Open Access Article
Open Access ArticleInterfacial defect engineering over fusiform bismuth vanadate photocatalyst enables to excellent solar-to-chemical energy coupling†
Chunjing Shi,
Xiaoli Dong *,
Jiawei Wang,
Xiuying Wang,
Hongchao Ma and
Xiufang Zhang
*,
Jiawei Wang,
Xiuying Wang,
Hongchao Ma and
Xiufang Zhang
School of Light Industry and Chemical Engineering, Dalian Polytechnic University, #1 Qinggongyuan, Dalian 116034, P R China. E-mail: dongxl@dlpu.edu.cn
First published on 18th May 2017
Abstract
The presence of surface oxygen vacancies over oxide semiconductors plays a versatile role in the development of light-driven organic degradation and energy production. Simultaneously, the role of defect sites successfully forms an ideal model for the photocatalytic responsiveness over BiVO4-OV in the NIR region.
With the development of industrialization and rapid growth of the population, the global energy crisis and the serious problems accompanying combustion of fossil fuels threaten our environment, climate and human health.1 Therefore, we suggest that a simple and efficient strategy can effectively solve aforementioned problems, that is quite important but challenging.2 The sustainable and environmentally friendly light-driven technologies have obtained considerable interdisciplinary attention in recent years. At present, metal oxide semiconductors as efficient catalysts for H2 production from water splitting and using solar light energy for the degradation of environmental pollutants have been investigated for various potential applications because of their high thermal conductivity, high chemical stability and low environmental toxicity. As previous report, the photocatalysts such as MoS2, ZnSnO3, g-C3N4 and 3C-SiC,3 were demonstrated profound improvement of its photocatalytic activity. They hold a great promise to realize the fast pollutant degradation and efficient energy generation via utilizing solar energy. However, sunlight basically consists of 44% visible light, 3% ultraviolet and the remainder infrared light.4 Unfortunately, the most of conventional photocatalysts only can utilize UV or/and visible light to achieve the solar-to-chemical energy conversion.5 To overcome the grant challenge, we tried best to effectively broaden the optical window of conventional photocatalysts and endow them with considerable utilization efficiency of near infrared light. This kind of photocatalysts could realize the remarkable enhancement of photocatalytic activity. Besides, the main limitation of energy conversion originates from two bottlenecks. They are poor transition probability of photoexcited electrons to the conduction band under solar light illumination and serious photogenerated electron–hole recombination. In order to solve the problems, it is very important for the researchers to seek a desirable material that could improve fundamentally properties of the sunlight harvesting and the carrier separation/transmission.
To date, only a few photocatalytic materials were reported that can efficiently utilize NIR light to achieve the pollutant decomposition and the clean energy production. In the regard, up-conversion material doped photocatalyst, narrow band gap photocatalyst and plasmon-assisted photocatalyst, have been successfully presented to improve the photocatalytic activity.6 However, some photocatalytic materials still suffer from intrinsic drawbacks. For instance, the applications of narrowband gap photocatalysts are severely restricted owing to their inappropriate energy band positions lead to weak reduction or oxidation power.7 Hence, the conventional narrow band gap photocatalysts cannot efficiently realize solar-to-chemical energy conversion. On the other hand, modified photocatalysts with complicated component such as nitrogen, sulfide and noble metal possibly result in environmental deterioration due to poisonous substances enter into the ecological environment. On this occasion, it is important meaning to explore an appropriate candidate for designing efficient semiconductor.
