Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A mesoporous titanium glycolate with exceptional adsorption capacity to remove multiple heavy metal ions in water†
Wei Han‡
ab,
Xiulin Yang‡ b,
Fuwen Zhaob,
Xiaofeng Shib,
Taishan Wangb,
Xiangdong Zhang*a,
Li Jiang
b,
Fuwen Zhaob,
Xiaofeng Shib,
Taishan Wangb,
Xiangdong Zhang*a,
Li Jiang *b and
Chunru Wangb
*b and
Chunru Wangb
aCollege of Chemistry, Liaoning University, Shenyang 110036, PR China. E-mail: xdzhang@lnu.edu.cn
bBeijing National Laboratory for Molecular Sciences, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: jiangli@iccas.ac.cn
First published on 12th June 2017
Abstract
A facile, green strategy is explored to obtain mesoporous titanium glycolate (Ti(OCH2CH2O)2) via a one-pot hydrothermal reaction. The mesoporous Ti(OCH2CH2O)2 nanomaterials take on a three-dimensional V-type stripe structure with a specific surface area of 246.5 m2 g−1 and present an excellent adsorption capacity of toxic heavy metal ions in aqueous solution, 225.73 mg g−1 for Pb(II), 131.93 mg g−1 for As(V) and 156.01 mg g−1 for As(III) with fast adsorption rates. The removal mechanism was found to derive from the electrostatic interaction or coordination interaction between Ti(OCH2CH2O)2 and heavy metal ions in aqueous solution since the mesoporous materials display a pH-dependent effect for arsenic removal.
Introduction
Drinking-water pollution induced by heavy metal ions is a serious threat for human health all over the world because of their serious health-toxic and non-biodegradable properties.1,2 For example, lead can accumulate in the apatite structure of bone to obstruct biosynthesis of heme as well as inhibit zinc enzymes and protein synthesis;3 Arsenic, being in the form of arsenite As(III) or arsenate As(V) in aqueous solution, may cause skin, lung or bladder cancer.4–6 However, because the toxic heavy metals are usually in low concentration and co-exist with nontoxic metal ions such as Fe, Ca, Na etc., the high efficiency and selective removal of these contaminants from drinking water remains a challenge.Recently, the rapid development of nanoscience and nanotechnology has greatly promoted drinking water treatment techniques by using nanomaterial adsorbents to remove the heavy metal contaminants. As is well known, nanomaterials usually possess large specific surface area and abundant active sites,7 which are expected to selectively absorb some specific heavy metals. For example, the nanomaterials based on titanium, iron and aluminum compounds or complexes8–10 have been observed to efficiently absorb Pb or As with remaining the innocuous metal ions unchanged.
In order to completely remove heavy metal contaminants, it is crucial to make clear both the usual chemical form of heavy metals in drinking water and how they interact with the applied nanomaterials. For most heavy metals such as Hg, Pb, Tl etc., a simple electrostatic absorption by some strong electronegative nanomaterials would suffice since they exist only in metal cationic form in water. However, some other toxic heavy metal contaminants such as As, Se, Te, etc., may have various chemical forms depending on the pH values in water. For example, arsenate exists as oxyanions (H2AsO4− or HAsO42−) in pH ranging from 2 to 12, while arsenite remains as neutral molecular species (H3AsO3) at pH < 9.2.11,12 In general, most nanomaterials composed of metal oxides or hydroxides are positive-charged when the pH value being lower than the isoelectric point, so they may effectively remove the negative-charged As(V) ions by electrostatic adsorption, but these nanomaterials are difficult to remove the neutral As(III) species.13,14
In order to improve the removal efficiency of As(III), it has been suggested to chemically transfer the charge state of As from As(III) to As(V) before the treatment,15–17 but this technique is either too complicated or kinetically too slow to be available. Undoubtedly, it would be perfect if an adsorbent can be developed to remove simultaneously both As(V) and As(III) without any additional oxidation process, which would not only simplify the water treatment procedure but also reduce the cost of water treatment to a great degree.4
To realize the simultaneous removal of As(V) and As(III), one way is to design a composite nanomaterial in which one part can adsorb the cationic heavy metals and the other part is able to adsorb the anionic ones.18 Up to now, unfortunately, such composite adsorbents are unavailable in application. Another way is to develop a material that can adsorb both metal cations and metal-containing anions in the meantime. Recently, several reports have shown the possibility of this technique. For example, nanoscale zero-valent iron and goethite (a-FeOOH) showed fast uptake rate and high adsorption capacity for multiple heavy metal ions simultaneously,19 and nanomaterials based on titanium compounds such as mesoporous titania beads,8 titanate nanobelts20 and titanate nanoflowers,21 also revealed potential applications in the heavy metal ions removal. However, some disadvantages of these materials, like low yields, complicated preparation processes, instability of iron-based nanomaterials and insufficient adsorption capacity of the titanium-based materials, largely limited their large-scale application in the practical drinking-water treatments.
