Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A binary polymer composite of graphitic carbon nitride and poly(diphenylbutadiyne) with enhanced visible light photocatalytic activity
Juying Lei a,
Fenghui Liub,
Lingzhi Wang
a,
Fenghui Liub,
Lingzhi Wang b,
Yongdi Liu*a and
Jinlong Zhang
b,
Yongdi Liu*a and
Jinlong Zhang *bc
*bc
aState Environmental Protection Key Laboratory of Environmental Risk Assessment and Control on Chemical Process, School of Resources and Environmental Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: ydliu@ecust.edu.cn
bKey Lab for Advanced Materials, Institute of Fine Chemicals, School of Chemistry and Chemical Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: jlzhang@ecust.edu.cn
cSuzhou Jukang New Materials Co. Ltd, 558 Fenhu Road, Suzhou, Jiangsu Province 201211, P. R. China
First published on 23rd May 2017
Abstract
New polymer composites consisting of poly(diphenylbutadiyne) (PDPB) and g-C3N4 have been successfully prepared. UV-vis diffuse reflectance spectra show that the existence of PDPB in composites can clearly increase the visible light absorption of the catalysts. Photoluminescence spectroscopy and photoelectrochemical measurements reveal that PDPB can effectively facilitate the charge carrier separation in the composites. Compared with pure g-C3N4 or pure PDPB, the composite catalysts exhibit observably enhanced visible-light photocatalytic activity for degradation of RhB and phenol. A possible mechanism for the charge separation and transfer in the composite catalysts is proposed. In addition, the composite catalysts show stable catalytic performance after five successive runs, displaying potential for applications in various fields of photocatalysis.
Introduction
Using semiconductor photocatalysts to degrade organic pollutants has attracted much attention due to the global energy crisis and environment pollution.1–3 To date, a variety of environmentally responsible photocatalysts have been developed, including TiO2,4–7 ZnO,8,9 CdS10,11 and so on. In particular, graphitic carbon nitride (g-C3N4), a novel metal-free material with a special layered structure, has unique properties, such that it is considered to be an ideal material for photocatalysis.12,13 In 2009, Wang et al. first reported that g-C3N4 has excellent photocatalytic ability.14 Since then, the catalytic performance of g-C3N4 for the photocatalytic degradation of organic pollutants in water has been extensively studied.However, the g-C3N4 has many weak points such as the small surface area, high recombination rate of photogenerated electron–hole pairs and low visible light utilization efficiency.15 Hence, various methods have been used to solve these problems, for example, synthesizing different structures,16,17 doping with metal or nonmetal elements,18–21 coupling with other semiconductors,22–26 and modifying by conjugated polymers.27,28 Among these methods, the conjugated polymer modification has attracted many attentions because the conjugated polymer not only can enhance the absorption of the visible light but also can act as a semiconductor. Yan et al. synthesized carbon nitride–poly(3-hexylthiophene) (g-C3N4–P3HT) composite photocatalyst.24 They found that the novel photocatalyst exhibited significantly enhanced photocatalytic activity. Thakare et al. reported on the synthesis of a ternary polymer composite of graphene, carbon nitride, and poly(3-hexylthiophene) (G–g-C3N4–P3HT) which had enhanced photocatalytic activity.29 Ge et al. prepared a novel polyaniline–graphitic carbon nitride (PANI/g-C3N4) composite photocatalyst which had intensive visible light photocatalytic activity.30 Wang et al. prepared an efficient visible-light photocatalyst using g-C3N4 and ordinary polyvinyl chloride (PVC) as main precursors.28 However, to the best of our knowledge, the report about using polymer modified g-C3N4 is still scarce.
Poly(diphenylbutadiyne) (PDPB) is a newly developed polymer semiconductor which was reported by Hynd Remita in 2015.31 The PDPB obtained by π-stacking of oligomers presents a high photocatalytic activity and long-term stability. Recent research has also shown that the composite of PDPB and semiconductor ZnO exhibited high visible light photocatalytic activity,32 due to the visible light harvesting of PDPB. Inspired by this, we speculate that if we combine PDPB with g-C3N4, based on the good visible light absorption of g-C3N4 itself, the combination of g-C3N4 and PDPB will bring a better absorption of visible light, leading to a better visible light activity.