Compared with the conventional and studied photocatalysts, oxygen vacancy defects (OVs) on the surface is the most common type of defects in oxide semiconductor. In particular, it provides perfect sites for enhanced O2 adsorption and activation. Meanwhile, OVs is a remarkable strategy to modify the band gap of semiconductors and adjust their electronic structures. In view of the point, it has been demonstrated that semiconductors surface with oxygen vacancy could efficaciously stretch photo-response from UV to visible light region.8 In this case, oxygen vacancy acts as the conduction band of semiconductor materials leading to optimizing electronic band structure, boosting electron–hole pair separation and suppressing the photoexcited electron–hole recombination in bulk materials. Thus, oxygen defects can make semiconductor materials enhance the ability of utilizing solar light.9 The result gives us an inspiration which semiconductor with OVs may be responsive to NIR light region. To validate this consideration, we selected bismuth vanadate (BiVO4) as the credible photocatalyst. Bismuth vanadate (BiVO4) is one of the most promising nano-materials, due to its low cost, chemical and thermal stability, and environmentally friendly properties.10 However, the widespread application of BiVO4 as an efficient photocatalyst is hindered by its poor light absorption and insufficient charge separation efficiency. In this study, we considered that OV-incorporated BiVO4 can be highly efficient degradation of pollutions under broad solar spectrum ranging from UV to NIR.
The desired BiVO4-OV was prepared by a conventional and facile one-step solvothermal method. During the process, glycerol acts as reducing agent. Based on redox reaction mechanism, the hydroxyl removed selectively oxygen atoms from the BiVO4 surface, resulting in the formation of oxygen vacancy. For comparison, the sample of BiVO4-OV was putted in an appropriate crucible. Then, it was annealed to 300 °C for 5 h in an air-rich atmosphere muffle furnace. After cooling to room temperature, the prepared of the sample without oxygen vacancy was obtained and marked as BiVO4-P. The detailed experimental procedure was provided in ESI.
The morphology of BiVO4-OV and BiVO4-P were charactered by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). The picture of FESEM indicated uniform fusiform shape with relatively porous and unsmooth surface, and the morphology had no obvious change between BiVO4-OV and BiVO4-P in Fig. 1a and b. Moreover, as shown in Fig. 1c and d, transmission electron microscopy (TEM) was performed which demonstrates the high crystallinity nature of semblable BiVO4 and have their (040) facets exposed. In addition, it can be seen from the high-resolution TEM images of BiVO4-OV that shows the crystal lattice fringes and the distance of neighboring fringes was measured to be 0.295 nm (Fig. 1f), which accorded with the (040) planes of BiVO4-OV. The lack of lattice was drawn with the dotted circle and a disordered layer of 1.59–1.77 nm thick could be obviously seen at the sample edges. The above appearance could reveal the surface damage due to oxygen atoms moved from the BiVO4 surface resulting in the formation of OVs (Fig. 1f).11 However, the edges of BiVO4-P are clearer lattice fringes. For compared with BiVO4-OV, BiVO4-P has no similar phenomenon (Fig. 1e).
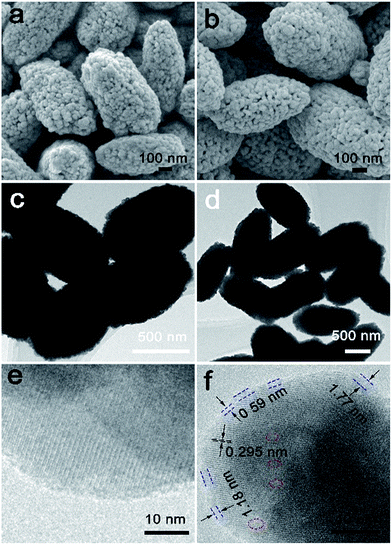 | ||
| Fig. 1 (a) FESEM images of BiVO4-P and (b) BiVO4-OV. (c) TEM images of BiVO4-P and (d) BiVO4-OV. (e) HRTEM image of BiVO4-P and (f) BiVO4-OV (the lattice distortion and lack of the lattice). | ||
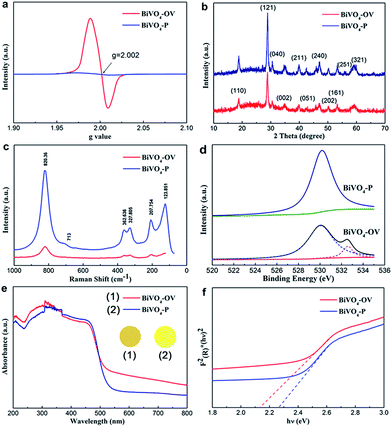
To further affirm the presence of OVs, the as-synthetized BiVO4-OV and BiVO4-P were charactered by electron spin resonance (ESR), a tool for examining unpaired electrons in materials. The spectrogram of BiVO4-OV uniquely exhibits a symmetrical ESR signal at g = 2.002, due to the electron trapping at oxygen vacancies (Fig. 2a).12 Therefore, the successful formation of OVs on BiVO4 surface via our facile one-step glycol-assisted solvothermal approach was verified. On the contrary, the ESR spectrum of the BiVO4-P could not emerge the corresponding signal. This phenomenon proves that the high temperature annealing process could eliminate all the OVs on BiVO4 surface.