In this context, we reported a new titanium glycolate nanomaterial of which three-dimensional V-type strip structure can not only absorb the heavy metal cations but also remove the heavy metal-containing anions simultaneously. Moreover, this material was able to be obtained using a very facile one-pot hydrothermal method, and expected to have practical applications in drinking water treatments due to its low-cost and environmentally friendly features.
Experimental
Materials
All chemical reagents used in this experiment were of analytical grade. Lead nitrate (Pb(NO3)2), sodium hydrogen arsenate heptahydrate (Na2HAsO4·7H2O), sodium arsenite (NaAsO2), tetrabutyl orthotitanate (Ti(OC4H9)4), ethylene glycol (HOCH2CH2OH), hydrochloric acid (HCl), sodium hydroxide (NaOH) and ethanol were procured commercially and were used as received without further purification.Synthesis of titanium glycolate
In a typical synthesis, 0.5 mL of Ti(OC4H9)4 was dissolved in 60 mL of HOCH2CH2OH and stirred at room temperature until a clear solution was formed, then the solution was transferred into a 100 mL Teflon-lined autoclave and heated up at 160 °C under vigorous stirring for 8 h. After cooling to room temperature naturally, the solid precipitate was collected through a PTFE membrane (pore size: 0.22 μm), and repeatedly washed with double-distilled water and ethanol several times. The obtained products were dried in a vacuum oven at 70 °C for 12 h.Spectroscopic characterizations
The morphology and microstructure of the samples were characterized by field emission scanning electron microscopy (FE-SEM, JEOL 6701F), transmission electron microscopy (TEM, JEOL 2010). X-ray diffraction (XRD) spectrometry was preformed on a Rigaku D/max-2500 diffractometer with Cu Kα radiation (λ = 1.5418 Å) at 40 kV and 30 mA. XPS was measured with an ESCALab220i-XL electron spectrometer (VG Scientific) using 300 W Al Kα radiation. Fourier transforms infrared spectrometry (FT-IR, Thermo Fisher Scientific) and Raman spectrometry (DXR Raman Microscope, America) were employed to analyze the surface chemical composition. Thermal gravity measurement was made on a TGA/STA409 PC module with a rising temperature rate of 10 °C min−1 from 50 to 1000 °C under continuous air flow. The specific surface area of the as-prepared products was measured on a Quantachrome Autosorb AS-1 instrument, and the pore size distribution was derived from the desorption branches of the isotherm with the Barrett–Joyner–Halenda (BJH) model. The pH value was measured using pH meter (Thermo Scientific, Model: 410p-13).Adsorption experiments
The solutions containing different concentration of Pb(II), As(V) and As(III) with 10, 20, 50, 100, 200 and 300 mg L−1 were prepared using Pb(NO3)2, Na2HAsO4·7H2O and NaAsO2 as the source of heavy metal ions, respectively. The initial pH value of the Na2HAsO4 and NaAsO2 solution was adjusted to 4 using hydrochloric acid (0.2 M). The time-dependant curves were preformed with the initial ion concentration on 10 ppm and the sample dose on 20 mg per 100 mL. At predetermined time intervals, 8 mL supernatant solutions were pipetted and filtered through 0.22 μm PTFE membranes. For the adsorption isotherms, 5 mg of the mesoporous titanium glycolate was added to 25 mL of the above solutions under stirring at room temperature. After 12 h, the samples were separated through 0.22 μm PTFE membrane and analyzed by inductively coupled plasma-optical emission spectroscopy (Shimazu ICPE-9000) to measure the concentration of metal ions in the remaining solution. The adsorption capacity of the adsorbents was calculated according to the following eqn (1):4,22