Herein, we prepare a polymer composite photocatalyst consisting of g-C3N4 and PDPB (CN-PDPB). The photocatalytic activity is investigated by evaluating the photocatalytic degradation of Rhodamine B (RhB) and phenol. The results exhibit that the CN–PDPB has higher photocatalytic activity than pure g-C3N4 and PDPB. It is maybe because the existence of PDPB can obviously improve the absorption of g-C3N4 in the visible light range. What's more, the interaction between g-C3N4 and PDPB improves the separation efficiency of the photo-generated charge carriers. In addition, the composites catalysts show stable catalytic performance after five successive runs.
Experimental
The preparation of photocatalysts
g-C3N4 was synthesized by directly heating melamine powder. Melamine powder was put into a covered alumina crucible and heated in a muffle furnace. Firstly, the powder was heated to 500 °C with a heating temperature rate of 2 °C min−1 and kept at this temperature for 2 h, then heated to 520 °C within 10 min and kept at this temperature for 2 h. Finally, we obtained a light yellow g-C3N4. The product was ground to powder for further usage.PDPB was synthesized following the previously method published by Hynd Remita.31
The composite catalysts of g-C3N4 and PDPB were prepared by impregnating g-C3N4 (0.5 g) with an ethanol solution of PDPB for a night, and then the solvent was vapored in oil bath at 363 K. The resultant samples were labeled as 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x CN–PDPB, where 50
x CN–PDPB, where 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x was the mass ratio of g-C3N4 and PDPB in the above-mentioned process for preparation of CN–PDPB photocatalysts.
x was the mass ratio of g-C3N4 and PDPB in the above-mentioned process for preparation of CN–PDPB photocatalysts.
Photocatalytic activity test
The photocatalytic activity of the samples was tested by degradation of RhB dyes and phenol at room temperature using a 300 W xenon lamp with AM 1.5 or 420 nm filter as light source. 10 mg of catalyst powder was added to the 50 mL of 10 mg L−1 RhB (or phenol) aqueous solution. Before irradiation, the suspensions were continuously stirred for 30 min in the dark in order to reach an adsorption–desorption equilibrium. At given time intervals, aliquots of the irradiated suspension were collected and centrifuged. The RhB was analyzed by the UV-vis spectra at 553 nm and the phenol was analyzed by a HPLC system.Results and discussion
Morphology and structural properties
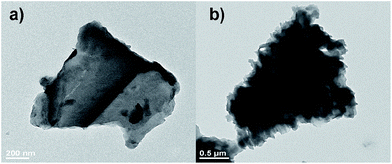
To confirm the structure and morphology of the polymer composites, the g-C3N4 and the polymer composite 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB were observed by TEM. As shown in Fig. 1, both pure g-C3N4 and 50
2 CN–PDPB were observed by TEM. As shown in Fig. 1, both pure g-C3N4 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB display layered structure. Pure g-C3N4 (Fig. 1a) exhibits a flat and smooth surface and it became rough after the modification of PDPB (Fig. 1b). It can be seen clearly that the PDPB distributes on the surface of g-C3N4. In addition to TEM, other characterizations were also carried out to prove the existence of PDPB in the composite CN–PPDB.
2 CN–PDPB display layered structure. Pure g-C3N4 (Fig. 1a) exhibits a flat and smooth surface and it became rough after the modification of PDPB (Fig. 1b). It can be seen clearly that the PDPB distributes on the surface of g-C3N4. In addition to TEM, other characterizations were also carried out to prove the existence of PDPB in the composite CN–PPDB.
