH3), 60.26 (OCH2CH3), 14.45 (CH3), 14.29 (CH3). MS (ESI): m/z 405 [M + H]+.
CH), 146.35 (Ar-C), 143.73 (Ar-C), 134.65 (C), 129.70 (Ar-C), 128.69 (C), 122.68 (Ar-CH), 117.52 (Ar-CH), 116.86 (Ar-CH), 102.67 (C), 64.65 (OCH2), 64.20 (OCH2), 61.60 (OCH2CH3), 60.26 (OCH2CH3), 14.45 (CH3), 14.29 (CH3). MS (ESI): m/z 405 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.67 (dd, J = 6.5; 2.3 Hz, 1H, Ar-H), 6.45 (br.s, 2H, NH2), 4.43 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.27 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.44 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.14 (C
CH), 7.67 (dd, J = 6.5; 2.3 Hz, 1H, Ar-H), 6.45 (br.s, 2H, NH2), 4.43 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.27 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.44 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.14 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.37 (C
O), 164.37 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.95 (NH2CS), 159.00 (d, J = 259.6 Hz, Ar-CF), 141.93 (d, J = 4.4 Hz, N
O), 160.95 (NH2CS), 159.00 (d, J = 259.6 Hz, Ar-CF), 141.93 (d, J = 4.4 Hz, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 133.35 (C), 132.84 (C), 128.96 (d, J = 3.8 Hz, Ar-CH), 127.79 (d, J = 33.5 Hz, Ar-C), 126.02 (d, J = 10.8 Hz, Ar-C), 123.03 (q, J = 265.0 Hz, CF3), 123.44 (Ar-CH), 122.74 (d, J = 18.2 Hz, Ar-C), 103.67 (C), 61.92 (CH2), 60.68 (CH2), 14.44 (CH3), 14.28 (CH3). MS (ESI): m/z 467.5 [M + H]+.
CH), 133.35 (C), 132.84 (C), 128.96 (d, J = 3.8 Hz, Ar-CH), 127.79 (d, J = 33.5 Hz, Ar-C), 126.02 (d, J = 10.8 Hz, Ar-C), 123.03 (q, J = 265.0 Hz, CF3), 123.44 (Ar-CH), 122.74 (d, J = 18.2 Hz, Ar-C), 103.67 (C), 61.92 (CH2), 60.68 (CH2), 14.44 (CH3), 14.28 (CH3). MS (ESI): m/z 467.5 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.54 (br.s, 2H, NH2), 4.39 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.41 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.31 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.15 (C
CH), 6.54 (br.s, 2H, NH2), 4.39 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.41 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.31 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.15 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.39 (C
O), 164.39 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.31 (NH2CS), 145.87 (d, J = 257.5 Hz, 2Ar-C), 141.99 (d, J = 258.1 Hz, Ar-C), 139.05 (d, J = 3.4 Hz, N
O), 161.31 (NH2CS), 145.87 (d, J = 257.5 Hz, 2Ar-C), 141.99 (d, J = 258.1 Hz, Ar-C), 139.05 (d, J = 3.4 Hz, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 137.68 (d, J = 235.5 Hz, 2Ar-C), 133.73 (C), 132.79 (C), 111.50 (Ar-C), 103.49 (C), 61.97 (CH2), 60.63 (CH2), 14.31 (CH3), 14.26 (CH3). MS (ESI): m/z 437 [M + H]+.
CH), 137.68 (d, J = 235.5 Hz, 2Ar-C), 133.73 (C), 132.79 (C), 111.50 (Ar-C), 103.49 (C), 61.97 (CH2), 60.63 (CH2), 14.31 (CH3), 14.26 (CH3). MS (ESI): m/z 437 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.95 (s, 2H, furan-H), 6.54 (br.s, 2H, NH2), 4.44 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.47 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.51 (C
CH), 6.95 (s, 2H, furan-H), 6.54 (br.s, 2H, NH2), 4.44 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.25 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.47 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.51 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.38 (C
O), 164.38 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.23 (NH2CS), 153.77 (furan-C), 153.30 (furan-C), 146.93 (Ar-C), 139.70 (N
O), 160.23 (NH2CS), 153.77 (furan-C), 153.30 (furan-C), 146.93 (Ar-C), 139.70 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 135.44 (furan-C), 134.05 (C), 130.69 (C), 124.61 (2Ar-CH), 124.43 (2Ar-CH), 116.52 (furan-CH), 111.64 (furan-CH), 103.35 (C), 61.74 (CH2), 60.48 (CH2), 14.47 (CH3), 14.29 (CH3). MS (ESI): m/z 458 [M + H]+.