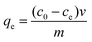 | (1) |
Results and discussion
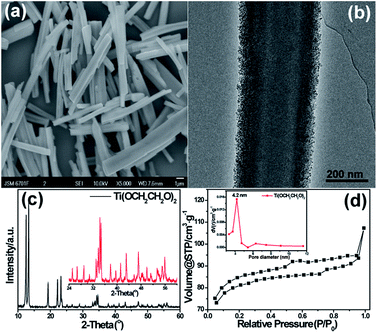
Spectroscopic characterizations of titanium glycolate. Fig. 1a and b are representative scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the titanium glycolate prepared by hydrothermal method in ethylene glycol solution. As shown in the SEM image of Fig. 1a, the titanium glycolate material shows V-type strip shape with a mean width of 0.4–1.0 μm and length of 6.0–10.0 μm. Significantly, the special V-type strip shape was never found in reported titanium glycolate nanostructure materials. The titanium glycolate strip took on well-defined multifaceted structure because of the existence of V-type groove, which undoubtedly would increase the specific surface area in favor of adsorption capacity. TEM image shown in Fig. 1b further revealed that the edge of V-type strips is constituted of a large number of nanoparticles. The energy dispersive X-ray spectrum (EDS) is recorded to identify the composition of the as-prepared sample (Fig. S1†), in which Ti, C, and O were observed as the main elements, indicating the formation of target titanium glycolate; the crystallographic structure of titanium glycolate is determined by X-ray powder diffraction (XRD), as shown in Fig. 1c, where the sharp diffraction peaks are mainly ascribed to C-center monoclinic titanium glycolate.23The specific surface area of the titanium glycolate is studied by nitrogen adsorption–desorption experiment. As shown in Fig. 1d, the BET surface area is estimated to be 246.5 m2 g−1, and combining the observed type IV isotherm with a distinct hysteresis loop, it is indicated that the titanium glycolate owns a mesopore structure with the pore size ranging from 3.3 to 5.4 nm and the average diameter ca. 4.2 nm (Fig. 1d inset).
X-ray photoelectron spectroscopy can provide valuable insight into the surface chemical composition of titanium glycolate. The survey XPS spectrum of Ti(OCH2CH2O)2 in Fig. 2a shows the composition of sample with Ti, O and C elements. As indicated in the insert of Fig. 2a, the two Ti 2p peaks at 458.9 and 464.6 eV correspond to binding energies of Ti 2p3/2 and Ti 2p1/2, respectively, indicating the presence of a Ti(IV) oxidation state in titanium glycolate;24 the high-resolution XPS of O 1s in Fig. 2b reveals two well-defined peaks at 531.3 and 532.0 eV, which are separately attributed to Ti–O and C–O species; the broad peak of C 1s (Fig. 2c) is well-fitted by two sub-peaks with binding energies of 285.2 and 286.2 eV, where the dominant peak at 285.2 eV is characteristic C–C/CHn, and the other peak is assigned as C–O.25 Moreover, survey scans of the Ti(OCH2CH2O)2 sample further reveals the atom ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C being about 1
C being about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.6, which is accordance to the theoretical value 1
4.6, which is accordance to the theoretical value 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The somewhat extortionate estimate of C and O is believed to be resulted from remnant ethylene glycol and/or the adventitious carbohydrates adsorbed on the sample.
4. The somewhat extortionate estimate of C and O is believed to be resulted from remnant ethylene glycol and/or the adventitious carbohydrates adsorbed on the sample.