XRD were employed to characterize the crystal phase of the sample BiVO4-P and BiVO4-OV. The pattern was depicted in Fig. 2b, the XRD diffraction peaks of BiVO4-P are corresponding to the standard well-indexed to the phase of monoclinic BiVO4 (JCPDS card no. 14-0688), which disclose the obtained sample without any impurities. Nevertheless, the typical XRD diffraction character of BiVO4-OV had no the homologous crystal facet (251) and the intensity of diffraction peak is severely weaker than those of BiVO4-P in Fig. 2b.13 The crystallinity weakening are attributed to the displacement of oxygen atoms resulting in compressive stress state. Previous reports also showed the similar phenomenon of OVs-induced crystallinity weakening.14
Raman spectroscopy can provide structural information to witness the existence of oxygen vacancy. To avert possible ambiguities in the structural information of sample, characteristic Raman peaks spectrogram of the sample BiVO4-OV and BiVO4-P were performed by Raman spectroscopy used different Raman laser lines. Based on Fig. 2c, it can found that the external mode (rotation/translation) occurred at around 210 cm−1 and 123.8 cm−1. The most intense peak at 820.36 cm−1 and the weak shoulder at about 713 cm−1 are attributed to the stretching modes of the two vibrational modes of the V–O bonds.15 The asymmetric and symmetric formations of the VO4 tetrahedron are detected at ca. 327.8 cm−1 and 362.6 cm−1, respectively.16 The peak of BiVO4-OV become weaker and wider than BiVO4-P, owing to the crystallinity weakening and the lattice distortion, which can manifest some oxygen atoms have been removed from BiVO4, resulting in the formation of oxygen vacancy defects.17 This similar phenomenon also appears in the Raman peaks spectrograms that were employed by laser lines 532 and 785 nm (Fig. S3 and S4†).
According to the X-ray photoelectron spectroscopy of the as-prepared BiVO4-P and BiVO4-OV, it seen that indicated the atomic chemical microenvironment on the surface. As we can see high resolution O 1s spectra in Fig. 2d, the ordinary peak at 529.9 eV for both samples is ascribed to the lattice oxygen ubiquitously in oxide semiconductors. Notably, an additional broad peak at 532.7 eV can be observed, as shown in Fig. 2d, which most probably arises from either oxygen vacancies or adsorbed oxygen species (O− and O2−) at oxygen vacancies.18 They also suggests that oxygen vacancies are stabilized by the adsorbed oxygen species, a typical feature for the defect-rich oxides. Moreover, based on the high resolution Bi 4f spectrum of BiVO4-OV (Fig. S5†), the level peaks of Bi 4f7/2 and Bi 4f5/2 spin-orbital splitting photoelectrons in the Bi3+ chemical state are at around 161.0 and 155.7 eV, respectively. The decreased binding energies of Bi 4f of BiVO4-OV are obvious due to the partial reduction of Bi3+ resulting from electrons localized at oxygen vacancy defects.19 For comparison with the BiVO4-P, the V 2p spectrum of the BiVO4-OV reveals that the binding energies of V 2p3/2 and V 2p1/2 peaks obviously shifted to corresponding to peak position due to the formation of OVs attracting more electrons (Fig. S6†). The similar observation was also reported in other literatures.20
To directly prove introduction oxygen vacancies, UV-Vis absorption spectroscopy of the sample was investigated to examine the optical properties of BiVO4-P and BiVO4-OV. As shown in Fig. 2e, this evidence ascertains the imperative roles of OVs during photocatalytic reactions. In intuitive sense, bright-yellow and black-yellow separately represent BiVO4-P and BiVO4-OV. The formation of oxygen vacancy on BiVO4 surface leads to change color of the sample. Thereby, the better light absorption and accompanied with a reduced band gap, which is reflected by UV-Vis absorption spectra. As shown in Fig. 2e and f, BiVO4-OV certified a much intense continuous absorption over Vis-NIR region. The occurrence of OVs-induced sub-bands excitation from defect states to CB could emerge this appearance, which may cause drastic changes in the optical property. In addition, the absorption edge of BiVO4-OV was obvious red-shifted, demonstrating the imperative contribution of OVs for band gap narrowing.21 Based on the Tauc plot (Fig. 2f), the red slash and blue slash stand for the band gaps of BiVO4-OV and BiVO4-P at approximated 2.10 and 2.26 eV, respectively. The phenomenon of OV-induced band gap narrowing could be ascribed to the down shift of CB minimum.22
Photoluminescence (PL) spectroscopy was provided to further ascertain the presence of oxygen vacancy detects. The measurement of PL emission was performed with the excitation at 425 nm and could reveal the efficiency of charge carries transfer and separation. To understand the fate of photogenerated electrons and holes in semiconductors, Fig. S7† shows the comparison of PL spectra of the BiVO4-P and BiVO4-OV. The both sample PL emission peaks center at about 473 nm. The PL emission intensity of BiVO4 with OVs stay lower than that of BiVO4-P, which is attributed to the suppressive recombination of electrons and holes, because of the formation of oxygen vacancies.23
To elucidate the presence of oxygen vacancy defects for the mechanism of enhanced photocatalytic performance, valence band XPS (VB XPS) analysis was proposed synchronously. It is readily seen from the illustration Fig. 3a, there are two estimated positions at 1.31 and 0.79 eV. They represent the VB maximum energy levels of BiVO4-P and BiVO4-OV, respectively. Combining the above results, corresponding to the CB minimum was speculated to be downshift, which validate the indispensable contribution of oxygen vacancy for changing the band edge of the BiVO4-OV. As delineated in Fig. 3b, the formation of sub-bands below CB is attributed to the introduction of OVs.25 Fig. 3b depicts the energy transfer process, there are three possible procedures to reflect that the electrons could be excited. For instance, (i) electrons transition from VB to CB; (ii) from VB to OV-induced defect states and (iii) from OV-induced defect states to CB.24 To look into above situation, the presence of OVs induced defect states serve as springboard for electrons transition, lead to improving the ability of capture more electrons under NIR light irradiation. This novel phenomenon confirms that NIR light could be harvested through the sub-bands excitation from the in-gap bands to CB, due to formation of oxygen vacancies.
