Open Access Article
Open Access ArticleIsolation and phototransformation of enantiomerically pure iridium(III) bis[(4,6-difluorophenyl)pyridinato-N,C2]picolinate†
Yue Wanga,
Takunori Haradab,
Yoshihito Shiotac,
Kazunari Yoshizawac,
Heng Wanga,
Sheng Wang‡
a,
Xichong Ye‡a,
Masamichi Ogasawarad and
Tamaki Nakano *ae
*ae
aInstitute for Catalysis (ICAT), Graduate School of Chemical Sciences and Engineering, Hokkaido University, N 21, W 10, Kita-ku, Sapporo 001-0021, Japan. E-mail: tamaki.nakano@cat.hokudai.ac.jp; Fax: +81-11-7069156; Tel: +81-11-7069155
bDepartment of Applied Chemistry, Faculty of Engineering, Oita University, 700, Dannoharu, Oita, Oita 870-1124, Japan
cInstitute for Materials Chemistry and Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
dGraduate School of Science and Technology, Tokushima University, 2-1, Minamijosanjima-cho, Tokushima 770-8506, Japan
eIntegrated Research Consortium on Chemical Sciences (IRCCS), Institute for Catalysis, Hokkaido University, N21W10, Kita-ku, Sapporo 001-0021, Japan
First published on 6th June 2017
Abstract
Here we report the resolution of phosphorescent light-emitting iridium(III) bis[(4,6-difluorophenyl)pyridinato-N,C2]-picolinate into its respective enantiomers by using chiral HPLC and the photo-induced transformation of the isolated enantiomers.
Phosphorescent light-emitting diodes (LEDs) are drawing more attention than fluorescent LEDs1–6 because the fluorescent LEDs have shown maximum internal quantum yields of ca. 25 while the phosphorescent LEDs can in theory reach internal quantum yields of nearly 100%.1 Therefore, various iridium complexes have been studied; with the blue light-emitting iridium bis[(4,6-difluorophenyl)pyridinato-N,C2]picolinate (FIrpic) being a representative complex.2 FIrpic is a heteroleptic, octahedral complex, for which four geometric isomers are possible, and the complex from commercial sources is known to be the mer isomer with cis C–C and trans N–N geometry, for which Λ- and Δ-enantiomers exist (Fig. 1, left pair).
 | ||
| Fig. 1 Structures of FIrpic: Λ- and Δ-enantiomers of mer–cis-C–C–trans-N–N isomer (left pair) and those of the mer–cis isomer (right pair) optimized by DFT calculations. Detailed conditions of calculations are found in ESI.† | ||
Herein we present the isolation of the Λ and Δ-enantiomers of FIrpic by preparative HPLC and their photo-induced transformation to the enantiomers of the mer–cis isomer (Fig. 1, right pair). Several examples of enantiomers of octahedral Ir complexes have been reported.3–10 Recently, the isolation and characterization of chiroptical properties of FIrpic enantiomers were reported by Cannazza and co-workers10 while the separation of these enantiomers in the present work was conducted independently. The phototransformation of an enantiomerically pure FIrpic complex has not been previously accomplished, to the best of our knowledge; the ability to carry out this transformation is important for developing applications for circularly polarized light (CPL) emitter designs where excited-state stability of light-emitting species would play a crucial role. In addition to LED applications, Ir complexes have been used as valuable catalysts in excited states11 where phototransformation of an Ir complex would have significant influences on the catalyzed reactions.
Full details of the current experiments are given in the ESI.† Preparative chromatographic resolution experiments were conducted using a Daicel ChiralPak IC column (25 cm × 2 cm (i.d.)), and analytical resolution using analytical-scale ChiralPak IC, IA, and OD columns (25 cm × 0.46 cm (i.d.)). These columns use polysaccharide derivatives as chiral selectors and have been applied for the resolution of a wide variety of racemic compounds.12 When carrying out analytical-scale resolution on the IA column, a mixture of hexane, 2-propanol, and dichloromethane as eluent led to a clearly better separation than did a mixture of hexane and 2-propanol (Fig. S1 in ESI†), and slightly better resolution than did a mixture of hexane, 2-propanol, and chloroform (Fig. S2 in ESI†). In the analytical resolution on the analytical IC column using a mixture of hexane, 2-propanol, and dichloromethane, base-line separation was achieved where the ratio of the three components affected the resolution profiles (Fig. S3 in ESI†). On the basis of these observations, preparative resolution was performed using a 40/40/20 (v/v/v) mixture of hexane, 2-propanol, and dichloromethane as the eluent.
The Chiralcel OD column was also effective in resolving FIrpic into its enantiomers; the circular dichroism (CD) signs at 250 nm of the first- and second-eluting enantiomers were opposite those reported in ref. 10 under the same separation condition (Fig. S4 in ESI†).
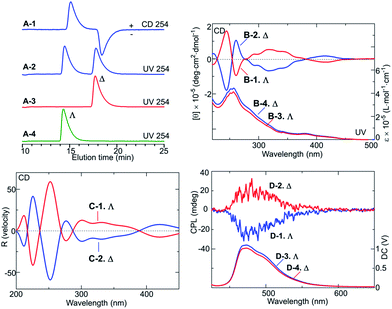
Fig. 2A(1–4) show the analytical high-performance liquid chromatography (HPLC) profiles obtained with the IA column, and CD, UV spectra, and CPL emission spectra13 of the isolated enantiomers, and theoretical CD spectra of Λ- and Δ-enantiomers obtained from density functional theory (DFT) calculations. The HPLC chromatograms (Fig. 2A) indicated that the enantiomers obtained by preparative HPLC resolution of FIrpic were almost enantiomerically pure; the separation factor in Fig. 1A-2 (α) was 1.29. The isolated enantiomers exhibited mirror-image CD spectra in methanol over the visible and UV ranges (Fig. 2B).
Furthermore, the isolated enantiomers showed clear, mirror-image CPL emission spectra13 in the range 450–550 nm on excitation at 286 nm (Fig. 2D) where maximum anisotropy (glum) values were 2.0 × 10−3 at 489 nm (Λ-enantiomer) and 2.1 × 10−3 at 490 nm (Δ-enantiomer); anisotropy in emission (glum) is defined as 2(IL − IR)/(IL + IR) where IL and IR are L- and R-CPL emission intensities, respectively. These results showed the isolated enantiomers to have enantiomeric structures both in the ground state and excited states. The clear CPL emission spectra of the FIrpic enantiomers indicated they are potentially applicable for CPL-emitting LEDs.
In addition, by comparing the experimental and theoretical CD spectra, the absolute configurations of the resolved enantiomers were determined. The theoretical spectra in Fig. 2C were obtained by carrying out TDDFT calculations with the B3LYP functional14,15 and the SDD basis set16 for Ir using the Gaussian 09 program package.17 The theoretical CD spectrum showed a rather sharp and intense negative Cotton band and a broad, weaker negative Cotton band in the range of ca. 300–380 nm, which roughly corresponded to the spectrum of the first enantiomer eluted in the chiral HPLC resolution of FIrpic (Fig. 2B-1). On the basis of these observations, the first-eluting enantiomer from the HPLC column was assigned to be the Λ-enantiomer and the second-eluting enantiomer was assigned to be the Δ-enantiomer. Thus, the Λ- and Δ-enantiomers of the mer–cis-C–C–trans-N–N isomer were successfully isolated and their absolute configurations were assigned.
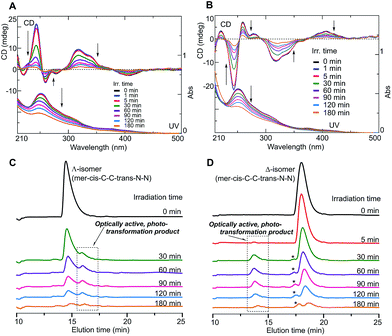
Furthermore, the isolated Δ- and Λ-enantiomers were found to undergo photo-isomerization and degradation. Since optically active Ir complexes may be used as CPL LED materials, the stability levels of such complexes in their excited states would be an important criterion in molecular design. In order to shed light on this aspect, the Δ- and Λ-enantiomers were independently irradiated in a MeOH solution (1.0 × 10−4 M) using non-polarized light (NPL) (ca. 200 mW cm−2) from an Hg–Xe lamp in a MeOH solution under an N2 atmosphere.
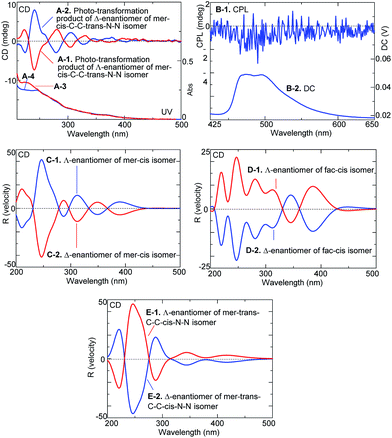
Fig. 3A and B show the changes in the CD and UV spectra of the Λ- and Δ-enantiomers, respectively, upon irradiation under an N2 atmosphere. CD spectral intensity remarkably decreased upon irradiation, and the shapes of the UV spectra also changed. The changes in UV spectra suggested that the CD spectral changes were not due to simple racemization but some other structural transformation. This assumption was supported by the HPLC profiles of the irradiated samples (Fig. 3C and D); upon irradiation, the intensity of the Λ- and Δ-enantiomer signals decreased and, at the same time, new signals emerged at retention times of 16.5 min and 13.5 min, respectively, for the systems with the Λ- and Δ-enantiomers: the new species isolated using HPLC exhibited clear mirror image CD spectra (Fig. 4A-1 and A-2).
Note the slightly different intensities of the CD spectra in Fig. 2B-1 and B-2; the Λ-isomer yielded a slightly lower intensity than did the Δ-isomer. A small amount of the Λ-isomer might have undergone the phototransformation before its CD spectrum was acquired.
In addition, a new species was obtained from the Δ-enantiomer as indicated from mass spectrometry results, which showed a main signal at a mass number (m/z) of 718, corresponding to an ion of FIrpic in the form of an [M + Na]+ sodium adduct, along with other weaker signals (Fig. S6 in ESI†). These results suggested that the Λ- and Δ-enantiomers of the mer–cis-C–C–trans-N–N isomer underwent, under the present experimental conditions, stereomutation to enantiomers of one of the three other geometric isomers of FIrpic, i.e., mer–cis, fac–cis, and mer–trans-C–C–cis-N–N, rather than simple racemization.
Moreover, the phototransformation product of the Δ-enantiomers of the mer–cis-C–C–trans-N–N isomer yielded CPL emission spectra with a maximum glum value appearing to be at about 2 × 10−3 (Fig. 4B-1 and B-2).
Since attempts at crystallizing the isolated, transformed enantiomers for X-ray analysis failed, their structures were identified by acquiring and comparing their experimental and theoretical CD spectra (Fig. 4C-1 and E-2). The theoretical CD spectra of the enantiomers of the three geometric isomers differed from one another. The shapes of the experimental spectra in Fig. 4A-1 and A-2 appeared most similar to those of the theoretical spectra of the enantiomers of the mer–cis isomer in Fig. 4C-1 and C-2: both the theoretical spectrum of the Λ-enantiomer (in Fig. 4C-1) and the experimental spectrum (in Fig. 4A-2) showed three positive and three negative Cotton bands in the range 200–400 nm and such a feature was not seen for the enantiomers of fac–cis isomer and mer–trans-C–C–cis-N–N isomer (Fig. 4D-1 and E-1). We hence propose that the Δ-enantiomer of the mer–cis-C–C–trans-N–N isomer mutated into the Λ-enantiomer of the mer–cis isomer.
Note that the intensities of all of the signals in the HPLC profiles in Fig. 3C and D decreased as the irradiation time was increased, and, after 180 min of irradiation, the overall intensities became much lower than those in the initial state (Fig. S7 in ESI†). The decreases in the CD intensities in Fig. 3A and B were thus not due only to the generation of the optically active transformed products but also to disappearance or decomposition of the enantiomers and their corresponding transformed derivatives.
In addition, changes in the CD/UV spectra and in the HPLC profiles of the Δ-enantiomer occurred more rapidly upon irradiation using NPL with a relatively high irradiation power (of ca. 200 mW cm−2) than when using LPL (ca. 40 mW cm−2) under an N2 atmosphere. Moreover, upon irradiation of the Δ-enantiomer using NPL, the changes in its CD and HPLC results were faster in the presence of air than under N2 (Fig. S7 in ESI†). These results suggested that the stereomutation and decomposition of the Λ- and Δ-enantiomers occurred in excited states, and were accelerated by O2 contained in air, possibly through interactions between unpaired electrons of O2 with electrons of FIrpic in its higher-energy orbitals. The photoreactions of FIrpic are assumed to have occurred through its triplet excited states. A photo-induced transformation of the Ir complex has been reported for iridium(III) tris(2-phenylpyridinato-N,C2′) (Ir(ppy)3)18 but has never been reported for FIrpic. The current work is the first to report on the photo-isomerization of enantiomers of FIrpic.
In conclusion, commercial FIrpic, i.e., the mer-isomer of FIrpic with cis C–C and trans N–N arrangements, was resolved into optically pure Λ- and Δ-enantiomers by using chiral HPLC, and upon photo-irradiation, the enantiomers underwent stereomutation and decomposition: we propose that the Λ- and Δ-enantiomers of the mer–cis-C–C–trans-N–N isomer mutated into the Δ- and Λ-enantiomers of the mer–cis geometrical isomer, respectively. The mutation and decomposition occurred not only in the presence of air but also under an N2 atmosphere. These results revealed an important cautionary note, that optically active FIrpic may be transformed in excited states even in a sealed device, which would need to be taken into consideration when designing LEDs using optically active FIrpic enantiomers. In addition, transformation of FIrpic may also play a role in catalyses using Ir complexes.11
Acknowledgements
This work was supported in part by the Mitsubishi Foundation and in part by MEXT program of Integrated Research Consortium on Chemical Synthesis (IRCCS).Notes and references
- (a) M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS; (b) D. F. O'Brien, M. A. Baldo, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 74, 442–444 CrossRef; (c) M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS; (d) M. Segal, M. A. Baldo, R. J. Holmes, S. R. Forrest and Z. G. Soos, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 075211 CrossRef; (e) M. A. Baldo, D. F. O'Brien, M. E. Thompson and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 14422 CrossRef CAS.