CH), 135.44 (furan-C), 134.05 (C), 130.69 (C), 124.61 (2Ar-CH), 124.43 (2Ar-CH), 116.52 (furan-CH), 111.64 (furan-CH), 103.35 (C), 61.74 (CH2), 60.48 (CH2), 14.47 (CH3), 14.29 (CH3). MS (ESI): m/z 458 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.75 (d, J = 3.3 Hz, 1H, furan-H), 6.42 (br.s, 2H, NH2), 6.10 (d, J = 3.3 Hz, 1H, furan-H), 4.39 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.23 (q, J = 7.1 Hz, 2H, OCH2CH3), 2.36 (s, 3H, furan-CH3), 1.41 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.69 (C
CH), 6.75 (d, J = 3.3 Hz, 1H, furan-H), 6.42 (br.s, 2H, NH2), 6.10 (d, J = 3.3 Hz, 1H, furan-H), 4.39 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.23 (q, J = 7.1 Hz, 2H, OCH2CH3), 2.36 (s, 3H, furan-CH3), 1.41 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.53 (C
O), 164.53 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.45 (NH2CS), 156.52 (furan-C), 150.56 (furan-C), 141.37 (N
O), 159.45 (NH2CS), 156.52 (furan-C), 150.56 (furan-C), 141.37 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 135.09 (C), 128.41 (C), 117.00 (furan-CH), 109.20 (furan-CH), 103.11 (C), 61.61 (CH2), 60.28 (CH2), 14.31 (furan-CH3, CH3), 14.09 (CH3). MS (ESI): m/z 351 [M + H]+.
CH), 135.09 (C), 128.41 (C), 117.00 (furan-CH), 109.20 (furan-CH), 103.11 (C), 61.61 (CH2), 60.28 (CH2), 14.31 (furan-CH3, CH3), 14.09 (CH3). MS (ESI): m/z 351 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.67 (s, 1H, furan-H), 6.34 (br.s, 2H, NH2), 4.39 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.23 (q, J = 7.1 Hz, 2H, OCH2CH3), 2.27 (s, 3H, furan-CH3), 1.96 (s, 3H, furan-CH3), 1.40 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.67 (C
CH), 6.67 (s, 1H, furan-H), 6.34 (br.s, 2H, NH2), 4.39 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.23 (q, J = 7.1 Hz, 2H, OCH2CH3), 2.27 (s, 3H, furan-CH3), 1.96 (s, 3H, furan-CH3), 1.40 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.67 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.59 (C
O), 164.59 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.23 (NH2CS), 152.54 (furan-C), 149.29 (furan-C), 141.56 (N
O), 159.23 (NH2CS), 152.54 (furan-C), 149.29 (furan-C), 141.56 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 135.41 (C), 128.02 (C), 119.08 (furan-CH), 117.96 (furan-C), 103.24 (C), 61.59 (CH2), 60.30 (CH2), 14.35 (2CH3), 12.00 (furan-CH3), 9.91 (furan-CH3). MS (ESI): m/z 365 [M + H]+.
CH), 135.41 (C), 128.02 (C), 119.08 (furan-CH), 117.96 (furan-C), 103.24 (C), 61.59 (CH2), 60.30 (CH2), 14.35 (2CH3), 12.00 (furan-CH3), 9.91 (furan-CH3). MS (ESI): m/z 365 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.84 (d, J = 4.3 Hz, 1H, thiophene-H), 7.19 (d, J = 4.3 Hz, 1H, thiophene-H), 6.54 (br.s, 2H, NH2), 4.43 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.26 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.46 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 164.87 (C
CH), 7.84 (d, J = 4.3 Hz, 1H, thiophene-H), 7.19 (d, J = 4.3 Hz, 1H, thiophene-H), 6.54 (br.s, 2H, NH2), 4.43 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.26 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.46 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.32 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 164.87 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.29 (C
O), 164.29 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.09 (NH2CS), 153.19 (thiophene-C), 148.93 (thiophene-C), 142.84 (N
O), 161.09 (NH2CS), 153.19 (thiophene-C), 148.93 (thiophene-C), 142.84 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 133.49 (C), 132.07 (C), 129.01 (thiophene-CH), 128.20 (thiophene-CH), 103.88 (C), 62.04 (CH2), 60.74 (CH2), 14.62 (CH3), 14.28 (CH3). MS (ESI): m/z 398 [M + H]+.