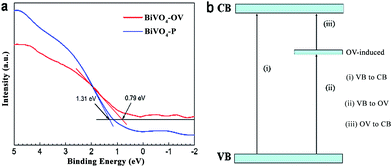 | ||
| Fig. 3 (a) VB XPS pattern of the BiVO4-P and BiVO4-OV, (b) illustration of BiVO4-OV with possible electrons excitation pathways. | ||
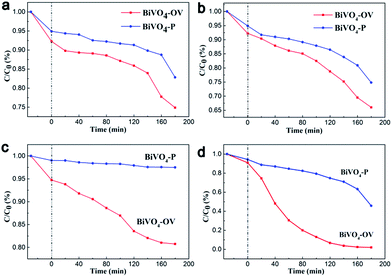
The photocatalytic activity of the obtained sample is evaluated by degradation of RhB solution at room temperature and atmospheric pressure under the different irradiation of UV, Vis, NIR light and simulated solar light. The photocatalytic performance experiment was carried through different experimental conditions. As shown in Fig. 4a and b, the degradation efficiency ratio of RhB using BiVO4-P and BiVO4-OV under the UV/Vis light irradiation for 3 h reaches the approximate 40% and 65%, respectively. Upon probing the achieved information, here are several key reasons why BiVO4-OV exhibit higher photocatalytic activity. Firstly, BiVO4-OV can absorb more light energy than BiVO4-P due to the presence of OVs played an indispensable role to construct new band gap. In addition, the photogenerated electrons that arrived at the surface of BiVO4-OV are trapped by the OVs, and then transferred immediately from the catalysts to the adsorbed molecules, thus hindering the photoexcited electron–hole recombination.25 Furthermore, the attraction between OVs and organic molecules would result in the formation of unexpected affinity interactions, which lower the energy barrier for interfacial charge transfer.26
The previous articles rarely reported that the photocatalytic performance of BiVO4 is response under NIR irradiation. Appreciable degradation rate was detected for BiVO4-P (Fig. 4c), which consistent with the theoretical expectation that pristine BiVO4 is not photocatalytic active under NIR light. However, by introduction surface OV detects, the degradation ability of BiVO4 with oxygen vacancy defects was distinctly higher than BiVO4-P under NIR irradiation. This demonstrates the presence of OV defects on the surface of BiVO4 could effectively decompose pollutions to in a noxious productions through gaining low photonic energy NIR light. As aforementioned, surface OVs played a pivotal role to promote the sub-bands excitation of electrons from OV-induced defect states to CB under long wavelength NIR light. To further examine the photocatalytic capability of BiVO4-OV for converting more energy, RhB degradation experiment was also carried out under simulated solar light irradiation (Fig. 4d). Anticipatorily, BiVO4-OV revealed an excellent light energy conversion, which was distinctly higher than BiVO4-P under individual UV, Vis and NIR irradiation.
On account of OVs on BiVO4 surface, capturing low-energy NIR photons could indirectly excite sub-bands. Compared to BiVO4-OV, the mechanism of organics side chain polyviologen over BiVO4-P only allowed direct excitation from VB to CB under UV-Vis light. Based on the analysis summary, the photocatalyst BiVO4-OV and BiVO4-P process of redox was schematically illustrated in Fig. 5a and b. Since the preparation of BiVO4-OV was employed by the facile solvothermal method and we consider its outstanding photocatalytic performance, it is no doubt that BiVO4-OV would be a promising candidate for a wide range of photocatalytic applications over broad spectrum of solar light.
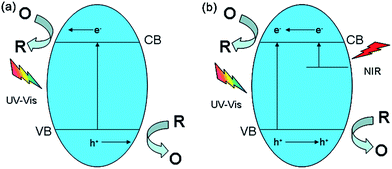 | ||
| Fig. 5 Schematic diagram for the possible mechanism of occurred redox reaction over (a) BiVO4-P and (b) BiVO4-OV. | ||
In conclusion, the aforementioned characterization results provide undeniable evidences for the successful formation of surface OV defects on BiVO4-OV, rendering prerequisite for studying the roles of OVs play in photocatalytic applications. BiVO4-OV photoresponse has been successfully attested under UV-Vis-NIR-irradiation, which reflected the presence of OVs. It can not only broaden the activity range of semiconductor oxide from UV to NIR light, but also enhance the carries transformation and effective separation without the incorporation of any noble metal or up-conversion materials. The obtained BiVO4 with the presence of OVs could not only harvest low photonic energy NIR light, simultaneously, it could provide a new train of thought to researchers in this work. Surface defect engineering as important and deep seated insights would maintain a huge promising value of research.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 21476033 and 21577008).Notes and references
- B. Z. Fang, Y. Xing, A. Bonakdarpour, S. C. Zhang and D. P. Wilkinson, ACS Sustainable Chem. Eng., 2015, 3, 2381–2388 CrossRef CAS; Z. Z. Wu, B. Z. Fang, Z. P. Wang, C. L. Wang, Z. H. Liu, F. Y. Liu, W. Wang, A. Alfantazi, D. Z. Wang and D. P. Wilkinson, ACS Catal., 2013, 3, 2101–2107 CrossRef; Z. Xiong, H. B. Wang, N. Y. Xu, H. L. Li, B. Z. Fang, Y. C. Zhao, J. Y. Zhang and C. G. Zheng, Int. J. Hydrogen Energy, 2015, 40, 10049–10062 CrossRef; Y. C. Hao, X. L. Dong, S. R. Zhai, X. Y. Wang, H. C. Ma and X. F. Zhang, Chem. Commun., 2016, 52, 6525 RSC.