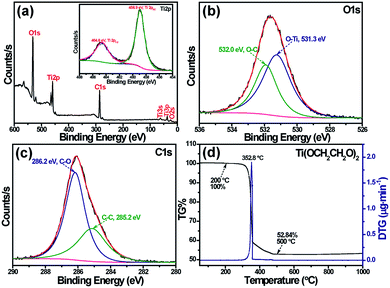 | ||
| Fig. 2 (a) XPS survey spectrum, and the inset shows a high-resolution XPS spectrum of Ti 2p; (b) O 1s of Ti(OCH2CH2O)2; (c) C 1s of Ti(OCH2CH2O)2; (d) TG and DTG curves of titanium glycolate. | ||
To investigate the thermal-stability properties of the titanium glycolate, TG-DTG analyses were performed in air (Fig. 2d). Referring to strong exothermic peak around 352.8 °C, the weight loss between 200 and 500 °C on the TG curve is caused by the decomposition of Ti(OCH2CH2O)2, in which the large one step weight loss of 47.16% is consistent with the calculated value (47.55%) from Ti(OCH2CH2O)2 to TiO2.26
Heavy metal were removed by the titanium glycolate. The high surface area and mesopore structure of the titanium glycolate gave a suggestion to be an excellent candidate for heavy metal removal in water treatments. Based on the previous studies of titanium-based nanomaterials on drinking water treatments, As(III), As(V) and Pb(II) ions were selected as typical toxic heavy metal ions to examine the efficiency of the titanium glycolate used in water treatments.
The surface charge of a sample is usually an important parameter to assess adsorption behaviour, so the zeta potential of Ti(OCH2CH2O)2 was firstly measured. Before measurements, the samples were dispersed in deionized water to form a homogeneous solution by ultrasonic followed by continuous stirring for 12 h. The pH values of the composite were initially adjusted from 2.0 to 10.0 by adding 0.2 M HCl or NaOH. As shown in Fig. S6,† the point of zero charge (pHpzc) of the Ti(OCH2CH2O)2 is about 5.15, which means the surface charge of Ti(OCH2CH2O)2 is positive at pH < 5.15, while the surface charge of that is negative at pH > 5.15.
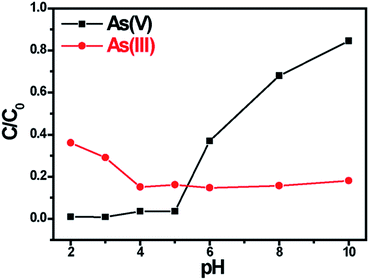
Because the different chemical forms of As(III) and As(V) in aqueous solution, the pH dependence of the titanium glycolate using on As(V) and As(III) adsorption is firstly studied. Na2HAsO4·7H2O and NaAsO2 were used to obtain As(V) and As(III) solution with 10 mg L−1 of initial arsenic concentrations, and 0.2 g L−1 dosage of Ti(OCH2CH2O)2 is used as adsorbent. As shown in Fig. 3, it is observed that the arsenic sorption behavior by Ti(OCH2CH2O)2 depends strongly on the pH values. Obviously, Ti(OCH2CH2O)2 has a high adsorption ability for As(V) at pH < 5, but the ability reduces rapidly when pH value being larger than 5. On the contrary, poor adsorbing efficiency for As(III) of Ti(OCH2CH2O)2 was investigated on low pH value but good efficiency can be obtained when pH > 4 and then keep constant until pH = 10.
The pH dependence of the titanium glycolate adsorbent for As(V) can be ascribed to the protonation of Ti(OCH2CH2O)2, which makes the material to carry positive charges under low pH condition and naturally adsorb the negative-charged As(V) of HAsO42−/H2AsO4− ions driven by electrostatic interactions. But along with the pH value increasing, more and more OH− ions are introduced into the solution that would compete with the As(V) species to be adsorbed on the titanium glycolate surface so that affect the As(V) absorption ability of this material. Otherwise, the electrostatic adsorption between As(III) and Ti(OCH2CH2O)2 would never happen since As(III) does not exist in the anions in water,27 leading to weak pH dependence of the Ti(OCH2CH2O)2 for As(III). In fact, it is suggested that the adsorption of As(III) on Ti(OCH2CH2O)2 follow by means of a coordination interacting mechanism.