CH), 133.49 (C), 132.07 (C), 129.01 (thiophene-CH), 128.20 (thiophene-CH), 103.88 (C), 62.04 (CH2), 60.74 (CH2), 14.62 (CH3), 14.28 (CH3). MS (ESI): m/z 398 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.05 (s, 1H, thiophene-H), 6.24 (br.s, 2H, NH2), 4.40 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.23 (q, J = 7.1 Hz, 2H, OCH2CH3), 2.36 (s, 3H, thiophene-CH3), 2.11 (s, 3H, thiophene-CH3), 1.43 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.57 (C
CH), 7.05 (s, 1H, thiophene-H), 6.24 (br.s, 2H, NH2), 4.40 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.23 (q, J = 7.1 Hz, 2H, OCH2CH3), 2.36 (s, 3H, thiophene-CH3), 2.11 (s, 3H, thiophene-CH3), 1.43 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.57 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.59 (C
O), 164.59 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.17 (NH2CS), 146.50 (N
O), 159.17 (NH2CS), 146.50 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 139.98 (thiophene-C), 137.58 (thiophene-C), 134.72 (C), 134.63 (thiophene-CH), 134.51 (thiophene-C), 128.30 (C), 103.18 (C), 61.57 (CH2), 60.34 (CH2), 14.59 (CH3), 14.30 (CH3), 14.03 (thiophene-CH3), 13.69 (thiophene-CH3). MS (ESI): m/z 381 [M + H]+.
CH), 139.98 (thiophene-C), 137.58 (thiophene-C), 134.72 (C), 134.63 (thiophene-CH), 134.51 (thiophene-C), 128.30 (C), 103.18 (C), 61.57 (CH2), 60.34 (CH2), 14.59 (CH3), 14.30 (CH3), 14.03 (thiophene-CH3), 13.69 (thiophene-CH3). MS (ESI): m/z 381 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.79 (s, 1H, pyrazole-H), 7.71 (s, 1H, pyrazole-H), 6.40 (br.s, 2H, NH2), 4.38 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.24 (q, J = 7.1 Hz, 2H, OCH2CH3), 3.90 (s, 3H, N-CH3), 1.40 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.81 (C
CH), 7.79 (s, 1H, pyrazole-H), 7.71 (s, 1H, pyrazole-H), 6.40 (br.s, 2H, NH2), 4.38 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.24 (q, J = 7.1 Hz, 2H, OCH2CH3), 3.90 (s, 3H, N-CH3), 1.40 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (CDCl3, 100 MHz, δ ppm): 165.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.55 (C
O), 164.55 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.26 (NH2CS), 145.73 (N
O), 159.26 (NH2CS), 145.73 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 139.82 (pyrazole-CH), 135.08 (C), 130.89 (pyrazole-CH), 127.69 (C), 121.24 (pyrazole-C), 102.75 (C), 61.52 (CH2), 60.26 (CH2), 39.33 (N–CH3), 14.42 (CH3), 14.32 (CH3). MS (ESI): m/z 351 [M + H]+.
CH), 139.82 (pyrazole-CH), 135.08 (C), 130.89 (pyrazole-CH), 127.69 (C), 121.24 (pyrazole-C), 102.75 (C), 61.52 (CH2), 60.26 (CH2), 39.33 (N–CH3), 14.42 (CH3), 14.32 (CH3). MS (ESI): m/z 351 [M + H]+.General procedure for the synthesis of compounds 4a–d and 5a–c
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.06 (2HNC
O), 164.06 (2HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 142.63 (2HNCS), 139.08 (2Ar-C), 133.60 (2Ar-CH), 132.20 (2Ar-C), 128.96 (2Ar-CH), 128.52 (2Ar-CH), 124.50 (2Ar-CH), 108.58 (2C), 61.39 (2CH2), 21.60 (2Ar-CH3), 14.43 (2CH3). MS (ESI): m/z 495 [M + H]+.