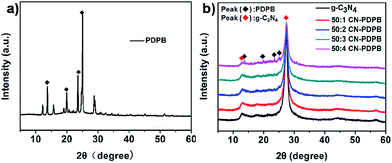
Fig. 2 shows the wide-angle XRD patterns of pure PDPB, pure g-C3N4 and CN–PDPB photocatalysts. The peak at 27.7° in the XRD pattern of g-C3N4 coming from the interlayer stacking of aromatic segments can be indexed as the (002) planes. Another pattern at 13.1° indexed as (100) planes is due to the in-plane structural packing motif.5,33,34 From the XRD patterns of CN–PDPB photocatalysts, we can see that the two characteristic peaks of g-C3N4 are clearly observed, indicating that PDPB does not change the crystalline structure of g-C3N4. However, the characteristic patterns of PDPB are not observed at first because of its low content in the composites but with the increase of content of PDPB, the XRD of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 CN–PDPB displays patterns at 14°, 20.4°, 23.6°, 24.7°, which are ascribed to the characteristic patterns of PDPB.31 This results further confirm the existence of PDPB in the CN–PDPB composites.
4 CN–PDPB displays patterns at 14°, 20.4°, 23.6°, 24.7°, which are ascribed to the characteristic patterns of PDPB.31 This results further confirm the existence of PDPB in the CN–PDPB composites.
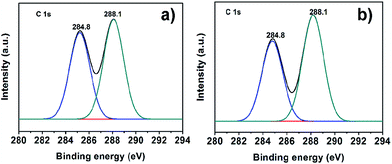
The surface chemical environment of the catalysts is analyzed by XPS. Fig. 3a and b show the C 1s XPS spectra of g-C3N4 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB, respectively. In Fig. 3b, the C 1s has two peaks at 284.8 and 288.1 eV. The C peak at 284.8 eV corresponds to graphitic carbon, which is usually observed in the XPS spectrum of carbon nitrides.35 The peak at 288.1 eV is ascribed to the sp2-bonded carbon (N–C
2 CN–PDPB, respectively. In Fig. 3b, the C 1s has two peaks at 284.8 and 288.1 eV. The C peak at 284.8 eV corresponds to graphitic carbon, which is usually observed in the XPS spectrum of carbon nitrides.35 The peak at 288.1 eV is ascribed to the sp2-bonded carbon (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from carbon nitride and carbon atoms in conjugated structure (–C
N) from carbon nitride and carbon atoms in conjugated structure (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) from PDPB.28,36–39 Compared with Fig. 3a, the intensity ratio of the peaks at 284.8 eV and 288.1 eV in Fig. 3b reduces, indicating the existence of –C
C–) from PDPB.28,36–39 Compared with Fig. 3a, the intensity ratio of the peaks at 284.8 eV and 288.1 eV in Fig. 3b reduces, indicating the existence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– on the surface of 50
C– on the surface of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB. This further confirms the existence of PDPB in the composite CN–PDPB.
2 CN–PDPB. This further confirms the existence of PDPB in the composite CN–PDPB.
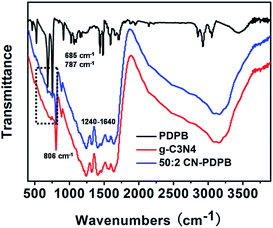
Fig. 4 shows the FTIR spectra of PDPB, g-C3N4 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB. For g-C3N4, the strong bands at 1241, 1322, 1406, 1571, and 1631 cm−1 are corresponding to the typical stretching modes of CN heterocycles and the band at 806 cm−1 is attributed to the characteristic breathing mode of triazine units. Compared with the FTIR of PDPB and g-C3N4, the FTIR band at 685 cm−1 and 787 cm−1 can be found in 50
2 CN–PDPB. For g-C3N4, the strong bands at 1241, 1322, 1406, 1571, and 1631 cm−1 are corresponding to the typical stretching modes of CN heterocycles and the band at 806 cm−1 is attributed to the characteristic breathing mode of triazine units. Compared with the FTIR of PDPB and g-C3N4, the FTIR band at 685 cm−1 and 787 cm−1 can be found in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB which is consistence with the previous report31 but the intensities of the peaks are weak. That is because the low content of PDPB in 50
2 CN–PDPB which is consistence with the previous report31 but the intensities of the peaks are weak. That is because the low content of PDPB in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB. All of these prove the existence of PDPB in 50
2 CN–PDPB. All of these prove the existence of PDPB in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB.
2 CN–PDPB.
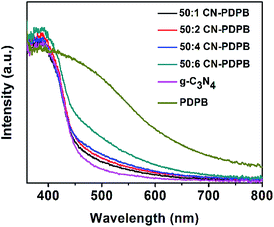
The UV-vis diffuse reflectance spectrum for the catalysts in Fig. 5 shows that the absorption of the CN–PDPB composites is higher than that of pure g-C3N4 in the range of 360–800 nm due to the existence of PDPB. Furthermore, the absorption intensity of the CN–PDPB increases with the increase of PDPB content. This improved absorption is helpful to the improvement of the photocatalytic activity of g-C3N4.
Photocatalytic performances
In order to evaluate the photocatalytic activity of the as-synthesized samples, a test reaction is carried out for the degradation of 10 mg L−1 of RhB with 10 mg of the catalyst under simulated sunlight or visible light and the results are shown in Fig. 6. In addition, Fig. 7 is the UV-vis spectra of the degraded RhB using the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB under simulated sunlight (with AM 1.5). As can be seen from Fig. 6, no matter under irradiation of simulated sunlight or visible light, the photocatalytic degradation of RhB with the CN–PDPB composites is clearly higher than that of the pure g-C3N4 and the pure PDPB. The reason may be ascribed to that the combination of g-C3N4 and PDPB can improve the separation efficiency of the photo-generated electrons and holes, meaning the recombination of electrons and holes is inhibited. However, from Fig. 6 we can also find that with the content of PDPB increased, the photocatalytic activity of CN–PDPB composites increases at first and then decreases, and the 50
2 CN–PDPB under simulated sunlight (with AM 1.5). As can be seen from Fig. 6, no matter under irradiation of simulated sunlight or visible light, the photocatalytic degradation of RhB with the CN–PDPB composites is clearly higher than that of the pure g-C3N4 and the pure PDPB. The reason may be ascribed to that the combination of g-C3N4 and PDPB can improve the separation efficiency of the photo-generated electrons and holes, meaning the recombination of electrons and holes is inhibited. However, from Fig. 6 we can also find that with the content of PDPB increased, the photocatalytic activity of CN–PDPB composites increases at first and then decreases, and the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB shows the highest photocatalytic activity. This is because with the contents of PDPB increase, extra PDPB will hinder the light exposed to g-C3N4 and less g-C3N4 can be excited to generate electron–hole pairs. As a result, the catalytic activity is reduced. Therefore, there is an optimal ratio to achieve the best photocatalytic activity.
2 CN–PDPB shows the highest photocatalytic activity. This is because with the contents of PDPB increase, extra PDPB will hinder the light exposed to g-C3N4 and less g-C3N4 can be excited to generate electron–hole pairs. As a result, the catalytic activity is reduced. Therefore, there is an optimal ratio to achieve the best photocatalytic activity.
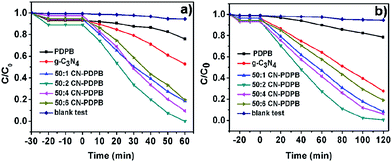 | ||
| Fig. 6 Photocatalytic degradation of RhB over different catalysts (a) under simulated sunlight (with AM 1.5), (b) under visible light irradiation (λ > 420 nm). | ||
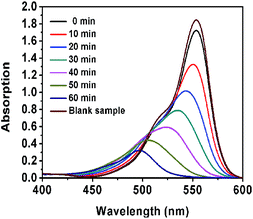 | ||
Fig. 7 The UV-vis spectra of the degraded RhB using the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB under simulated sunlight (with AM 1.5). 2 CN–PDPB under simulated sunlight (with AM 1.5). | ||
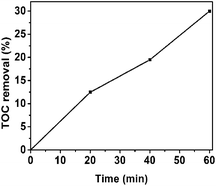
In addition, we use TOC measurement to further confirm the degree of mineralization of RhB solution. The RhB solution is almost decolorized after 60 min irradiation (in Fig. 6a) and about 30% removal of TOC has been seen in Fig. 8, manifesting that RhB has been not only decolorized but also mineralized partly.
Phenol was chosen as another model pollutant to eliminate the sensitization effect of RhB.5 Fig. 9 presents the results of photocatalytic degradation of phenol catalyzed by pure g-C3N4 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB under visible light irradiation. We can see that the photocatalytic degradation efficiency of phenol with 50
2 CN–PDPB under visible light irradiation. We can see that the photocatalytic degradation efficiency of phenol with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
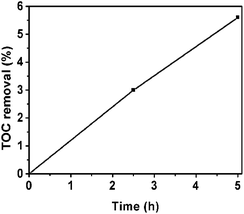
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB is obviously higher than that of pure g-C3N4, further illustrating that the composite has increscent photocatalytic activity. In addition, we use TOC measurement to further confirm the degree of mineralization of phenol solution. From Fig. 10, it can be seen that the TOC removal values are increased. The degradation of phenol takes time until being finally mineralized.
2 CN–PDPB is obviously higher than that of pure g-C3N4, further illustrating that the composite has increscent photocatalytic activity. In addition, we use TOC measurement to further confirm the degree of mineralization of phenol solution. From Fig. 10, it can be seen that the TOC removal values are increased. The degradation of phenol takes time until being finally mineralized.
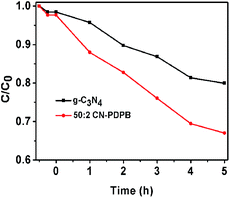 | ||
| Fig. 9 Photocatalytic degradation of phenol over different catalysts under visible light irradiation. | ||
Mechanism for the enhanced photocatalytic activity
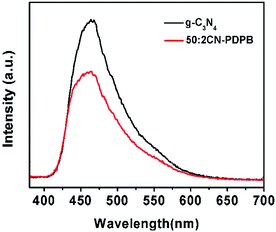
To investigate the mechanism for the enhancement of the photocatalytic activity by the modification of g-C3N4 with PDPB, photoluminescence (PL) spectra were measured. Fig. 11 shows the results of PL spectra of pure g-C3N4 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB excited at 360 nm. As can be seen from the figure, the pure g-C3N4 exhibits a PL emission band at 460 nm. In comparison, the PL intensity of 50
2 CN–PDPB excited at 360 nm. As can be seen from the figure, the pure g-C3N4 exhibits a PL emission band at 460 nm. In comparison, the PL intensity of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB composites is lower than that of g-C3N4 due to the easy transfer of carriers between g-C3N4 and PDPB, indicating the increase in the separation efficiency of photo-generated electron–hole pairs.40–42
2 CN–PDPB composites is lower than that of g-C3N4 due to the easy transfer of carriers between g-C3N4 and PDPB, indicating the increase in the separation efficiency of photo-generated electron–hole pairs.40–42
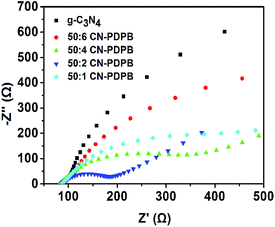
Besides the photoluminescence measurement, electrochemical impedance spectroscopy (EIS) is also applied to investigate the charge transfer resistance of the catalysts.43–45 The EIS results of prepared samples are presented in Fig. 12 in the form of Nyquist plots. It can be clearly found that the g-C3N4 has the highest charge transport resistance for its biggest arc radius and the arc radius of CN–PDPB is significantly smaller than that of the pure g-C3N4. In addition, the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB has the smallest arc radius among the prepared materials, revealing an effective separation of photogenerated electron–hole pairs and faster charge transfer occurred on 50
2 CN–PDPB has the smallest arc radius among the prepared materials, revealing an effective separation of photogenerated electron–hole pairs and faster charge transfer occurred on 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB.
2 CN–PDPB.
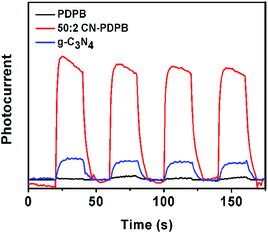
To further investigate the photocatalytic mechanism of the CN–PDPB, the transient photocurrent responses of pure g-C3N4 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB were tested by on–off cycles of irradiation.