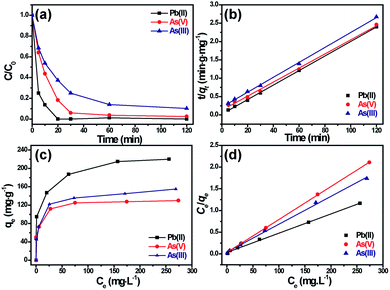
The adsorption speed of titanium glycolate for Pb(II), As(V) and As(III) is investigated, as shown in Fig. 4a, which is undoubtedly one of the most important features for materials using in water treatments. It can be clearly observed that adsorption ability of Ti(OCH2CH2O)2 for different heavy metal ions is quite different, i.e., the adsorption/desorption reach an equilibrium in 20 min for Pb(II), in 30 min for As(V) and in 60 min for As(III), respectively. If we set the initial ion concentration at 10 mg L−1, the maximum removal efficiency of the Ti(OCH2CH2O)2 for Pb(II), As(V) and As(III) is separately 100%, 97.6% and 89.8%, indicating an excellent performance of this nanomaterial.
The rapid adsorption speed of Ti(OCH2CH2O)2 for heavy metal ions shows an excellent adsorption kinetic property of this material. A linear form of pseudo-second-order rate model (k2, g·mg−1 min−1) was adopted to well fit the experimental data, as shown in Fig. 4b, which suggests a chem-sorption process with the rate limiting step.28 Briefly, the pseudo-second-order model is shown as:
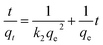 | (2) |
| Heavy metal ions | Experimental qe (mg g−1) | Calculated qe (mg g−1) | k2 (g mg−1 min−1) | R2 |
|---|---|---|---|---|
| Pb(II) | 50.00 | 50.51 | 1.829 × 10−2 | 0.9997 |
| As(V) | 48.79 | 52.16 | 2.876 × 10−3 | 0.9961 |
| As(III) | 44.90 | 49.16 | 1.957 × 10−3 | 0.9990 |
The adsorption capacity of Ti(OCH2CH2O)2 for Pb(II), As(V) and As(III) ions is evaluated using the equilibrium adsorption isotherm. In Fig. 4c, the Ce represents the equilibrium concentration of heavy metal ions (mg L−1) with different initial amounts of the heavy metals from 10 to 200 mg L−1, and qe is the amounts of adsorbed heavy metal (mg g−1) while the adsorption/desorption process reaching equilibrium. It can be observed that while the initial amount of heavy metal is little, all of the heavy metal ions are adsorbed by Ti(OCH2CH2O)2, but along with the increasing of the initial concentration of heavy metal ions, the adsorption capacity qe of Ti(OCH2CH2O)2 slowly reaches maximum.
In order to explore the adsorption mechanism, two empirical isotherms models, i.e., the Langmuir and Freundlich models, are adopted to analyze the adsorption process of Ti(OCH2CH2O)2 for Pb(II), As(V) and As(III) ions, which are expressed as follow equations:
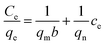 | (3) |
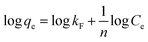 | (4) |
Comparing the fitting results of the two isotherm models with that of experiments, it was found that the adsorption process of Ti(OCH2CH2O)2 for Pb(II), As(V) and As(III) ions follow the Langmuir model (Fig. 4d) as no expected linear plot of Freundlich isotherm is observed (Fig. S2†). Therefore, the Pb(II), As(V) and As(III) ions adopt a monolayer adsorption on the surface of Ti(OCH2CH2O)2.
Based on the Langmuir adsorption isotherm, the correlation coefficients, equilibrium constant and maximum adsorption capacity of the as-prepared Ti(OCH2CH2O)2 were summarized in Table 2, which indicates clearly a high adsorption capacity of Ti(OCH2CH2O)2 for all the Pb(II), As(V) and As(III) ions.
| Heavy metal ions | qm (mg g−1) | b (L mg−1) | R2 |
|---|---|---|---|
| Pb(II) | 225.73 | 0.1253 | 0.9977 |
| As(V) | 131.93 | 0.2119 | 0.9999 |
| As(III) | 156.01 | 0.1444 | 0.9978 |
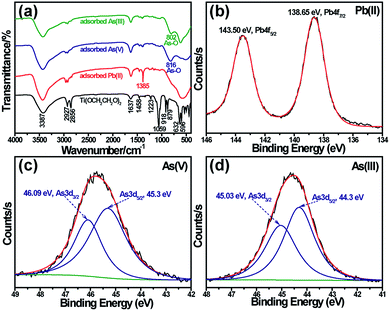
To further explore the adsorption mechanism of Ti(OCH2CH2O)2 for heavy metal ions, FTIR spectroscopic characterizations of Ti(OCH2CH2O)2 were performed to understand how the surface species changes before and after heavy metal ion adsorption on the titanium glycolate, as shown in Fig. 5a. For the Ti(OCH2CH2O)2 sample, the broad band at 3387 cm−1 is ascribed to stretching vibrations of –OH groups; the bands at 2927 and 2856 cm−1 are assigned to the stretching vibrations of C–H; the weak band at 1637 cm−1 can be assigned to the hydroxyl deformation mode of the adsorbed water; the band at 1458 cm−1 is the bending vibration of –CH2 and the bands at 1223 and 1059 cm−1 can be ascribed to C–C and C–O stretching vibration, respectively. As for the four bands at 918, 879, 632 and 596 cm−1, they can be assigned to the characteristic of ethylene glycolate ligands linked to titanium of C–O–Ti, and the other three bands at 536, 498 and 442 cm−1 are assigned to the Ti–O–Ti stretching and bending vibrations.23
After the heavy metal ion adsorption, it was observed that the IR spectra features largely changed due to the adsorbed species. First, due to the large coverage of heavy metal ions on the surface of Ti(OCH2CH2O)2, most characteristic peaks of Ti(OCH2CH2O)2 become weak or even disappear after the heavy metal ion adsorption; secondly, some new spectral bands appear in the spectra corresponding to different heavy metal adsorption, for example, a sharp peak at 1385 cm−1 appears in the spectrum of Pb(II)-adsorbed Ti(OCH2CH2O)2, which is attributed to the stretching vibration of the adsorbed NO3− group.29 For the As(V) and As(III) adsorption, the spectrum separately showed a new peak at 816 cm−1 and 802 cm−1, corresponding to the stretching vibrations of As(V)–O and As(III)–O, respectively.27,30 Similar spectral change of Ti(OCH2CH2O)2 before and after heavy metal adsorption was also observed in Raman spectra (Fig. S3†).
XPS spectrometry is used to further characterize the oxidation state of the adsorbed heavy metals on Ti(OCH2CH2O)2 nanostructures. Fig. S4† shows the full-range XPS spectra of Ti(OCH2CH2O)2 nanostructures after Pb(II), As(V) and As(III) adsorptions, and the high-resolution XPS spectrum for the adsorbed heavy metals are shown in Fig. 5b to d. In general, Pb(NO3)2 shows the 4f7/2 peak at 138.5 eV, whereas Pb hydroxides shows corresponding peak at around 137.75 eV, so it is obvious that the two XPS peaks at 138.65 and 143.5 eV in Fig. 5b can be attributed to Pb4f in the adsorbed Pb(NO3)2, and this result is consistent with that of FTIR spectrometry. As for the As(V), the 45.8 eV peak in Fig. 5c can be deconvoluted into two peaks at 45.3 (3d5/2) and 46.09 eV (3d7/2), corresponding to As3d(V). Therefore, it is suggested that phys-adsorption is occurred for the above heavy metal ions on titanium glycolate nanostructures.
The XPS curves of Ti(OCH2CH2O)2 samples after absorbing As(III) were analysed carefully as shown in Fig. 5d. Different from the assignment of As(V), the 44.6 eV peak can be deconvoluted into a pair of peaks at 44.3 (3d5/2) and 45.03 eV (3d7/2), which can be assigned to As(III)–O and As(V)–O.31–33 This indicates that there are As(V) to appear on the surface of the mesoporous Ti(OCH2CH2O)2 nanomaterials, which probably resulted from the oxidization of a part of As(III). In addition, there are a large number of hydroxyl groups on the surface of the mesoporous Ti(OCH2CH2O)2 (Fig. 5a), which contribute to the effective adsorption through the coordination reaction between –OH and As(III) in aqueous solution. Therefore, we can infer that the adsorption of As(III) on the surface of mesoporous Ti(OCH2CH2O)2 nanomaterials is a complex process involved of both oxidization and coordination reaction simultaneously. Namely, the whole adsorption process includes the adsorption of As(III) directly onto Ti(OCH2CH2O)2, the oxidation of As(III) to As(V) and adsorption of As(V) onto Ti(OCH2CH2O)2.