- S. Q. Fan, C. Kim, B. Z. Fang, K. X. Liao, G. J. Yang, C. J. Li, J. J. Kim and J. Ko, J. Phys. Chem. C, 2011, 115, 7747–7754 CAS; B. Z. Fang, A. Bonakdarpour, K. Reilly, Y. Xing, F. Taghipour and D. P. Wilkinson, ACS Appl. Mater. Interfaces, 2014, 6, 15488–15498 Search PubMed; Y. C. Hao, X. L. Dong, S. R. Zhai, X. Y. Wang, H. C. Ma and X. F. Zhang, J. Mater. Chem. A, 2016, 4, 8298 Search PubMed.
- Z. Z. Wu, C. Y. Tang, P. Zhou, Z. H. Liu, Y. S. Xu, D. Z. Wang and B. Z. Fang, J. Mater. Chem. A, 2015, 3, 13050–13056 Search PubMed; S. Y. Dong, J. Y. Sun, Y. k. Li, C. F. Yu, Y. H. Li and J. H. Sun, Appl. Catal., B, 2014, 144, 386–393 CrossRef CAS; Y. Zheng, Y. Jiao, Y. H. Zhu, Q. Cai, A. Vasileff, L. H. Li, Y. Han, Y. Chen and S. Z. Qiao, J. Am. Chem. Soc., 2017, 139, 3336–3339 CrossRef PubMed; J. D. Zhang, J. J. Chen, L. P. Xin and M. M. Wang, Mater. Sci. Eng., B, 2014, 179, 6–11 CrossRef.
- S. B. Gawande and S. R. Thakar, Int. Nano Lett., 2012, 2, 11 CrossRef CAS; S. Fu, Y. D. Liu, Y. Ding, X. Q. Du, F. Y. Song, R. Xiang and B. C. Ma, Chem. Commun., 2014, 50, 2167 RSC.
- H. Tong, S. X. Quyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229 CrossRef CAS PubMed.
- J. Tian, Y. Leng, Z. Zhao, Y. Xia, Y. Sang, P. Hao, J. Zhan, M. Li and H. Liu, Nano Energy, 2015, 11, 419 CrossRef CAS; Y. Sang, Z. Zhao, M. Zhao, P. Hao, Y. Leng and H. Liu, Adv. Mater., 2015, 27, 363 CrossRef PubMed; J. Yan, T. Wang, G. Wu, W. Dai, N. Guan, L. Li and J. Gong, Adv. Mater., 2015, 27, 1580 CrossRef PubMed.
- C. Guo, J. Shao, S. Yuan, C. Su, W. Ya, H. Pin, C. Jerome, L. Hong and Y. Guan, Nanoscale, 2015, 7, 3117 RSC.
- I. Justicia, P. Ordejón, G. Canto, J. L. Mozos, J. Fraxedas, G. A. Battiston, R. Gerbasi and A. Figueras, Adv. Funct. Mater., 2002, 14, 1399 CrossRef CAS; X. Pan, M.-Q. Yang, X. Fu, N. Zhang and Y.-J. Xu, Nanoscale, 2013, 5, 3601 RSC; J. Wang, Z. Wang, B. Huang, Y. Ma, Y. Liu, X. Qin, X. Zhang and Y. Dai, ACS Appl. Mater. Interfaces, 2012, 4, 4024 Search PubMed; M. Guan, C. Xiao, J. Zhang, S. Fan, R. An, Q. Cheng, J. Xie, M. Zhou, B. Ye and Y. Xie, J. Am. Chem. Soc., 2013, 135, 10411 CrossRef PubMed.
- J. Du, J. Qi, D. Wang and Z. Tang, Energy Environ. Sci., 2012, 5, 6914 CAS.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990 CrossRef CAS PubMed.
- Y. H. Lv, Y. f. Liu, Y. Y. Zhu and Y. F. Zhu, J. Mater. Chem. A, 2014, 2, 1174 CAS.
- L. F. Sun, Y. Liu, K. Gao, S. Liang, L. Pan and B. Xie, J. Am. Chem. Soc., 2014, 136, 6826 CrossRef PubMed.
- Y. Yu, C. Cao, H. Liu, P. Li, F. Wei, Y. Jiang and W. Song, J. Mater. Chem. A, 2014, 2, 1677 CAS.
- H. Li, J. Shi, K. Zhao and L. Zhang, Nanoscale, 2014, 6, 14168 RSC.
- G. M. Wang, Y. X. Ling, X. H. Lu, F. Qian, Y. X. Tong, J. Z. Zhang, V. Lordi, C. R. Leao and Y. Li, J. Phys. Chem. C, 2013, 117, 10957 CAS.
- S. M. Thalluri, Phys. Chem. Chem. Phys., 2015, 17, 17821 RSC.
- H. Li, J. G. Shi, K. Zhao and L. L. Zhang, Nanoscale, 2014, 6, 14168 RSC; Y. H. Lv, Y. F. Liu, Y. Y. Zhu and Y. F. Zhu, J. Mater. Chem. A, 2014, 2, 1174 Search PubMed; X. Y. Kong, W. P. Cathie Lee, W. J. Ong, S.-P. Chai and A. R. Mohamed, ChemCatChem, 2016, 8, 1–9 CrossRef CAS.
- S. P. Berglund, A. J. E. Rettie, S. Hoang and C. B. Mullins, Phys. Chem. Chem. Phys., 2012, 14, 7065 RSC.
- X. Y. Kong, W. P. C. Lee, W.-J. Ong, S.-P. Chai and A. R. Mohamed, ChemCatChem, 2016, 8, 3074 CrossRef CAS.
- Z. P. Nie, D. K. Ma, G. Y. Fang, W. Chen and S. M. Huang, J. Mater. Chem. A, 2016, 4, 2438 CAS.
- L. Ye, L. Zan, L. Tian, T. Peng and J. Zhang, Chem. Commun., 2011, 47, 6951 RSC.
- G. Zhang, Z. Hu, M. Sun, Y. Liu, L. Liu, H. Liu, C. P. Huang, J. Qu and J. Li, Adv. Funct. Mater., 2015, 25, 3726 CrossRef CAS.
- X. D. Jiang, Y. P. Zhang, J. Jiang, Y. S Rong, Y. C. Wang, Y. C. Wu and C. X. Pan, J. Phys. Chem. C, 2012, 116, 22619–22624 Search PubMed; S. Y. Dong, Y. R. Cui, Y. F. Wang, Y. K. Li, L. M. Hu, J. Y. Sun and J. H. Sun, Chem. Eng. J., 2014, 249, 102–110 CrossRef CAS.
- H. Li, J. Shi, K. Zhao and L. Zhang, Nanoscale, 2014, 6, 14168 RSC.
- W. J. Ong, L. L. Tan, S. P. Chai, S. T. Yong and A. R. Mohamed, Nanoscale, 2014, 6, 1946 RSC; W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Chem. Rev., 2016, 116, 7159 CrossRef CAS PubMed.
- K. Xin, C. Yen and C. Siang-Piao, Chem. Commun., 2016, 99, 14242 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental section, photocatalytic activity test of BiVO4-OV and BiVO4-P. Raman spectra, high resolution Bi 4f and V 2p XPS spectra of BiVO4-OV and BiVO4-P, PL spectra. See DOI: 10.1039/c7ra04328g |
| This journal is © The Royal Society of Chemistry 2017 |