2 CN–PDPB were tested by on–off cycles of irradiation.
Transient photocurrent analysis is also a widely used typical method to investigate the electron–hole separation effect for a material.46 In Fig. 13, it can be seen that the photocurrent of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB is much larger than the photocurrent of pure g-C3N4, indicating higher separation and transfer efficiency of photo-generated electrons and holes due to the synergetic effect of g-C3N4 and PDPB.
2 CN–PDPB is much larger than the photocurrent of pure g-C3N4, indicating higher separation and transfer efficiency of photo-generated electrons and holes due to the synergetic effect of g-C3N4 and PDPB.
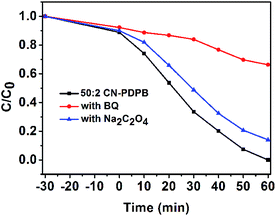
The effects of benzoquinone (BQ, a typical ˙O2− scavenger) and disodium oxalate (Na2C2O4, a typical hole scavenger) on the photodegradation of RhB in the presence of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB are investigated. From the Fig. 14, we can see that the BQ clearly inhibits the photodegradation of RhB, but the Na2C2O4 lightly decreases the photodegradation of RhB, indicating that the ˙O2− and the holes are all effective for the degradation of RhB but the ˙O2− is the main active species for the RhB degradation.
2 CN–PDPB are investigated. From the Fig. 14, we can see that the BQ clearly inhibits the photodegradation of RhB, but the Na2C2O4 lightly decreases the photodegradation of RhB, indicating that the ˙O2− and the holes are all effective for the degradation of RhB but the ˙O2− is the main active species for the RhB degradation.
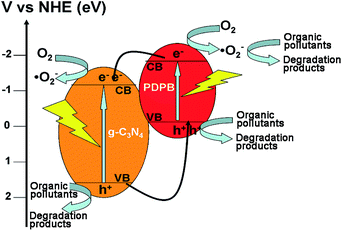
On the basis of the above experiments and results, the mechanism is explained in Fig. 15. With irradiation of light, both g-C3N4 and PDPB can be excited to produce photo-generated electrons and holes. The conduction band (CB) position of PDPB (−1.78 eV) is more negative than that of g-C3N4 (−1.13 eV), and the valence band (VB) position of g-C3N4 (+1.57 eV) is more positive than that of PDPB (+0.18 eV).31 Hence, the photo-generated electrons of PDPB can easily transfer to the conduction band (CB) of g-C3N4, while the holes of g-C3N4 can transfer to the valence band (VB) of PDPB. Thus, the recombination of photo-generated electrons and holes can be inhibited and an effective charge separation is achieved, resulting in a remarkable enhancement of the photocatalytic activity. Moreover, the major possible reactions can be displayed as follows:
| CN–PDPB + hv → g-C3N4*–PDPB* |
| g-C3N4*–PDPB* → g-C3N4 (e−e−/h+h+)–PDPB (e−e−/h+h+) |
| g-C3N4 (e−e−/h+h+)–PDPB (e−e−/h+h+) → g-C3N4 (e−e−e−/h+)–PDPB (e−/h+h+h+) |
| e− + O2 → ˙O2− |
| ˙O2− + organic pollutants → degradation products |
| h+ + organic pollutants → degradation products |
The stability of photocatalyst is another important issue for a photocatalyst. The recycling runs in the photodegradation of RhB in the presence of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB are carried out. Fig. 16 shows that the photocatalytic degradation degree of RhB still remains high after 5 cycles, manifesting the catalyst has good photocatalytic stability.
2 CN–PDPB are carried out. Fig. 16 shows that the photocatalytic degradation degree of RhB still remains high after 5 cycles, manifesting the catalyst has good photocatalytic stability.