- E. Baranoff and B. F. Curchod, Dalton Trans., 2015, 44, 8318–8329 RSC.
- X. Chen, Y. Okamoto, T. Yano and J. Otsuki, J. Sep. Sci., 2007, 30, 713–716 CrossRef CAS PubMed.
- F. J. Coughlin, M. S. Westrol, K. D. Oyler, N. Byrne, C. Kraml, E. Zysman-Colman, M. S. Lowry and S. Bernhard, Inorg. Chem., 2008, 47, 2039–2048 CrossRef CAS PubMed.
- M. Ashizawa, L. Yang, K. Kobayashi, H. Sato, A. Yamagishi, F. Okuda, T. Harada, R. Kuroda and M. A. Haga, Dalton Trans., 2009, 1700–1702 RSC.
- K. Tsuchiya, E. Ito, S. Yagai, A. Kitamura and T. Karatsu, Eur. J. Inorg. Chem., 2009, 2104–2109 CrossRef CAS.
- H. Sato, K. Tamura, M. Taniguchi and A. Yamagishi, New J. Chem., 2010, 34, 617–622 RSC.
- T.-Y. Li, Y.-M. Jing, X. Liu, Y. Zhao, L. Shi, Z. Tang, Y.-X. Zheng and J.-L. Zuo, Sci. Rep., 2015, 14912, 1–9 Search PubMed.
- G. Mazzeo, M. Fusè, G. Longhi, I. Rimoldi, E. Cesarotti, A. Crispini and S. Abbate, Dalton Trans., 2016, 45, 992–999 RSC.
- C. Citti, U. M. Battisti, G. Ciccarella, V. Maiorano, G. Gigli, S. Abbate, G. Mazzeo, E. Castiglioni, G. Longhi and G. Cannazza, J. Chromatogr. A, 2016, 1467, 335–346 CrossRef CAS PubMed.
- (a) A. Millet, Q. Lefebvre and M. Rueping, Chem.–Eur. J., 2016, 22, 13464–13468 CrossRef CAS PubMed; (b) J. D. Nguyen, E. M. D'Amato, J. M. Narayanam and C. R. Stephenson, Nat. Chem., 2012, 4, 854–859 CrossRef CAS PubMed; (c) N. J. Treat, B. P. Fors, J. W. Kramer, M. Christianson, C.-Y. Chiu, J. Read de Alaniz and C. J. Hawker, ACS Macro Lett., 2014, 3, 580–584 CrossRef CAS; (d) L. Zhang and E. Meggers, Acc. Chem. Res., 2017, 50, 320–330 CrossRef CAS PubMed; (e) A. Gualandi, E. Matteucci, F. Monti, A. Baschieri, N. Armaroli, L. Sambri and P. G. Cozzi, Chem. Sci., 2017, 8, 1613–1620 RSC.
- (a) J. Shen and Y. Okamoto, Chem. Rev., 2015, 116, 1094–1138 CrossRef PubMed; (b) Y. Okamoto, M. Kawashima, K. Yamamoto and K. Hatada, Chem. Lett., 1984, 13, 739–742 CrossRef; (c) Y. Okamoto and E. Yashima, Angew. Chem., Int. Ed., 1998, 37, 1020–1043 CrossRef; (d) E. Yashima, J. Chromatogr. A, 2001, 906, 105–125 CrossRef CAS PubMed.
- T. Harada, H. Hayakawa, M. Watanabe and M. Takamoto, Rev. Sci. Instrum., 2016, 87, 075102 CrossRef PubMed.
- (a) A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 37, 785–789 CrossRef.
- D. Andrae, U. Haeussermann, M. Dolg, H. Stoll and H. Preuss, Theor. Chem. Acc., 1990, 77, 123–141 CrossRef CAS.
- Gaussian 09, Revision E01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- K. Tsuchiya, E. Ito, S. Yagai, A. Kitamura and T. Karatsu, Eur. J. Inorg. Chem., 2009, 2104–2019 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and selected chromatograms and spectra. See DOI: 10.1039/c7ra04141a |
| ‡ On leave from College of Chemistry & Molecular Engineering, Peking University as visiting students. |
| This journal is © The Royal Society of Chemistry 2017 |