O), 142.63 (2HNCS), 139.08 (2Ar-C), 133.60 (2Ar-CH), 132.20 (2Ar-C), 128.96 (2Ar-CH), 128.52 (2Ar-CH), 124.50 (2Ar-CH), 108.58 (2C), 61.39 (2CH2), 21.60 (2Ar-CH3), 14.43 (2CH3). MS (ESI): m/z 495 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.11 (2HNC
O), 161.11 (2HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 141.83 (2HNCS), 134.39 (2Ar-C), 132.96 (q, J = 34.0 Hz, 4Ar-C), 127.86 (4Ar-CH), 126.33 (2Ar-CH), 122.90 (d, J = 272.9 Hz, 4CF3), 110.08 (2C), 61.96 (2CH2), 14.36 (2CH3). MS (ESI): m/z 739 [M + H]+.
O), 141.83 (2HNCS), 134.39 (2Ar-C), 132.96 (q, J = 34.0 Hz, 4Ar-C), 127.86 (4Ar-CH), 126.33 (2Ar-CH), 122.90 (d, J = 272.9 Hz, 4CF3), 110.08 (2C), 61.96 (2CH2), 14.36 (2CH3). MS (ESI): m/z 739 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.80 (2HNC
O), 160.80 (2HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.57 (dd, J = 253.3; 10.3 Hz, 4Ar-CF), 142.99 (dt, J = 259.0; 11.4 Hz, 2Ar-CF), 142.11 (2HNCS), 128.14 (Ar-C), 112.45 (dd, J = 16.3; 6.6 Hz, 4Ar-CH), 109.43 (2C), 61.84 (2CH2), 14.39 (2CH3). MS (ESI): m/z 575 [M + H]+.
O), 151.57 (dd, J = 253.3; 10.3 Hz, 4Ar-CF), 142.99 (dt, J = 259.0; 11.4 Hz, 2Ar-CF), 142.11 (2HNCS), 128.14 (Ar-C), 112.45 (dd, J = 16.3; 6.6 Hz, 4Ar-CH), 109.43 (2C), 61.84 (2CH2), 14.39 (2CH3). MS (ESI): m/z 575 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 163.26 (2HNC
O), 163.26 (2HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 153.58 (4Ar-C), 142.64 (2HNCS), 142.17 (2Ar-C), 127.46 (2Ar-C), 108.58 (2C), 105.05 (4Ar-CH), 61.39 (2CH2), 61.15 (2OCH3), 56.53 (4OCH3), 14.41 (2CH3). MS (ESI): m/z 647 [M + H]+.
O), 153.58 (4Ar-C), 142.64 (2HNCS), 142.17 (2Ar-C), 127.46 (2Ar-C), 108.58 (2C), 105.05 (4Ar-CH), 61.39 (2CH2), 61.15 (2OCH3), 56.53 (4OCH3), 14.41 (2CH3). MS (ESI): m/z 647 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.24 (C
O), 165.24 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.36 (HNC
O), 161.36 (HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 154.73 (NH2CS), 148.82 (HNCS), 134.63 (Ar-C), 134.05 (Ar-C), 132.73 (Ar-CH), 130.37 (Ar-CH), 127.14 (Ar-CH), 122.97 (Ar-CH), 111.22 (C), 102.37 (C), 61.51 (CH2), 60.48 (CH2), 14.57 (CH3), 14.34 (CH3). MS (ESI): m/z 408 [M + H]+.
O), 154.73 (NH2CS), 148.82 (HNCS), 134.63 (Ar-C), 134.05 (Ar-C), 132.73 (Ar-CH), 130.37 (Ar-CH), 127.14 (Ar-CH), 122.97 (Ar-CH), 111.22 (C), 102.37 (C), 61.51 (CH2), 60.48 (CH2), 14.57 (CH3), 14.34 (CH3). MS (ESI): m/z 408 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.38 (C
O), 165.38 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 163.48 (HNC
O), 163.48 (HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 154.50 (NH2CS), 153.56 (2Ar-C), 142.14 (HNCS), 135.61 (Ar-C), 127.34 (Ar-C), 110.14 (C), 104.88 (2Ar-CH), 102.20 (C), 61.18 (CH2), 61.13 (OCH3), 60.34 (CH2), 56.49 (2OCH3), 14.55 (CH3), 14.35 (CH3). MS (ESI): m/z 453 [M + H]+.
O), 154.50 (NH2CS), 153.56 (2Ar-C), 142.14 (HNCS), 135.61 (Ar-C), 127.34 (Ar-C), 110.14 (C), 104.88 (2Ar-CH), 102.20 (C), 61.18 (CH2), 61.13 (OCH3), 60.34 (CH2), 56.49 (2OCH3), 14.55 (CH3), 14.35 (CH3). MS (ESI): m/z 453 [M + H]+.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.24 (C
O), 165.24 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 162.15 (HNC
O), 162.15 (HNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.95 (NH2CS), 155.58 (HNCS), 154.70 (Ar-C), 149.11 (Ar-CH), 137.63 (Ar-CH), 134.52 (Ar-C), 126.95 (C), 124.73 (Ar-CH), 102.30 (C), 61.49 (CH2), 60.47 (CH2), 14.56 (CH3), 14.33 (CH3). MS (ESI): m/z 398.5 [M + H]+.
O), 160.95 (NH2CS), 155.58 (HNCS), 154.70 (Ar-C), 149.11 (Ar-CH), 137.63 (Ar-CH), 134.52 (Ar-C), 126.95 (C), 124.73 (Ar-CH), 102.30 (C), 61.49 (CH2), 60.47 (CH2), 14.56 (CH3), 14.33 (CH3). MS (ESI): m/z 398.5 [M + H]+.Biology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells and cultured at 37 °C in a humidified CO2 incubator (95% air, 5% CO2) for 24 h. Three times diluted compound was added cell monolayer and continued to cultivate for 48 h. Then, the cytopathic effects were recorded. The 50% cytotoxic concentration (TC50) values of active compounds were calculated by the method of Reed and Muench.
000 cells and cultured at 37 °C in a humidified CO2 incubator (95% air, 5% CO2) for 24 h. Three times diluted compound was added cell monolayer and continued to cultivate for 48 h. Then, the cytopathic effects were recorded. The 50% cytotoxic concentration (TC50) values of active compounds were calculated by the method of Reed and Muench.Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC) (Grant No. 21550110495) and Chinese Academy of Sciences President's International Fellowship Initiative (Grant No. 2016PT014).Notes and references
- M. Congreve, G. Chessari, D. Tisi and A. J. Woodhead, J. Med. Chem., 2008, 51, 3661–3680 CrossRef CAS PubMed.
- D. C. Rees, M. Congreve, C. W. Murray and R. Carr, Nat. Rev. Drug Discovery, 2004, 3, 660–672 CrossRef CAS PubMed.
- M. G. Siegel and M. Vieth, Drug Discovery Today, 2007, 12, 71–79 CrossRef CAS PubMed.
- P. Bamborough and C.-w. Chung, MedChemComm, 2015, 6, 1587–1604 RSC.
- J. Wang and T. Hou, J. Chem. Inf. Model., 2010, 50, 55–67 CrossRef CAS PubMed.
- S. Marino, D. Stachurska-Buczek, D. Huggins, B. Krywult, C. Sheehan, T. Nguyen, N. Choi, J. Parsons, P. Griffiths, I. James, A. Bray, J. White and R. Boyce, Molecules, 2004, 9, 405 CrossRef CAS PubMed.
- L. C. Blum and J.-L. Reymond, J. Am. Chem. Soc., 2009, 131, 8732–8733 CrossRef CAS PubMed.
- G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887–2893 CrossRef CAS PubMed.
- G. W. Bemis and M. A. Murcko, J. Med. Chem., 1999, 42, 5095–5099 CrossRef CAS PubMed.
- G. Schneider, M.-L. Lee, M. Stahl and P. Schneider, J. Comput.-Aided Mol. Des., 2000, 14, 487–494 CrossRef CAS PubMed.
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2016, 66, 7–30 CrossRef PubMed.
- C. Mugnaini, V. Pedani, D. Giunta, B. Sechi, M. Solinas, A. Casti, M. P. Castelli, G. Giorgi and F. Corelli, RSC Adv., 2014, 4, 1782–1793 RSC.
- R. Romagnoli, P. G. Baraldi, C. Lopez-Cara, M. K. Salvador, D. Preti, M. A. Tabrizi, J. Balzarini, P. Nussbaumer, M. Bassetto, A. Brancale, X.-H. Fu, G. Yang, J. Li, S.-Z. Zhang, E. Hamel, R. Bortolozzi, G. Basso and G. Viola, Bioorg. Med. Chem., 2014, 22, 5097–5109 CrossRef CAS PubMed.
- D. Ye, Y. Zhang, F. Wang, M. Zheng, X. Zhang, X. Luo, X. Shen, H. Jiang and H. Liu, Bioorg. Med. Chem., 2010, 18, 1773–1782 CrossRef CAS PubMed.
- Y. Sun, J. Fan, Z. Zhu, X. Guo, T. Zhou, W. Duan and X. Shen, Eur. J. Pharmacol., 2015, 762, 202–213 CrossRef CAS PubMed.
- J. Thomas, A. Jejcic, P. Vervaeke, R. Romagnoli, S. Liekens, J. Balzarini and W. Dehaen, ChemMedChem, 2014, 9, 2744–2753 CrossRef CAS PubMed.
- A. Martorana, C. Gentile, U. Perricone, A. P. Piccionello, R. Bartolotta, A. Terenzi, A. Pace, F. Mingoia, A. M. Almerico and A. Lauria, Eur. J. Med. Chem., 2015, 90, 537–546 CrossRef CAS PubMed.
- A. Abu-Hashem, M. El-Shehry and F. Badria, Acta Pharm., 2010, 60, 311–323 CrossRef CAS PubMed.
- A. E. Rashad, A. H. Shamroukh, R. E. Abdel-Megeid, A. Mostafa, R. El-Shesheny, A. Kandeil, M. A. Ali and K. Banert, Eur. J. Med. Chem., 2010, 45, 5251–5257 CrossRef CAS PubMed.
- S. Massari, G. Nannetti, L. Goracci, L. Sancineto, G. Muratore, S. Sabatini, G. Manfroni, B. Mercorelli, V. Cecchetti, M. Facchini, G. Palù, G. Cruciani, A. Loregian and O. Tabarrini, J. Med. Chem., 2013, 56, 10118–10131 CrossRef CAS PubMed.
- S. Lepri, G. Nannetti, G. Muratore, G. Cruciani, R. Ruzziconi, B. Mercorelli, G. Palù, A. Loregian and L. Goracci, J. Med. Chem., 2014, 57, 4337–4350 CrossRef CAS PubMed.
- K. Bozorov, J.-Y. Zhao, B. Elmuradov, A. Pataer and H. A. Aisa, Eur. J. Med. Chem., 2015, 102, 552–573 CrossRef CAS PubMed.
- F. Han, S. Lin, P. Liu, X. Liu, J. Tao, X. Deng, C. Yi and H. Xu, ACS Med. Chem. Lett., 2015, 6, 434–438 CrossRef CAS PubMed.
- A. A. Gryshchenko, V. G. Bdzhola, A. O. Balanda, N. V. Briukhovetska, I. M. Kotey, A. G. Golub, T. P. Ruban, L. L. Lukash and S. M. Yarmoluk, Bioorg. Med. Chem., 2015, 23, 2287–2293 CrossRef CAS PubMed.
- S. Bugge, I. U. Moen, K.-O. Kragseth Sylte, E. Sundby and B. H. Hoff, Eur. J. Med. Chem., 2015, 94, 175–194 CrossRef CAS PubMed.
- F. Bysting, S. Bugge, E. Sundby and B. H. Hoff, RSC Adv., 2017, 7, 18569–18577 RSC.
- K. Bozorov, H.-R. Ma, J.-Y. Zhao, H.-Q. Zhao, H. Chen, K. Bobakulov, X.-L. Xin, B. Elmuradov, K. Shakhidoyatov and H. A. Aisa, Eur. J. Med. Chem., 2014, 84, 739–745 CrossRef CAS PubMed.
- A. M. Ginsberg, M. W. Laurenzi, D. J. Rouse, K. D. Whitney and M. K. Spigelman, Antimicrob. Agents Chemother., 2009, 53, 3720–3725 CrossRef CAS PubMed.
- R. Tiwari, G. C. Moraski, V. Krchňák, P. A. Miller, M. Colon-Martinez, E. Herrero, A. G. Oliver and M. J. Miller, J. Am. Chem. Soc., 2013, 135, 3539–3549 CrossRef CAS PubMed.
- L. Zhou, G. Stewart, E. Rideau, N. J. Westwood and T. K. Smith, J. Med. Chem., 2013, 56, 796–806 CrossRef CAS PubMed.
- R. Mukkavilli, J. Pinjari, B. Patel, S. Sengottuvelan, S. Mondal, A. Gadekar, M. Verma, J. Patel, L. Pothuri, G. Chandrashekar, P. Koiram, T. Harisudhan, A. Moinuddin, D. Launay, N. Vachharajani, V. Ramanathan and D. Martin, Eur. J. Pharm. Sci., 2014, 65, 147–155 CrossRef CAS PubMed.
- A. M. Thompson, P. D. O'Connor, A. Blaser, V. Yardley, L. Maes, S. Gupta, D. Launay, D. Martin, S. G. Franzblau, B. Wan, Y. Wang, Z. Ma and W. A. Denny, J. Med. Chem., 2016, 59, 2530–2550 CrossRef CAS PubMed.
- A. C. Véras of Aguiar, R. O. of Moura, J. F. Bezerra Mendonça Junior, H. A. de Oliveira Rocha, R. B. Gomes Câmara and M. dos Santos Carvalho Schiavon, Biomed. Pharmacother., 2016, 84, 403–414 CrossRef PubMed.
- K. Snegaroff, F. Lassagne, G. Bentabed-Ababsa, E. Nassar, S. C. S. Ely, H. Stephanie, E. Perspicace, A. Derdour and F. Mongin, Org. Biomol. Chem., 2009, 7, 4782–4788 CAS.
- M. Bourgeaux, S. Vomscheid and W. G. Skene, Synth. Commun., 2007, 37, 3551–3558 CrossRef CAS.
- C. Isanbor and D. O'Hagan, J. Fluorine Chem., 2006, 127, 303–319 CrossRef CAS.
- W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed.
- E. Asadi, M. Abdouss, R. M. Leblanc, N. Ezzati, J. N. Wilson and S. Azodi-Deilami, RSC Adv., 2016, 6, 37308–37318 RSC.
- E. A. K. Nivethaa, S. Dhanavel, V. Narayanan, C. A. Vasu and A. Stephen, RSC Adv., 2015, 5, 1024–1032 RSC.
- M. Dauria and G. Mauriello, Photochem. Photobiol., 1994, 60, 542–545 CrossRef CAS.
- R. R. Tykwinski, A. Hilger, F. Diederich, H. P. Lüthi, P. Seiler, V. Gramlich, J.-P. Gisselbrecht, C. Boudon and M. Gross, Helv. Chim. Acta, 2000, 83, 1484–1508 CrossRef CAS.
- P. Thomson, M. A. Naylor, S. A. Everett, M. R. L. Stratford, G. Lewis, S. Hill, K. B. Patel, P. Wardman and P. D. Davis, Mol. Cancer Ther., 2006, 5, 2886–2894 CrossRef CAS PubMed.
- J. Weinstock, J. Wu, P. Cao, W. D. Kingsbury, J. L. McDermott, M. P. Kodrasov, D. M. McKelvey, K. G. Suresh Kumar, S. J. Goldenberg, M. R. Mattern and B. Nicholson, ACS Med. Chem. Lett., 2012, 3, 789–792 CrossRef CAS PubMed.
- R. W. Pero, A. Olsson, A. Amiri and D. Chaplin, Cancer Detect. Prev., 1998, 22, 225–236 CrossRef CAS PubMed.
- Y. Zhang, L. Jin, H. Xiang, J. Wu, P. Wang, D. Hu, W. Xue and S. Yang, Eur. J. Med. Chem., 2013, 66, 335–344 CrossRef CAS PubMed.
- J. Zhao and H. A. Aisa, Bioorg. Med. Chem. Lett., 2012, 22, 2321–2325 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04808d |
| This journal is © The Royal Society of Chemistry 2017 |