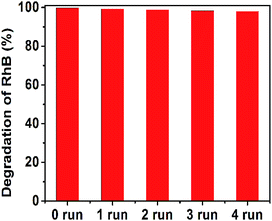 | ||
Fig. 16 Effect of cycling runs on RhB degradation in the presence of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB under visible-light irradiation. 2 CN–PDPB under visible-light irradiation. | ||
Conclusions
In summary, a new photocatalyst has been prepared by compounding g-C3N4 and the polymer PDPB via a facile method. The photocatalytic activity of CN–PDPB composites is much higher than that of the pure catalysts (g-C3N4 or PDPB), which is confirmed by the photocatalytic degradation of RhB and phenol under simulated solar light or visible light. With the increase of PDPB content, the photocatalytic activity increases at first and then decreases, and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CN–PDPB has the highest photocatalytic activity. The enhanced photocatalytic activity may be caused by the increased charge separation which can be attributed to the synergistic effect between g-C3N4 and PDPB. In addition, the photocatalytic stability of the catalyst is good. This study is hopeful to provide some experience for the synthesis of other polymer composited g-C3N4 or other semiconductor composited PDPB in the application of photocatalysis and other fields.
2 CN–PDPB has the highest photocatalytic activity. The enhanced photocatalytic activity may be caused by the increased charge separation which can be attributed to the synergistic effect between g-C3N4 and PDPB. In addition, the photocatalytic stability of the catalyst is good. This study is hopeful to provide some experience for the synthesis of other polymer composited g-C3N4 or other semiconductor composited PDPB in the application of photocatalysis and other fields.
Acknowledgements
The authors wish to express their deepest and sincerest recognition of Prof. András Dombi a key figure in the topic of photocatalytic materials for the degradation of contaminants of environmental concern. This work was financially supported by National Nature Science Foundation of China (21407049, 21377038, 21237003 and 21677048), China Postdoctoral Science Foundation (2015T80409), Shanghai Pujiang Program (14PJ1402100), the Science and Technology Commission of Shanghai (16JC1401400) and the Science and Technology Commission of Jiangsu Province (BC2015135).References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- S. Dong, J. Feng, M. Fan, Y. Pi, L. Hu, X. Han, M. Liu, J. Sun and J. Sun, RSC Adv., 2015, 5, 14610–14630 RSC.
- W. Wang, Y. Liu, J. Qu, Y. Chen and Z. Shao, RSC Adv., 2016, 6, 40923–40931 RSC.
- H. Li, L. Zhou, L. Z. Wang, Y. D. Liu, J. Lei and J. L. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 17406–17412 RSC.
- H. Li, X. Shen, Y. Liu, L. Wang, J. Lei and J. Zhang, J. Alloys Compd., 2016, 687, 927–936 CrossRef CAS.
- M. Nasir, J. Lei, W. Iqbal and J. Zhang, Appl. Surf. Sci., 2016, 364, 446–454 CrossRef CAS.
- S. G. Kumar and K. S. R. K. Rao, RSC Adv., 2015, 5, 3306–3351 RSC.
- Y. Xu, H. Xu, H. Li, J. Xia, C. Liu and L. Liu, J. Alloys Compd., 2011, 509, 3286–3292 CrossRef CAS.
- Y. Liu, P. Zhang, B. Z. Tian and J. L. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 13849–13858 CAS.
- S. Singh and N. Khare, RSC Adv., 2015, 5, 96562–96572 RSC.
- J. Lei, Y. Chen, F. Shen, L. Wang, Y. Liu and J. Zhang, J. Alloys Compd., 2015, 631, 328–334 CrossRef CAS.
- H. Li, L. Wang, Y. Liu, J. Lei and J. Zhang, Res. Chem. Intermed., 2016, 42, 3979–3998 CrossRef CAS.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–82 CrossRef CAS PubMed.
- Z. W. Zhao, Y. J. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- J. Hong, X. Xia, Y. Wang and R. Xu, J. Mater. Chem., 2012, 22, 15006–15012 RSC.
- Y. Xu, M. Xie, S. Huang, H. Xu, H. Ji, J. Xia, Y. Li and H. Li, RSC Adv., 2015, 5, 26281–26290 RSC.
- X. F. Chen, J. S. Zhang, X. Z. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- L. Zhang, X. Chen, J. Guan, Y. Jiang, T. Hou and X. Mu, Mater. Res. Bull., 2013, 48, 3485–3491 CrossRef CAS.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- J. Lei, Y. Chen, L. Wang, Y. Liu and J. Zhang, J. Mater. Sci., 2015, 50(9), 3467–3476 CrossRef CAS.
- L. Zhou, L. Wang, J. Lei, Y. Liu and J. Zhang, Catal. Commun., 2017, 89, 125–128 CrossRef CAS.
- L. Zhou, L. Wang, J. Zhang, J. Lei and Y. Liu, Eur. J. Inorg. Chem., 2016, 5387–5392 CrossRef CAS.
- Y. S. Xu and W. D. Zhang, ChemCatChem, 2013, 5, 2343–2351 CrossRef CAS.
- S. Z. Wu and K. Li, Appl. Surf. Sci., 2015, 324, 324–331 CrossRef CAS.
- H. J. Yan and Y. Huang, Chem. Commun., 2011, 47, 4168–4170 RSC.
- D. S. Wang, H. T. Sun, Q. Z. Luo, X. L. Yang and R. Yin, Appl. Catal., B, 2014, 156, 323–330 CrossRef.
- S. Gawande and S. R. Thakare, ChemCatChem, 2012, 4, 1759–1763 CrossRef CAS.
- L. Ge, C. C. Han and J. Liu, J. Mater. Chem., 2012, 22, 11843–11850 RSC.
- S. Ghosh, L. Ramos, S. Remita, A. Dazzi and H. Remita, Nat. Mater., 2015, 14, 505–511 CrossRef CAS PubMed.
- S. Sardar, P. Kar, H. Remita, B. Liu, P. Lemmens, S. K. Pal and S. Ghosh, Sci. Rep., 2015, 5, 17313–17326 CrossRef CAS PubMed.
- Y. Xu, M. Xie, S. Huang, H. Xu, H. Ji, J. Xia, Y. Li and H. Li, RSC Adv., 2015, 5, 26281–26290 RSC.
- Y. Xu, S. Huang, M. Xie, Y. Li, H. Xu, L. Huang, Q. Zhang and H. Li, RSC Adv., 2015, 5, 95727–95735 RSC.
- X. Jian, X. Liu, H. M. Yang, J. G. Li, X. L. Song, H. Y. Dai and Z. H. Liang, Appl. Surf. Sci., 2016, 370, 514–521 CrossRef CAS.
- S. W. Cao, Y. P. Yuan, J. Fang, M. M. Shahjamali, F. Y. C. Boey, J. Barber, S. C. J. Loo and C. Xue, Int. J. Hydrogen Energy, 2013, 8, 1258–1266 CrossRef.
- L. Ge, C. Han, J. Liu and Y. Li, Appl. Catal., A, 2011, 409–410, 215–222 CrossRef CAS.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- A. Vinu, Adv. Funct. Mater., 2008, 18, 816–827 CrossRef CAS.
- B. Subash, B. Krishnakumar, M. Swaminathan and M. Shanthi, Res. Chem. Intermed., 2013, 39, 3181–3197 CrossRef CAS.
- K. Yadav, M. Giri and N. Jaggi, Res. Chem. Intermed., 2015, 41, 9967–9978 CrossRef CAS.
- A. K. L. Sajjad, S. Shamaila, B. Z. Tian, F. Chen and J. L. Zhang, Appl. Catal., B, 2009, 91, 397–405 CrossRef CAS.
- C. Zeng, M. Guo, B. Tian and J. Zhang, Chem. Phys. Lett., 2013, 575, 81–85 CrossRef CAS.
- C. Zeng, B. Tian and J. Zhang, J. Colloid Interface Sci., 2013, 405, 17–21 CrossRef CAS PubMed.
- X. Liu, L. J. Chen, R. Y. Chen, Z. Chen, X. Chen and X. Zheng, Res. Chem. Intermed., 2015, 41(6), 3623–3636 CrossRef CAS.
- K. Dai, L. Lu, Q. Liu, G. Zhu, X. Wei, J. Bai, L. Xuan and H. Wang, Dalton Trans., 2014, 43, 6295–6299 RSC.
| This journal is © The Royal Society of Chemistry 2017 |