Besides of FTIR and XPS, SEM, TEM, EDS and element mapping are also performed to characterize the surface species after the Ti(OCH2CH2O)2 adsorbing heavy metal ions. As shown in Fig. S5,† SEM images reveal that the structure of the Ti(OCH2CH2O)2 adsorbent is slightly damaged after heavy metal adsorption, and EDS analysis confirms the composition of adsorbents after heavy metal ion adsorption containing relevant metals. Moreover, TEM and element mapping further demonstrate that the heavy metal ions are uniformly adsorbed on the Ti(OCH2CH2O)2 surface.
Conclusions
A novel titanium-based nanomaterial with large surface area deriving from three-dimensional V-type stripe structure was synthesized by a convenient and green technique. The eco-friend material is well characterized by various spectroscopic techniques, and is found to own mesopore structure with surface area as high as 246.5 m2 g−1. Being an excellent candidate in drinking water treatments, the titanium-based nanomaterial exhibits fast adsorption rates and high removal capacity for multiple typical toxic heavy metal ions such as Pd(II), As(V) and As(III), no matter which form these metal exists, in cation or anion in the water.Acknowledgements
This work is supported by National Basic Research Program (No. 2016YFA0202503) and the National Natural Science Foundation of China (No. 21673257, 51672281).Notes and references
- G. Lofrano, M. Carotenuto, G. Libralato, R. F. Domingos, A. Markus, L. Dini, R. K. Gautam, D. Baldantoni, M. Rossi, S. K. Sharma, M. C. Chattopadhyaya, M. Giugni and S. Meric, Water Res., 2016, 92, 22–37 CrossRef CAS PubMed
.
- R. P. Schwarzenbach, B. I. Escher, K. Fenner, T. B. Hofstetter, C. A. Johnson, U. von Gunten and B. Wehrli, Science, 2006, 313, 1072–1077 CrossRef CAS PubMed
.
- L. Wang, J. Li, Q. Jiang and L. Zhao, Dalton Trans., 2012, 41, 4544–4551 RSC
.
- X.-Y. Yu, R.-X. Xu, C. Gao, T. Luo, Y. Jia, J.-H. Liu and X.-J. Huang, ACS Appl. Mater. Interfaces, 2012, 4, 1954–1962 CAS
.
- F. Zhao, P. Severson, S. Pacheco, B. W. Futscher and W. T. Klimecki, Toxicol. Appl. Pharmacol., 2013, 271, 72–77 CrossRef CAS PubMed
.
- K. Dutta, P. Prasad and D. Sinha, Int. J. Hyg. Environ. Health, 2015, 218, 564–574 CrossRef CAS PubMed
.
- P. Kampalanonwat and P. Supaphol, ACS Appl. Mater. Interfaces, 2010, 2, 3619–3627 CAS
.
- N. Wu, H. Wei and L. Zhang, Environ. Sci. Technol., 2011, 46, 419–425 CrossRef PubMed
.
- X. Yang, X. Wang, X. Liu, Y. Zhang, W. Song, C. Shu, L. Jiang and C. Wang, J. Mater. Chem. A, 2013, 1, 8332 CAS
.
- X. Yang, X. Wang, Y. Feng, G. Zhang, T. Wang, W. Song, C. Shu, L. Jiang and C. Wang, J. Mater. Chem. A, 2013, 1, 473–477 CAS
.
- H. Lu, Z. Zhu, H. Zhang, J. Zhu, Y. Qiu, L. Zhu and S. Kuppers, ACS Appl. Mater. Interfaces, 2016, 8, 25343–25352 CAS
.
- E. Roy, S. Patra, R. Madhuri and P. K. Sharma, Chem. Eng. J., 2016, 304, 259–270 CrossRef CAS
.
- W. Yan, M. A. V. Ramos, B. E. Koel and W.-X. Zhang, J. Phys. Chem. C, 2012, 116, 5303–5311 CAS
.
- B. A. Manning, S. E. Fendorf and S. Goldberg, Environ. Sci. Technol., 1998, 32, 2383–2388 CrossRef CAS
.
- L. C. Jones, B. J. Lafferty and D. L. Sparks, Environ. Sci. Technol., 2012, 46, 6548–6555 CrossRef CAS PubMed
.
- C. Hu, H. Liu, G. Chen, W. Jefferson and J. Qu, Environ. Sci. Technol., 2012, 46, 6776–6782 CrossRef CAS PubMed
.
- I. K. Levy, M. Mizrahi, G. Ruano, G. Zampieri, F. G. Requejo and M. I. Litter, Environ. Sci. Technol., 2012, 46, 2299–2308 CrossRef CAS PubMed
.
- F. Zhang, J. Lan, Z. Zhao, Y. Yang, R. Tan and W. Song, J. Colloid Interface Sci., 2012, 387, 205–212 CrossRef CAS PubMed
.
- B. Wang, H. Wu, L. Yu, R. Xu, T. T. Lim and X. W. Lou, Adv. Mater., 2012, 24, 1111–1116 CrossRef CAS PubMed
.
- T. Wen, Z. Zhao, C. Shen, J. Li, X. Tan, A. Zeb, X. Wang and A. W. Xu, Sci. Rep., 2016, 6, 20920 CrossRef CAS PubMed
.
- J. Huang, Y. Cao, Z. Liu, Z. Deng, F. Tang and W. Wang, Chem. Eng. J., 2012, 180, 75–80 CrossRef CAS
.
- V. Rocher, J.-M. Siaugue, V. Cabuil and A. Bee, Water Res., 2008, 42, 1290–1298 CrossRef CAS PubMed
.
- D. Wang, R. Yu, N. Kumada and N. Kinomura, Chem. Mater., 1999, 11, 2008–2012 CrossRef CAS
.
- S. S. Thind, G. Wu and A. Chen, Appl. Catal., B, 2012, 111–112, 38–45 CrossRef CAS
.
- C. H. Kim, B.-H. Kim and K. S. Yang, Carbon, 2012, 50, 2472–2481 CrossRef CAS
.
- D. Wang, R. Yu, Y. Chen, N. Kumada, N. Kinomura and M. Takano, Solid State Ionics, 2004, 172, 101–104 CrossRef CAS
.
- T. Yang, M.-L. Chen, L.-H. Liu, J.-H. Wang and P. K. Dasgupta, Environ. Sci. Technol., 2012, 46, 2251–2256 CrossRef CAS PubMed
.
- A. K. Bhattacharya, T. K. Naiya, S. N. Mandal and S. K. Das, Chem. Eng. J., 2008, 137, 529–541 CAS
.
- J. Gong, T. Liu, X. Wang, X. Hu and L. Zhang, Environ. Sci. Technol., 2011, 45, 6181–6187 CrossRef CAS PubMed
.
- C.-Y. Cao, J. Qu, W.-S. Yan, J.-F. Zhu, Z.-Y. Wu and W.-G. Song, Langmuir, 2012, 28, 4573–4579 CrossRef CAS PubMed
.
- G.-S. Zhang, J.-H. Qu, H.-J. Liu, R.-P. Liu and G.-T. Li, Environ. Sci. Technol., 2007, 41, 4613–4619 CrossRef CAS PubMed
.
- S. Ouvrard, P. de Donato, M.-O. Simonnot, S. Begin, J. Ghanbaja, M. Alnot, Y.-B. Duval, F. Lhote, O. Barres and M. Sardin, Geochim. Cosmochim. Acta, 2005, 69, 2715–2724 CrossRef CAS
.
- R. Li, W. Yang, Y. Su, Q. Li, S. Gao and J.-K. Shang, J. Mater. Sci. Technol., 2014, 30, 949–953 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03439c |
| ‡ W. H., X. L. Y. contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |