Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of Ag3VO4/β-AgVO3 nanowires with efficient visible-light photocatalytic activity
Lei Gaoab,
Zhonghua Li *b and
Jiawen Liu*c
*b and
Jiawen Liu*c
aSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, P. R. China
bKey Laboratory of Microsystems and Microstructures Manufacturing, Ministry of Education, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: lizh@hit.edu.cn
cKey Laboratory for Photochemical Biomaterials and Energy Storage Materials, College of Chemistry and Chemical Engineering, Harbin Normal University, Harbin 150025, Heilongjiang Province, P. R. China. E-mail: jiawen86@163.com
First published on 23rd May 2017
Abstract
Ag3VO4/β-AgVO3 nanocomposites were successfully fabricated by chemical precipitation and hydrothermal method. The composites displayed excellent photocatalytic activity in comparision with those of pure β-AgVO3 and Ag3VO4, which may be primarily ascribed to the matched energy structures. The sample with a molar ratio of 30% Ag3VO4 to β-AgVO3 showed the highest photocatalytic activity for RhB degradation, which was almost 9 and 2.4 times higher than those of pure β-AgVO3 and Ag3VO4, respectively. In addition, the trapping experiments also confirmed that holes and hydroxyl radicals are the active species for RhB degradation, and the possible mechanism for enhanced photocatalytic activity was proposed.
1 Introduction
Heterogenerous photocatalysis has gained widespread attention from researchers because it is one of the most green and effective methods to solve the energy shortage and environmental problems.1–3 Over the past several decades, more and more photocatalysts have been developed, including TiO2, ZnO, Ta2O5 and CuO.4–10 However, most of them had a high rate of charge carrier recombination and no response in the visible light range. It is very important to effectively utilize solar energy to overcome the above two limitations. Heterostructured photocatalysts,11–16 an effective media, have been developed to avoid the recombination of photoinduced electron–hole and increase the charge carrier separation efficiency because of the interface electric field. Hence, many different composites have been prepared and reported, such as Ag/AgX,17 Ag/AgX/GO,18 RGO/Bi3.64M0.36O6.55,19,20 AgX/Ag3PO4,21 InVO4/BiVO4 (ref. 22–26) and BiPO4/Ag3PO4.27The monoclinic scheelite Ag3VO4 with a band energy of 2.3 eV has received much attention due to its good photocatalytic activity under visible light irradiation. Although the pure Ag3VO4 exhibits high photocatalytic performance, its photocatalytic activity is limited because of its low efficiency in separating photogenerated electrons and holes. Hence, many Ag3VO4-based heterostructure photocatalysts such as Ag3VO4/TiO2,28 Ag2O/Ag3VO4/Ag4V2O7,29 Ag2O/Ag3VO4/AgVO3,30 Ag3VO4/Ag3PO4 (ref. 31) and Ag3VO4/g-C3N4,32 have been developed to enhance the separation of photoinduced charge carriers.
AgVO3 with excellent optical absorption in the visible light region has also attracted considerable attention due to its narrow band gap, high stability and well crystallization.33–35 However, compared with other Ag-based materials such as Ag3PO4,36 AgX,37 and Ag2CO3,38 there are only few studies concentrated on degradation of pollutants about AgVO3, which may be attributed to low quantum yield and the poor absorption efficiency in visible light. Therefore, a suitable material coupled with AgVO3 is of great importance to solve its limited application in photocatalysis.
In this study, we reported novel Ag3VO4/β-AgVO3 composites through a chemical precipitation approach. We chose one-dimensional β-AgVO3 nanowires as the substrate materials because they have lots of advantages such as visible light responding and supporting materials. A facile chemical precipitation can efficiently suppress the aggregation of Ag3VO4 and boost the contact between Ag3VO4 and β-AgVO3. The Ag3VO4/β-AgVO3 hybrid materials were used for the photodegradation of RhB under visible light and exhibited much higher photocatalytic activity than single Ag3VO4 and β-AgVO3. Moreover, a possible photocatalytic mechanism and the stability of the Ag3VO4/β-AgVO3 heterojunction were also investigated.
2 Experimental
2.1 Preparation of β-AgVO3 nanowires
All reagents for synthesis and analysis were analytical grade and used without further purification. β-AgVO3 nanowires were synthesized by the previously reported hydrothermal method.39 1 mmol NH4VO3 (Tianjin bo di reagent co., LTD, Certified China) was dissolved in 60 mL deionized water (Harbin zhong jia chemical reagent co., LTD, Certified China) with magnetic stirring for 5 min to obtain a transparent solution under room temperature. Then, 1 mmol AgNO3 (Shanghai shi yi chemical reagent co., LTD, Certified China) was slowly added into the above solution and stirred for another 3 min. The pH value of the solution was adjusted to 8–8.2 by using NH3·H2O (25–28%) and then the mixture was homogeneously transferred into five 20 mL Teflon-lined stainless vessel and heated at 180 °C for 12 h. The β-AgVO3 nanowires were collected by centrifugation, washed with deionized water three times, dried at 60 °C for 10 h.2.2 Synthesis of Ag3VO4/β-AgVO3 composites
Ag3VO4/β-AgVO3 composites were prepared by a chemical precipitation method. 0.1034 g as-prepared β-AgVO3 nanowires and 0.1010 g AgNO3 (Shanghai shi yi chemical reagent co., LTD, Certified China) dispersed in 40 mL deionized water with magnetic stirring for 5 min. Subsequently, 20 mL of a certain amount of Na3VO4·12H2O (Beijing chemical reagent co., LTD, Certified China) aqueous solution was dropped into the above solution and stirred for 3 h. The obtained yellow precipitate was washed with distilled water three times and dried in oven at 60 °C for 6 h. Ag3VO4/β-AgVO3 composites with different molar ratios of Ag3VO4 were obtained: 5%, 10%, 20%, 30%, 40% and the samples prepared were denoted as 5% Ag3VO4/β-AgVO3, 10% Ag3VO4/β-AgVO3, 20% Ag3VO4/β-AgVO3, 30% Ag3VO4/β-AgVO3 and 40% Ag3VO4/β-AgVO3, respectively.2.3 Characterization of Ag3VO4/β-AgVO3 composites
The crystalline structures of the samples were recorded on a Rigaku D/MAX-rA XRD (Japan) with Cu Kα radiation (λ = 0.15405 nm) in the range of 20–70° (2θ). The images of the micro-morphology were determined by scanning electron microscopy (SEM, Hitachi, SU8010) and Transmission electron microscope (TEM, Tecnai, G2F30). The surface analysis was studied by X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, ESCALAB250Xi) with a Mg Kα source. The binding energies were calibrated with the C 1s peak of surface adventitious carbon at 284.8 eV. The Ultraviolet-visible Diffuse Reflectance Spectra (UV-vis DRS) were obtained in the range of 255–800 nm using a UV-vis spectrophotometer (UV-2550, Shimadzu, Japan). BaSO4 was used as the reflectance standard material. The BET surface area was measured by N2 adsorption using a surface analysis instrument (Beishide, 3H-2000PSI, China). The photoluminescence spectra (PL) of the catalysts were recorded using a FluoroMax-4 photoluminescence instrument (HORIBA JobinYvon) with a 350 nm excitation wavelength.2.4 Evaluation of photocatalytic performance
The photocatalytic properties of the Ag3VO4/β-AgVO3 composite samples were evaluated by the degradation of rhodamine B (RhB) dye under visible light irradiation. A 300 W Xe lump was employed as visible light (λ > 400 nm) source and NaNO2 solution was poured between the lump and the photocatalyst to filter out UV light (λ < 400 nm). The vertical distance between the light source and the surface of the solution was about 12 cm. 0.0500 g photocatalyst was dispersed into the 70 mL of RhB (10 mg L−1) in a quartz reactor. Prior to the light illumination, the suspension was stirred in the dark for 30 min to achieve the adsorption–desorption equilibrium on the surface of photocatalyst. At defined time (10 min), about 3 mL of the reaction solution was withdrawn and centrifuged (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 1 min) to remove the photocatalyst particles. The residual amount of RhB in the solution was recorded by measuring its characteristic optical absorption at 554 nm using UV-vis spectrophotometer.
000 rpm, 1 min) to remove the photocatalyst particles. The residual amount of RhB in the solution was recorded by measuring its characteristic optical absorption at 554 nm using UV-vis spectrophotometer.
3 Results and discussion
The typical XRD patterns for the pure β-AgVO3, Ag3VO4 and Ag3VO4/β-AgVO3 composites are shown in Fig. 1. The pure β-AgVO3 patterns match well with the JCPDS (29-1154) standard data, which suggests that the prepared β-AgVO3 has a monoclinic structure.40 The diffraction peaks of Ag3VO4 can be indexed as the JCPDS (43-0542) standard data.41 It was clearly found that all the Ag3VO4/β-AgVO3 composites exhibit a cooccurrence of both β-AgVO3 and Ag3VO4 phases. Furthermore, with the Ag3VO4 amounts increasing, the diffraction peak intensity of Ag3VO4 becomes stronger gradually. Meanwhile, the diffraction peak positions of β-AgVO3 do not shift, indicating that the introduction of Ag3VO4 does not influence the crystal structure of β-AgVO3.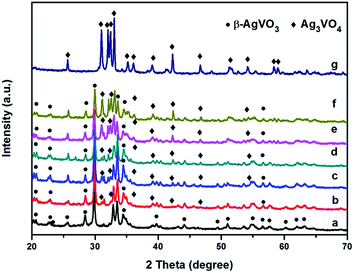 | ||
| Fig. 1 XRD patterns of β-AgVO3 nanoribbons (a), 5% Ag3VO4/β-AgVO3 (b), 10% Ag3VO4/β-AgVO3 (c), 20% Ag3VO4/β-AgVO3 (d), 30% Ag3VO4/β-AgVO3 (e), 40% Ag3VO4/β-AgVO3 (f) and Ag3VO4 (g). | ||
Fig. 2(a) shows the UV-vis diffuse reflectance spectra of pure β-AgVO3, Ag3VO4 and Ag3VO4/β-AgVO3 composite photocatalysts in order to investigate the optical absorption properties. As depicted from Fig. 2(a), β-AgVO3 and Ag3VO4 display excellent optical absorption in visible light. Their absorption edges were approximately 640 nm and 615 nm respectively. From the formula: αhν = A(hν − Eg)n/2, where α is the absorption coefficient, hν is the energy, A is a constant, and Eg is the band gap. The value of n depends on whether the transition is direct (n = 1) or indirect (n = 4) discrete photon in a semiconductor. As shown in Fig. 2(b), we can get the band gap Eg of pure β-AgVO3 and Ag3VO4 to be 2.20 eV and 2.37 eV, respectively. Moreover, the potential of conduction band of Ag3VO4 and β-AgVO3 could be calculated by the following Mulliken electronegativity theory: ECB = χ − Ee − 0.5Eg, where χ is the absolute electronegativity of the semiconductor, Ee is the energy of free electrons (4.5 eV), and Eg is the band gap energy of semiconductor. The χ values of Ag3VO4 and β-AgVO3 are 5.64 and 5.88, respectively. So the CB values of Ag3VO4 and β-AgVO3 were calculated to be −0.045 and 0.28 eV, respectively. Based on the equation EVB = ECB + Eg, the corresponding EVB values were also predicted to be 2.325 and 2.48 eV, respectively. The results indicate that the composite could successfully fabricate the matched energy structures between Ag3VO4 and β-AgVO3.
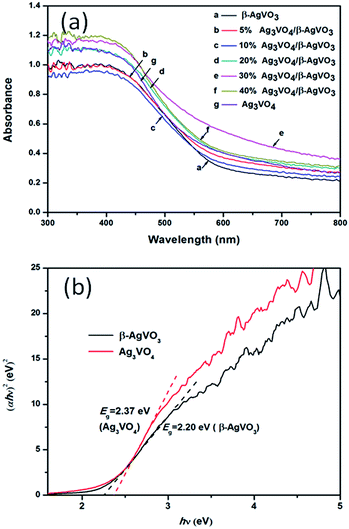 | ||
| Fig. 2 (a) UV-vis diffuse reflectance spectra of β-AgVO3, Ag3VO4 and Ag3VO4/β-AgVO3 composites. (b) Plots of (αhν)2 versus hν of pure β-AgVO3, Ag3VO4 and Ag3VO4/β-AgVO3 composites. | ||
The microstructures of pure β-AgVO3, Ag3VO4 and Ag3VO4/β-AgVO3 composite photocatalysts were measured by SEM. As shown in Fig. 3(a), pure β-AgVO3 displays a number of nanowires with about 100 nm in width and more than 20 μm in length and its surface is very smooth. As depicted in Fig. 3(b)–(f), the composites of Ag3VO4/β-AgVO3 do not influence the shape of β-AgVO3 and the size of Ag3VO4 particles becomes larger with the Ag3VO4 content increasing, which is in agreement with the enhanced intensity of Ag3VO4 XRD patterns.
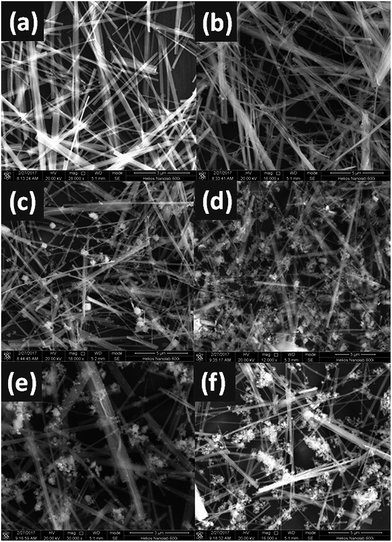 | ||
| Fig. 3 SEM images of β-AgVO3 nanoribbons (a), 5% Ag3VO4/β-AgVO3 (b), 10% Ag3VO4/β-AgVO3 (c), 20% Ag3VO4/β-AgVO3 (d), 30% Ag3VO4/β-AgVO3 (e) and 40% Ag3VO4/β-AgVO3 (f). | ||
The morphology of 30% Ag3VO4/β-AgVO3 composite is further illustrated by TEM and HRTEM. Fig. 4(a)–(c) show that some Ag3VO4 nanoparticles appeared on the smooth surface of β-AgVO3 nanowires.42 HRTEM in Fig. 4(d) is the location of the circle in Fig. 4(c). By measuring the lattice fringes, the interplanar spacing is 0.317 nm and 0.243 nm, which are corresponding to the (−301) plane of β-AgVO3 and the (202) plane of Ag3VO4.
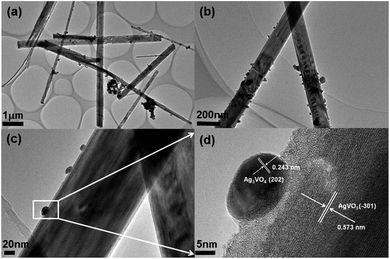 | ||
| Fig. 4 (a)–(c) TEM images of 30% Ag3VO4/β-AgVO3 composite and (d) HRTEM images of the designated square area in panel of (c). | ||
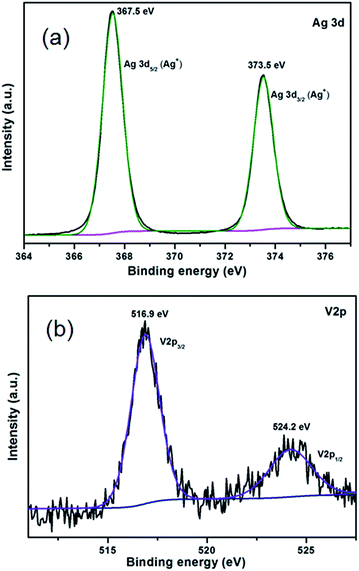
XPS analysis was carried out to further clarify the surface chemical composition and bonding environment of 30% Ag3VO4/β-AgVO3 composite and the results are displayed in Fig. 5. The binding energy at 248.8 eV of C 1s is calibrated. The Fig. 5(a) and (b) demonstrates the predominant presence of silver and vanadium. The two peaks at 367.5 and 373.5 eV can be corresponding to the Ag 3d5/2 and Ag 3d3/2 of Ag+ both in Ag3VO4 and β-AgVO3.41,43 The V 2p peaks of 30% Ag3VO4/β-AgVO3 at 516.9 and 524.2 eV can be assigned to V 2p5/2 and V 2p3/2 of V5+ both in Ag3VO4 and β-AgVO3.41,43
The photocatalytic performance of pure β-AgVO3, Ag3VO4 and Ag3VO4/AgVO3 composites was evaluated by photocatalytic degradation of RhB under visible light illumination. Fig. 6 displays the experimental results. It can be seen that after 60 min of visible light irradiation the degradation efficiency of pure β-AgVO3 and Ag3VO4 were 23% and 57% respectively. Obviously, all the composites have more effective photocatalytic performance than the pure samples, which can be attributed to the presence of Ag3VO4 on the surface of β-AgVO3, accelerating the separation of photoinduced electrons and holes. The best photocatalytic performance comes from 30% Ag3VO4/β-AgVO3 composite, which is 9 times and 2.4 times than pure β-AgVO3 and Ag3VO4. However, with the content of Ag3VO4 enhanced to 40%, the decomposition efficiency of RhB declines, suggesting that the excessive Ag3VO4 particles deposited on the surface of β-AgVO3 might intervene the photocatalytic activity sites.
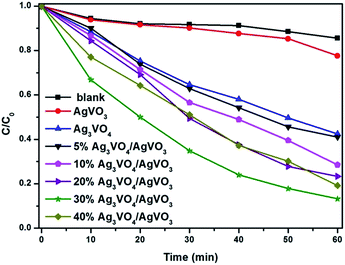 | ||
| Fig. 6 Photocatalytic degradation of RhB over Ag3VO4/β-AgVO3 nanowire composites under visible light irradiation. | ||
Fig. 7 exhibits the obvious changes of RhB solution in UV-visible absorption spectra of 30% Ag3VO4/β-AgVO3 composite at different times. It can be clearly observed that the absorbance maximum peak of RhB shifts from 554 nm to 504 nm, which is in good accordance with the previous reports of the degradation of RhB over TiO2 (ref. 44) and Ag2O/Ag3VO4/Ag4V2O7.29 The color of RhB solution changes from pink to almost colourless after 60 min illumination, which indicates the cleavage of conjugated chromophore structure of RhB.
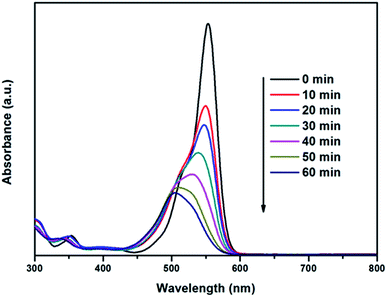 | ||
| Fig. 7 Changes in UV-visible absorption spectra of 30% Ag3VO4/β-AgVO3 photocatalyst at different times under visible light irradiation. | ||
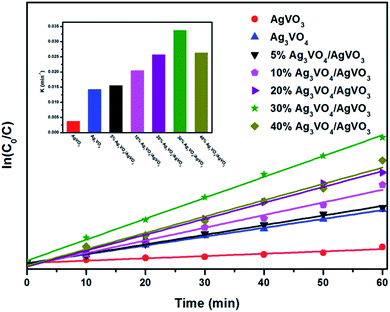
As depicted from Fig. 8, the experimental results were fitted to pseudo-first-order and all Ag3VO4/β-AgVO3 composites exhibit higher photocatalytic performance than single Ag3VO4 and β-AgVO3 nanowires. The apparent rate constant k was shown in inset. It can be seen that the composite with mole ratios of 30% Ag3VO4/β-AgVO3 shows the highest value of apparent rate constant k, which was 9 and 2.4 times higher than pure β-AgVO3 and Ag3VO4. This indicates that the composite with 30% molar ratio is the most suitable for β-AgVO3 and Ag3VO4, which could boost the separation of charge carriers and enhance the photocatalytic performance.
Furthermore, as is shown in Fig. 9, 30% Ag3VO4/β-AgVO3 composite has weak adsorption and desorption. The surface areas of β-AgVO3 and 30% Ag3VO4/β-AgVO3 composite were 3.096 and 4.610 m2 g−1, respectively. It was worthwhile to note that the surface area of 30% Ag3VO4/β-AgVO3 composite becomes larger with the Ag3VO4 nanoparticles deposited on the β-AgVO3 nanoribbons, which can provide more active sites and enhance photocatalytic activity.
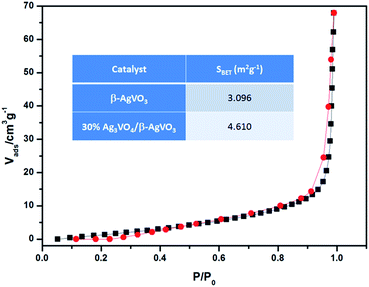 | ||
| Fig. 9 N2 adsorption–desorption isotherm of 30% Ag3VO4/β-AgVO3 photocatalyst at 393.15 K and the surface areas inset of β-AgVO3 and 30% Ag3VO4/β-AgVO3 composite. | ||
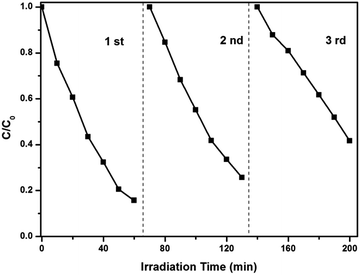
The stability of photocatalysts is an important factor for the further application. As is shown in Fig. 10, the photocatalytic activity of the 30% Ag3VO4/β-AgVO3 composite still reached 67%, even though it had been used 3 times.
Additionally, to further reveal the photocatalytic mechanism, the trapping experiments were performed, as shown in Fig. 11. Under visible illumination the photodegradation of RhB is slightly inhibited by adding the IPA (hydroxyl radical scavenger) and ammonium oxalate (hole scavenger), which indicates that hydroxyl radical and hole are the main species that can degenerate RhB.
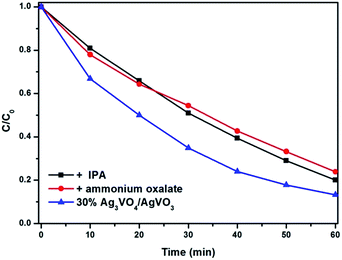 | ||
| Fig. 11 The plots of photogenerated carrier trapping in the system of photodegradation of RhB under visible light irradiation. | ||
In order to investigate the process of charge carriers trapping, migration and separation efficiency, the PL spectra of β-AgVO3, Ag3VO4, and 30% Ag3VO4/β-AgVO3 samples under the excitation wavelength of 350 nm, as shown in Fig. 12. It was generally acknowledged that the lower PL intensity indicates that the higher separation capacity of charge carriers, which results in higher photocatalytic activity. It was observed that the main emission peak of β-AgVO3, Ag3VO4, and Ag3VO4/β-AgVO3 composites all center at about 595 nm. Compared with pure β-AgVO3 and Ag3VO4 samples, the lower PL peak intensity of 30% Ag3VO4/β-AgVO3 composites implies that the interface between Ag3VO4 and β-AgVO3 can migrates the photogenerate electrons and holes more effectively.
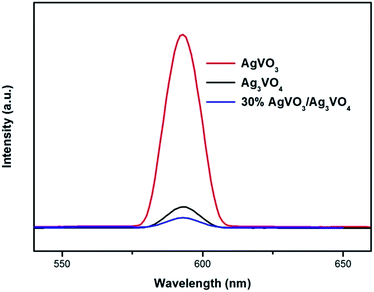 | ||
| Fig. 12 Photoluminescence (PL) spectra of β-AgVO3, Ag3VO4, and 30% Ag3VO4/β-AgVO3 samples under the excitation wavelength of 350 nm. | ||
In fact, the pathway of RhB degradation over Ag3VO4/β-AgVO3 composite under visible light is rather complex. Based on the above experimental results and photocatalytic degradation reports, the possible reactions are summarized by eqn (1)–(4).
| Ag3VO4 + hν → Ag3VO4 (eCB− + hVB+) | (1a) |
| β-AgVO3 + hν → β-AgVO3 (eCB− + hVB+) | (1b) |
| Ag+ (in β-AgVO3) + eCB− → Ag45 | (2a) |
| Ag + hν → Ag (SPR) | (2b) |
| 4hVB++ 2H2O → 4H+ + O2 | (3a) |
| 4hVB++ 2OH− → 2H+ + O2 | (3b) |
| hVB++ H2O → ˙OH + H+30 | (3c) |
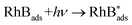 | (4a) |
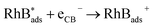 | (4b) |
| RhBads+ + ˙OH → N-ethyl rhodamine → CO2 + H2O46 | (4c) |
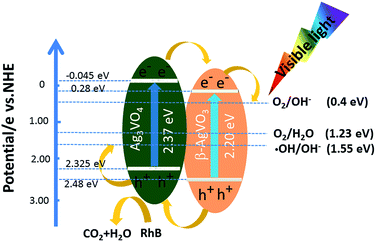
In accordance with eqn (1)–(4) and the Scheme 1, the possible mechanism of migration of carriers in the Ag3VO4/β-AgVO3 composite heterostructures was discussed. Both Ag3VO4 and β-AgVO3 can be excited under visible light illumination according to the UV-vis diffuse reflectance spectra (eqn (1a) and (1b)). The electrons on the conduction band of Ag3VO4 can transfer into the conduction band of β-AgVO3 and the holes on the valence band of Ag3VO4 can transfer into the valence band of β-AgVO3, because the conduction band of Ag3VO4 is negative than β-AgVO3 and the valence band of Ag3VO4 is positive than β-AgVO3.47–50 So, photoinduced electrons and holes can separate effectively, which is crucial for enhancing photocatalytic activities.
After the photoreaction on the Ag3VO4/β-AgVO3 composite photocatalyst, the photogenerated electrons of VB transferred to the CB of β-AgVO3 could reduce the Ag+ to Ag0 nanoparticles45 (eqn (2a) and (2b)). However, Ag0 nanoparticles with SPR in the visible light region could disrupt the ordered transfer of electrons and holes of Ag3VO4/β-AgVO3 composite, which results in the gradual declining of photocatalytic activity over Ag3VO4/β-AgVO3 hybrid. This is in agreement with decreased photocatalytic performance after three cycling runs.
In addition, the VB potential of Ag3VO4 (2.325 eV vs. NHE) and β-AgVO3 (2.48 eV vs. NHE) were more positive than the standard reduction potential of O2/OH− (0.4 eV vs. NHE) (3a), O2/H2O (1.23 eV vs. NHE) (3b) and ˙OH/OH− (1.55 eV vs. NHE) (3c). Therefore, holes on the VB of both Ag3VO4 and β-AgVO3 could react with H2O and OH− to form ˙OH, H+ and O2, according to eqn (3a)–(3c). Thus, holes and ˙OH radicals play important roles in the photocatalytic degradation of RhB, which is in good agreement with the experimental result of trapping experiment. Furthermore, the redox potential of RhB (−1.09 eV vs. NHE) is more negative than the CB potential of Ag3VO4 (−0.045 eV vs. NHE) resulting in the self-sensitized degradation of RhB according to eqn (4a)–(4c).
4 Conclusions
In summary, we have successfully synthesized Ag3VO4/β-AgVO3 heterojunctions via chemical precipitation and hydrothermal method. They show much better photocatalytic activity than pure Ag3VO4 and β-AgVO3 toward RhB degradation under visible light and the 30% Ag3VO4/β-AgVO3 composite exhibits the highest. It is found that the photocatalytic activity increased with the Ag3VO4 amount increasing, which can be ascribed to the matched energy structure in the Ag3VO4/β-AgVO3 heterojunction that can efficiently enhanced separation of photoinduced carriers. The holes and hydroxyl radicals play significant roles in the photocatalytic degradation of RhB under visible light.Acknowledgements
The authors would like to thank the partial financial support from the National Natural Science Foundation of China (No. 51272052 and No. 50902040).Notes and references
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- M. Zhang, C. C. Chen, W. H. Ma and J. C. Zhao, Angew. Chem., Int. Ed., 2008, 47, 9730–9733 CrossRef CAS PubMed.
- P. Zhou, J. G. Yu and M. Jaroniec, Adv. Mater., 2014, 26, 4920–4935 CrossRef CAS PubMed.
- R. A. Lucky and P. A. Charpentier, Adv. Mater., 2008, 20, 1755–1759 CrossRef CAS.
- L. Ge, M. X. Xu and H. B. Fang, Mater. Lett., 2007, 61, 63–66 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- J. G. Yu and X. X. Yu, Environ. Sci. Technol., 2008, 42, 4902–4907 CrossRef CAS PubMed.
- Y. Takahara, J. N. Kondo, T. Takata, D. Lu and K. Domen, Chem. Mater., 2001, 13, 1194–1199 CrossRef CAS.
- W. J. Chun, A. Ishikawa, H. Fujisawa, T. Takata, J. N. Kondo, M. Hara, M. Kawai, Y. Matsumoto and K. Domen, J. Phys. Chem. B, 2003, 107, 1798–1803 CrossRef CAS.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406–8407 CrossRef CAS PubMed.
- L. Q. Ye, J. Y. Liu, C. Q. Gong, L. H. Tian, T. Y. Peng and L. Zan, ACS Catal., 2012, 2, 1677–1683 CrossRef CAS.
- J. X. Wang, H. Ruan, W. J. Li, D. Z. Li, Y. Hu, J. Chen, Y. Shao and Y. Zheng, J. Phys. Chem. C, 2012, 116, 13935–13943 CAS.
- C. L. Yu, G. Li, S. Kumar, K. Yang and R. C. Jin, Adv. Mater., 2014, 26, 892–898 CrossRef CAS PubMed.
- X. Yu, Z. H. Li, J. W. Liu and P. G. Hu, Appl. Catal., B, 2017, 205, 271–280 CrossRef CAS.
- Q. Yuan, L. Chen, M. Xiong, J. He, S. L. Luo, C. T. Au and S. F. Yin, Chem.–Eur. J., 2014, 255, 394–402 CAS.
- Q. X. Xiang, J. G. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai, J. Y. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931–7933 CrossRef CAS PubMed.
- M. S. Zhu, P. L. Chen and M. H. Liu, ACS Nano, 2011, 6, 4529–4536 CrossRef PubMed.
- X. Lin, S. Y. Yu, Z. Y. Gao, X. X. Zhang and G. B. Che, J. Mol. Catal. A: Chem., 2016, 411, 40–47 CrossRef CAS.
- X. Lin, B. Wei, X. X. Zhang, M. S. Song, S. Y. Yu, Z. Y. Gao, H. J. Zhai, L. N. Zhao and G. B. Che, Sep. Purif. Technol., 2016, 169, 9–16 CrossRef CAS.
- Y. P. Bi, S. X. Ouyang, J. Y. Cao and J. H. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071–10075 RSC.
- F. Guo, W. L. Shi, X. Lin, X. Yan, Y. Guo and G. B. Che, Sep. Purif. Technol., 2015, 141, 246–255 CrossRef CAS.
- X. Lin, X. Y. Guo, W. L. Shi, L. N. Zhao, Y. S. Yan and Q. W. Wang, J. Alloys Compd., 2015, 635, 256–264 CrossRef CAS.
- X. Lin, D. Xu, J. Zheng, M. S. Song, G. B. Che, Y. S. Wang, Y. Yang, C. Liu, L. N. Zhao and L. M. Chang, J. Alloys Compd., 2016, 688, 891–898 CrossRef CAS.
- X. Lin, Y. S. Wang, J. Zheng, C. Liu, Y. Yang and G. B. Che, Dalton Trans., 2015, 44, 19185–19193 RSC.
- X. Lin, D. Xu, Y. Xi, R. Zhao, L. N. Zhao, M. S. Song, H. J. Zhai, G. B. Che and L. M. Chang, Colloids Surf., A, 2017, 513, 117–124 CrossRef CAS.
- H. L. Lin, H. F. Ye, B. Y. Xu, J. Cao and S. F. Chen, Catal. Commun., 2013, 37, 55–59 CrossRef CAS.
- J. X. Wang, H. Ruan, W. J. Li, D. Z. Li, Y. Hu, J. Chen, Y. Shao and Y. Zheng, J. Phys. Chem. C, 2012, 116, 13935–13943 CAS.
- R. Ran, J. G. McEvoy and Z. S. Zhang, Mater. Res. Bull., 2016, 74, 140–150 CrossRef CAS.
- R. Ran, X. C. Meng and Z. S. Zhang, Appl. Catal., B, 2016, 196, 1–15 CrossRef CAS.
- E. Akbarzadeh, S. R. Setayesh and M. R. Gholami, RSC Adv., 2016, 6, 14909–14915 RSC.
- T. T. Zhu, Y. H. Song, H. Y. Ji, Y. G. Xu, Y. X. Song, J. X. Xia, S. Yin, Y. P. Li, H. Xu, Q. Zhang and H. M. Li, Chem.–Eur. J., 2015, 271, 96–105 CAS.
- X. Lin, D. Xu, S. S. Jiang, F. Xie, M. S. Song, H. J. Zhai, L. N. Zhao, G. B. Che and L. M. Chang, Catal. Commun., 2017, 89, 96–99 CrossRef CAS.
- J. M. Song, Y. Z. Lin, H. B. Yao, F. J. Fan, X. G. Li and S. H. Yu, ACS Nano, 2009, 3, 653–660 CrossRef CAS PubMed.
- H. S. Lin and P. A. Maggard, Inorg. Chem., 2008, 47, 8044–8052 CrossRef CAS PubMed.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- P. Wang, B. B. Huang, X. Y. Zhang, X. Y. Qin, H. Jin, Y. Dai, Z. Y. Wang, J. Y. Wei, J. Zhan, S. Y. Wang, J. P. Wang and M. H. Whangbo, Chem.–Eur. J., 2009, 15, 1821–1824 CrossRef CAS PubMed.
- G. P. Dai, J. G. Yu and G. Liu, J. Phys. Chem. C, 2012, 116, 15519–15524 CAS.
- Y. M. Yang, Y. Y. Liu, B. B. Huang, R. Zhang, Y. Dai, X. Y. Qin and X. Y. Zhang, RSC Adv., 2014, 4, 20058–20061 RSC.
- H. F. Shi, C. L. Zhang and C. P. Zhou, RSC Adv., 2015, 5, 50146–50154 RSC.
- S. M. Wang, Y. Guan, L. P. Wang, W. Zhao, H. He, J. Xiao, S. G. Yang and C. Sun, Appl. Catal., B, 2015, 168, 448–457 CrossRef.
- H. D. Lu, J. X. Wang, Z. Y. Du, Y. P. Liu, M. Li, P. Chen and L. Z. Zhang, Mater. Lett., 2015, 157, 231–234 CrossRef CAS.
- X. Lin, X. Y. Guo, W. L. Shi, F. Guo, H. J. Zhai, Y. S. Yan and Q. W. Wang, Catal. Commun., 2015, 66, 67–72 CrossRef CAS.
- X. C. Shi and D. H. Wang, Langmuir, 2010, 26, 9686–9694 CrossRef PubMed.
- R. C. Oliveira, M. Assis, M. M. Teixeira, M. D. P. Silva, M. S. Li, J. Andres, L. Gracia and E. Longo, J. Phys. Chem. C, 2016, 120, 12254–12264 Search PubMed.
- J. Zhao, C. Chen and W. Ma, Top. Catal., 2005, 35, 269–278 CrossRef CAS.
- X. K. Li and J. H. Ye, J. Phys. Chem. C, 2007, 111, 13109–13116 CAS.
- H. B. Fu, C. S. Pan, W. Q. Yao and Y. F. Zhu, J. Phys. Chem. B, 2005, 109, 22432–22439 CrossRef CAS PubMed.
- H. M. Sung-Suh, J. R. Choi, H. J. Hah, S. M. Koo and Y. C. Bae, J. Photochem. Photobiol., A, 2004, 163, 37–44 CrossRef CAS.
- A. Sari, C. Alkan and C. Bilgin, Appl. Energy, 2014, 136, 217–227 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |