Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancing the water repellency of wood surfaces by atmospheric pressure cold plasma deposition of fluorocarbon film
Pedro Henrique Gonzalez de Cademartori a,
Luc Staffordb,
Pierre Blanchet
a,
Luc Staffordb,
Pierre Blanchet c,
Washington Luiz Esteves Magalhães
c,
Washington Luiz Esteves Magalhães d and
Graciela Ines Bolzon de Muniz
d and
Graciela Ines Bolzon de Muniz *a
*a
aPrograma de Pós-Graduação em Engenharia Florestal, Universidade Federal do Paraná, 632 Lothário Meissner Avenue, 80210-170, Curitiba, Paraná, Brazil. E-mail: pedrocademartori@gmail.com; gbmunize@ufpr.br; Tel: +55-41-3360-5328 Tel: +55-41-88445311
bDépartement de Physique, Université de Montréal, H3C 3J7, Montréal, Québec, Canada. E-mail: luc.stafford@umontreal.ca
cNSERC Industrial Research Chair on Ecoresponsible Wood Construction, Wood and Forest Department, Laval University, G1V0A6, Quebec City, Quebec, Canada. E-mail: pierre.blanchet@sbf.ulaval.ca
dEmbrapa Florestas, Centro Nacional de Pesquisa de Florestas, 83411-000, Colombo, Paraná, Brazil. E-mail: washington.magalhaes@embrapa.br
First published on 5th June 2017
Abstract
In this study, fluorocarbon thin films were deposited on the surface of white spruce and Brazilian cedar woods via atmospheric pressure dielectric barrier discharge in the afterglow mode with octafluoropropane gas. A pre-treatment with oxygen plasma was introduced before the thin film deposition to determine if this plasma condition could increase the wood nano/micro roughness, helping to improve the hydrophobicity of the surface. The optimal conditions of water repellency for both woods were examined in terms of their resistance to aging in controlled conditions. The water contact angle measurements showed a hydrophobic surface with a stable angle for both woods. X-ray photoelectron spectroscopy reveals the presence of CF, CF2 and CF3 functional groups after the Ar/C3F8 plasma, especially for longer treatments. The open-air system of the plasma reactor leads to the simultaneous fluorination and incorporation of oxygen-containing groups. The pre-treatment of oxygen plasma increases the wood roughness; however, the chemical attachment of oxygen molecules is more significant, negatively affecting the degree of repellency of the plasma-coated woods. On the other hand, the fluorocarbon deposition increases the wood roughness and creates a hydrophobic surface simultaneously. Even with partial defluorination after aging, both plasma-coated woods retain a similar degree of water repellency, which partially avoid chemical reorganization of their surface due to environmental exposure.
Introduction
Wood is a sustainable and renewable material but its hygroscopic surface can limit its use for several applications, and thus requires repellency against polar and non-polar liquids. Expanding the functionalization of wood surfaces is receiving great interest to produce new materials with better properties, especially using methods based on materials science such as nanotechnology. Many techniques have been applied to change the wetting properties of the wood surface, most of which involve wet chemistry, such as hydrothermal processes,1 sol–gel processes2 and spraying of nanoparticles.3Among the techniques for surface modification, plasma treatment is extensively used to change the surface of this material. It is more interesting than the usual wet techniques because it is a dry and a clean process with little environmental impact. In certain cases, traditional chemical treatments are often expensive and can result in the leaching of toxic substances.4 Some characteristics of plasma treatment are often impossible to reproduce using common chemical methods. For example, specific components in the plasma discharge present a higher energy density than that by common chemical methods and plasma has a high concentration of charged chemical species, such as ions, electrons and metastables, which help to reduce the activation energy for chemical reactions.5,6 Moreover, plasma treatment can be used to change the surface roughness of materials by etching and introducing reactive groups on their surface without any modification of their bulk properties.7,8 Etching and chemical changes by the introduction of reactive groups on the surface and surface cleaning and crosslinking of near-surface molecules are considered the four major effects during plasma discharge which can act individually or in synergistically.9 These major effects drive the application of plasma treatments for different goals, such as antifouling properties,10 biocompatibility,11 superhydrophobicity,12 improving surface adhesion,13,14 oxygen barrier properties15 and antibacterial properties.16
The application of plasma in wood and wood-based materials involves two main goals, the improvement of surface adhesion17–21 and the deposition of low surface energy thin films to increase the barrier properties against polar and non-polar liquids and gases. The deposition of low surface energy films on the wood surface is relatively well understood in low-pressure plasma reactors from the many reports published, which mainly use siloxanes, fluorine and alkanes as precursors.8,22–26
On the other hand, in the last two decades there has been growing interest in thin film deposition via atmospheric pressure cold plasma technologies;27 however, unlike other natural and synthetic materials, this field of study has not been fully explored for wood and wood-based materials, especially using fluorine precursors. The few studies published in the literature focused on functionalization with hexamethyldisiloxane (HMDSO),28–30 ZnO–SiO2 coatings31 and other precursors, such as ethane, methane, ethylene, chlorotrifluoroethylene and hexafluoropropylene.32,33 At the same time, the importance of atmospheric pressure plasma technology is highlighted by the fact that is an interesting alternative for use in the wood industry due to both the generation of cold plasma, which means plasma discharge generated at room temperature, in open systems34 and the easy implementation in a continuous production line.35
The intrinsic characteristics of wood lead to strong aging effects mainly through the wood inactivation phenomenon. This phenomenon can induce changes in the chemical properties of the wood surface, thus reducing the plasma effect on the wood surface with time. Additionally, the plasma–wood interactions are complex because the plasma source produces active species that can provide a large number of chemical and physical changes in the main constituents presented in the wood surface, including cellulose, lignin, hemicelluloses and extractives.36 Depending on the plasma conditions applied and the initial conditions of the substrate, the surface energy of the wood increases or decreases.
In the case of dielectric barrier discharges (DBD), Levasseur et al.28 confirmed that the treatment of wood and textiles is a challenge compared to other conventional materials since the plasma deposition dynamics not only depends on the chemical structure of these polymers but also on their porous microstructure and the presence of impurities on their surface. As a matter of fact, wood surface treatment by inert or reactive gases is controversial since both hydrophobic and hydrophilic characteristics have been reported using the same precursor. For example, as expected, previous studies observed the hydrophilization of the wood surface by oxygen (O2) treatment, in some cases followed by an increase in roughness.37–39 On the other hand, Prégent et al.36 reported recently the hydrophobization of a wood surface treated by N2/O2 plasma in an afterglow system at atmospheric pressure due to the increase of neutral gas at a temperature of 75 °C, which led to similar conditions to wood heat treatment at temperatures lower than 150 °C. Levasseur et al.28 observed the time dependence of DBD treatment because short periods led to the deposition of an SiOx hydrophilic layer, whereas longer times (>10 min) resulted in a hydrophobic layer containing Si(CH3)3–O–Si(CH3)2, Si(CH3)3, and Si(CH3)2 functional groups.
In this context, this study investigates the deposition of fluorocarbon thin films with octafluoropropane (C3F8) on the surface of white spruce and Brazilian cedar woods via DBD afterglow plasma by simulating an industrial line and focusing on the optimal conditions for high water repellency. Moreover, a pre-treatment of O2 plasma is introduced to understand if the physical and/or chemical changes imposed by O2 plasma before thin film deposition can improve the hydrophobicity of the wood-coated surface. The optimal conditions are evaluated towards the resistance of plasma treated wood against aging in controlled conditions.
Material and methods
Wood samples preparation
White spruce (Picea glauca (Moench)) and Brazilian Cedar (Erisma uncinatum Warm.) woods were used as raw materials. The original boards were cut into small wood samples measuring 12.5 × 12.5 × 2 mm (length × width × thickness) in the tangential direction. Before the plasma treatments, the wood samples were stored under vacuum (10−6 torr) overnight to remove the outgassed gases and volatile organic compounds. Then, they were sanded with 180-grit sandpaper and cleaned with a compressed air flow to remove surface sawdust.DBD afterglow plasma treatments
Plasma treatments were performed in a planar DBD ATMOS reactor (Plasmionique, Canada) equipped with a conveyer system which simulates an industrial line. This device operates at atmospheric pressure with a variable alternating current generator connected to an amplifier for ignition and sustention of the discharge. The discharge was open between two metallic electrodes with area of 285 cm2 covered by quartz plates separated by a distance of 0.14 mm and the samples were placed in the flowing afterglow of the DBD. More details can be obtained in the report by Prégent et al.36The plasma treatments were conducted at 6 kHz under different conditions (Table 1) using argon (Ar) as the carrier gas for both O2 and C3F8 plasma discharges. The gases were mixed before entering the discharge zone and their flow was controlled by a mass flow system. The distance between the wood samples and the DBD outlet was set at 3 mm40 and the speed of the conveyer was set at 0.5 cm s−1. The low speed increased the plasma exposure time of the wood samples for each pass in the conveyer. The time of treatment was estimated according to the number of passes in the plasma afterglow discharge. One pass corresponds to approximately 0.33 s.
| Parameters | DBD treatment | |
|---|---|---|
| Ar/O2 | Ar/C3F8 | |
| a High argon flow rates were used to guarantee that the active species reached the wood surface.40 | ||
| Flow rate – precursor gas (sccm) | 20 | 5 |
| Peak-to-peak applied voltage (kV) | 12–14 | 10–11 |
| Number of passes (time of treatment) | 20 (∼6.6 s) and 60 (∼19, 8 s) | 20 (∼6.6 s), 100 (∼33 s) and 200 (∼66 s) |
| Argon flow (carrier gas, in sccm)a | 30 | 40 |
The plasma discharge characteristics (current–voltage, I–V) were measured using a high-voltage probe (Tektronix P6015A, USA) and recorded with an oscilloscope (Tektronix TDS 2012B, USA). The measurements were performed three times: in the first few seconds, in the middle and in the final discharge.
Contact angle measurements
The wettability of the untreated and plasma treated samples was measured with an Attention goniometer (Biolin Scientific, Sweden) using the sessile drop method at room temperature. The measurements were performed before and after plasma treatments on each sample, assuming the natural heterogeneity of the wood surface. Three deionized water (surface tension of 72.80 mN m−1) droplets with a 5 μL volume were dispensed on the surface of each wood sample. The water contact angle (WCA), the droplet volume and the baseline were recorded for 10 s on the tangential wood face and in the perpendicular direction to the fibers. Measurements with the non-polar solvent diiodomethane were also performed to determine the surface free energy based on the Wu theory (eqn (1)).41,42
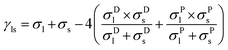 | (1) |
The WCA measurements were performed after 40 days of exposure in controlled conditions (temperature of 20 °C and 65% relative humidity) to evaluate the aging effect on the untreated and plasma treated wood surfaces. All the collected data were analyzed by descriptive statistics.
Profilometer measurements
The surface roughness of the untreated and plasma treated samples were analyzed with a non-contact 3D Contour GT-K1 profilometer from Bruker (USA). Measurements were performed in the VSI mode using a 5× objective lens and a green laser. The parameters Spk (reduced peak height), Sk (core roughness depth) and Svk (reduced valley depth) were determined in triplicate for each wood sample. The cut-off used to analyze the roughness parameters was 0.025 mm and the Gaussian regression filter was applied for the subsequent data collection. The collected data were analyzed by the analysis of variance (ANOVA) at 5% probability of error. When the null hypothesis was rejected (p < 0.05), the average values were compared using the Tukey HSD (honestly significant difference) test at 5% probability of error.XPS analysis
The elemental and chemical composition of the surface of the untreated and plasma treated wood samples were determined via X-ray photoelectron spectroscopy (Axis-Ultra, Kratos UK). General survey and high-resolution spectra (C 1s, O 1s and F 1s) were recorded using a monochromatic Al Kα X-ray source at 300 W with a neutralizer to avoid the electrostatic charge effect. Calculation of the apparent relative atomic concentrations and peak fitting were performed with the CasaXPS software assuming that the analyzed spot (approximately 800 μm × 400 μm × 5 nm) was homogeneous.Results and discussion
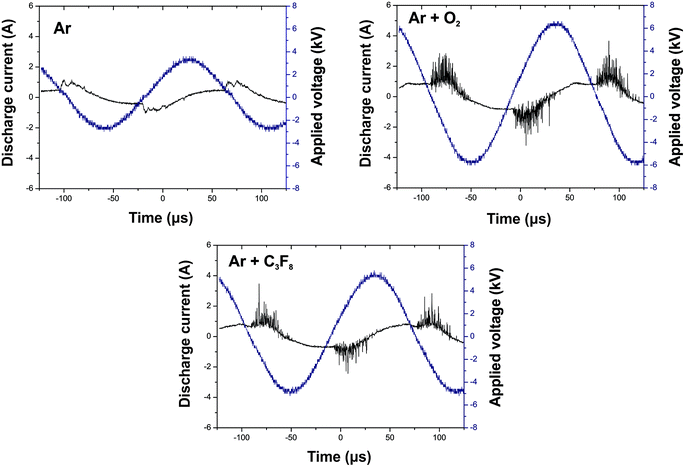
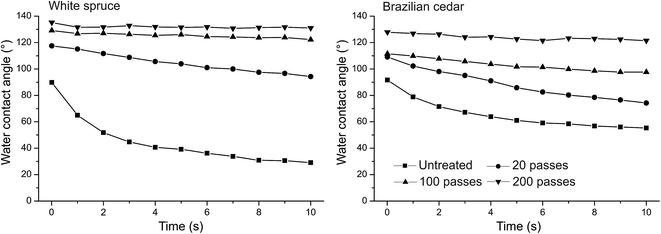
The current–voltage waveforms of the DBD afterglow plasma in Ar, Ar/O2 and Ar/C3F8 are given in Fig. 1. The discharge current for all DBD plasma treatments operates in the filamentary mode, which is characterized by multiple peaks with a duration of nanoseconds. The density and the amplitude of the spikes generated by the microdischarges can characterize the quality of the glow obtained.43 The number of microdischarges increased after the addition of both reactive O2 and fluoropolymer gases with large molecular weight, which disturbed the regime and caused a shift to a highly filamentary mode.The untreated wood samples for both species display hydrophilic behavior. The hydrophilicity of the untreated samples is most pronounced for white spruce wood (Fig. 2). After 2 s of droplet deposition, the WCA decreases to ∼50% and ∼28% for the white spruce and Brazilian cedar woods, respectively. During 10 s of measurement, both spreading and absorption phenomena can be seen in the untreated samples due to the decrease in the volume and baseline of the droplets (Table 2).
| Treatment | Volume (μL) | Baseline (mm) | ||
|---|---|---|---|---|
| 1 s | 10 s | 1 s | 10 s | |
| a WS = white spruce wood and BC = Brazilian cedar wood. *Baseline means the border between the droplet shape and the surface of the sample. | ||||
| WS untreated | 2.15 (0.48) | 0.81 (0.39) | 2.00 (0.26) | 2.50 (0.44) |
| WS 20 passes | 2.80 (0.29) | 1.71 (0.39) | 1.65 (0.10) | 1.79 (0.25) |
| WS 100 passes | 3.16 (0.24) | 2.80 (0.41) | 1.46 (0.21) | 1.53 (0.29) |
| WS 200 passes | 2.94 (0.29) | 2.85 (0.33) | 1.36 (0.16) | 1.35 (0.17) |
| BC untreated | 2.29 (0.39) | 1.55 (0.41) | 2.02 (0.26) | 2.41 (0.38) |
| BC 20 passes | 3.00 (0.21) | 2.29 (0.27) | 1.87 (0.14) | 2.35 (0.32) |
| BC 100 passes | 2.94 (0.23) | 2.37 (0.39) | 1.68 (0.13) | 1.92 (0.33) |
| BC 200 passes | 2.91 (0.30) | 2.78 (0.40) | 1.47 (0.16) | 1.56 (0.28) |
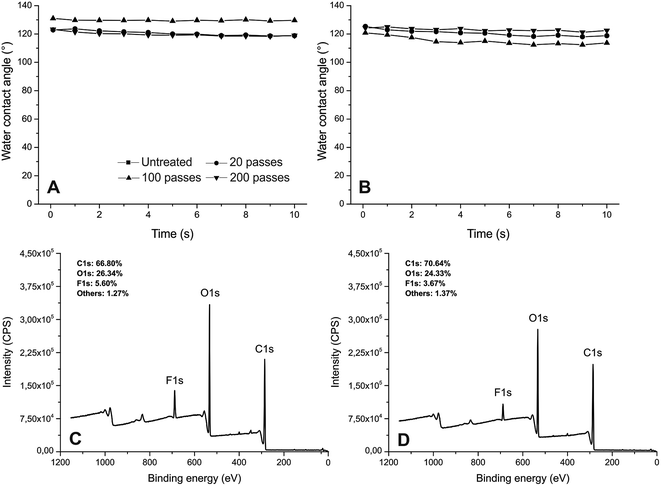
The Ar/C3F8 treatments in the DBD afterglow at atmospheric pressure increased the hydrophobicity of the woods surfaces, especially in the white spruce. The WCA showed “sticky” droplets on the surface, which indicates the absence of superhydrophobicity. The water repellency increased with an increase in the number of passes (i.e. time of treatment) under the plasma discharges. After 20 passes (= ∼6 s), the WCA of the white spruce wood increased from 90° to 117.4°, whereas the WCA of the Brazilian cedar wood increases in lower proportion (from 91.6° to 109°). The use of 100 passes (∼33 s) also resulted in a significant increment in the hydrophobicity for both woods, especially for the white spruce. However, the best results were found after 200 passes (∼66 s), which suggests that the DBD afterglow polymerized fluorocarbon film has sufficient fluorination. The WCA of the white spruce wood increased to 135.20°, whereas that of the Brazilian cedar reached 129.83°. This degree of fluorination significantly changed the surface free energy (SFE) of the woods. Based on the Wu calculation, the SFE decreased from 58 ± 4 mN m−1 to 21 ± 5 mN m−1 in the white spruce and from 53 ± 2 mN m−1 to 18 ± 5 mN m−1 in the Brazilian cedar after the Ar/C3F8 treatment with 200 passes. As expected, both the dispersive and polar parts decreased after the fluorination, where the latter reached close to zero for both the white spruce and Brazilian cedar woods.
The most pronounced characteristic for the longer treatments with Ar/C3F8 was the stable WCA. With an increase in the number of passes, the WCA remained practically stable for the white spruce wood without significant changes in both volume and baseline characteristics (Table 2). The same characteristic was observed for the Brazilian cedar to a lesser degree. Besides the fluorination on the surface, the better behavior of the white spruce could be related to the wood surface roughness, which will be discussed later. The difference in the WCA stabilization between 20, 100 and 200 passes may be related to the insufficient deposition of fluorocarbon-containing groups, which results in a higher affinity between wood surface and liquid water. According to a previous report,44 for post-discharge configurations, the low concentration of active species that reaches the surface results in lower deposition rates. Moreover, the presence of oxygen-containing groups due to the open-air system in the afterglow plasma reactor and the lower quantity of positive ions and metastable atoms and molecules45 could reduce the fluorocarbon deposition, as can be seen for 20 passes. The same unstable contact angle was observed by Liu et al. (2009) in plasma-coated paper with fluorocarbon films at atmospheric pressure.
The XPS analysis shows that the untreated wood samples are composed mainly of oxygen (19–20%) and carbon (77–79%) with varying percentages according to the wood species (Table 3). Some traces of contamination were detected mainly because the plasma discharges were performed at atmospheric pressure in an open system. The concentration of carbon decreased, whereas the concentration of oxygen increased after the Ar/C3F8 deposition at atmospheric pressure, regardless of the wood species and the number of passes used in the plasma treatment. The F/C and O/C ratios increased after the Ar/C3F8 plasma treatments for both 100 and 200 passes, which indicates fluorination and the incorporation of oxygen-containing groups at the same time, where the latter is due to the treatment at atmospheric pressure.
| Wood species | Passes-Ar/C3F8 | Atomic concentration (%) | Atomic ratio | ||||
|---|---|---|---|---|---|---|---|
| C 1s | F 1s | O 1s | Othera | F/C | O/C | ||
| a Many atoms in no significant percentage were considered as contaminants: Ca, Cl, Si, Al, N and S. | |||||||
| White spruce | Untreated | 78.31 | — | 19.46 | 2.18 | — | 0.248 |
| 100 | 68.97 | 7.56 | 22.12 | 1.36 | 0.109 | 0.320 | |
| 200 | 69.22 | 9.46 | 20.01 | 1.31 | 0.136 | 0.289 | |
| Brazilian cedar | Untreated | 77.06 | — | 19.37 | 3.38 | — | 0.251 |
| 100 | 68.84 | 7.67 | 22.03 | 1.45 | 0.111 | 0.320 | |
| 200 | 65.49 | 9.84 | 23.77 | 0.9 | 0.150 | 0.363 | |
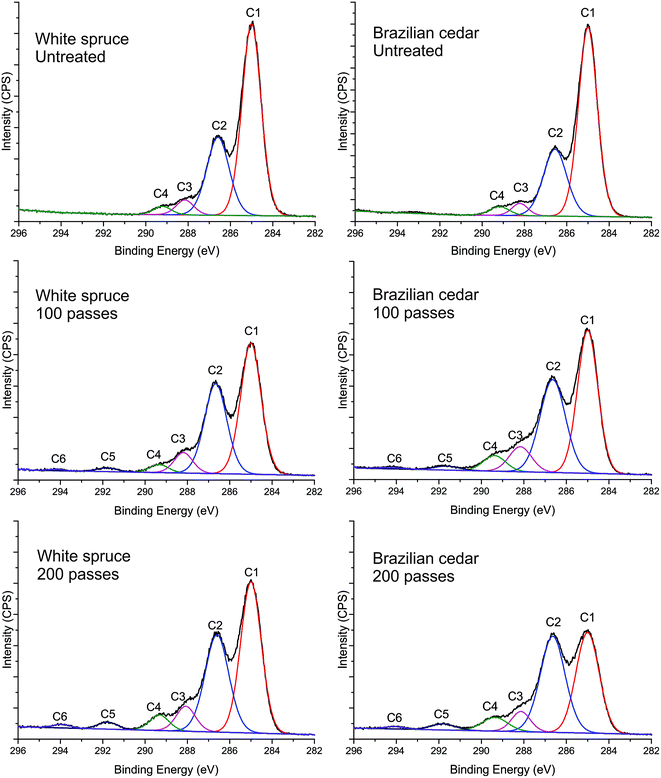
Fitting of the high-resolution C 1s spectra was performed to obtain better insight into the chemical changes resulting from the DBD afterglow plasma treatments (Fig. 3).
 | ||
| Fig. 3 High-resolution C 1s spectra of the woods before and after the Ar/C3F8 DBD afterglow plasma treatments. | ||
The C 1s spectra of the untreated samples of white spruce and Brazilian cedar wood were decomposed into four main peaks, C1 (C–C and C–H) at 285.0 eV, C2 (C–O) at 286.59 eV, C3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–C–O and C–CFn) at 288.15 eV and C4 (O
O, O–C–O and C–CFn) at 288.15 eV and C4 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O and –CF) at 289.22 eV. The fluorination with C3F8 for both 100 and 200 passes resulted in the addition of new functional groups to the wood surface, where a decrease in C–C/C–H groups and increase in oxygen-containing groups, especially ester (C2 peak) and carbonyl (C3 peak) groups were observed. This increase in C2 and C3 peaks is mainly attributed to the interaction of oxygen molecules during the plasma discharge since the treatments were performed at atmospheric pressure. The appearance of CF groups in the C 1s spectrum can be observed through two main peaks, C5 (–CF2) at 291.8 eV and C6 (–CF3) at 294.2 eV. Furthermore, the addition of CF groups can be observed on a lesser scale by the C–CFn and –CF groups at 288.15 and 289.22 eV. A higher percentage of CF2/CF3 results in higher hydrophobicity and higher water contact angles of the materials.46 Also, it should be highlighted that the C5 peak attributed to –CF2 groups is higher than C6 peak (–CF3). This higher proportion can be seen for the treatment with 100 passes and it increases with an increase in the treatment time (200 passes). This is in accord with the hydrophobic characteristic of the plasma-treated wood surfaces, including their high and stable WCA, as seen previously for the treatment with 200 passes in Fig. 2. Furthermore, the high-resolution F 1s spectra (Fig. 4) for both wood species show the presence of fluorinated groups at 685.2 eV, 687.7 eV and 689.2 eV, which may represent various C–F, CF2 and CF3 bonds with or without O.
C–O and –CF) at 289.22 eV. The fluorination with C3F8 for both 100 and 200 passes resulted in the addition of new functional groups to the wood surface, where a decrease in C–C/C–H groups and increase in oxygen-containing groups, especially ester (C2 peak) and carbonyl (C3 peak) groups were observed. This increase in C2 and C3 peaks is mainly attributed to the interaction of oxygen molecules during the plasma discharge since the treatments were performed at atmospheric pressure. The appearance of CF groups in the C 1s spectrum can be observed through two main peaks, C5 (–CF2) at 291.8 eV and C6 (–CF3) at 294.2 eV. Furthermore, the addition of CF groups can be observed on a lesser scale by the C–CFn and –CF groups at 288.15 and 289.22 eV. A higher percentage of CF2/CF3 results in higher hydrophobicity and higher water contact angles of the materials.46 Also, it should be highlighted that the C5 peak attributed to –CF2 groups is higher than C6 peak (–CF3). This higher proportion can be seen for the treatment with 100 passes and it increases with an increase in the treatment time (200 passes). This is in accord with the hydrophobic characteristic of the plasma-treated wood surfaces, including their high and stable WCA, as seen previously for the treatment with 200 passes in Fig. 2. Furthermore, the high-resolution F 1s spectra (Fig. 4) for both wood species show the presence of fluorinated groups at 685.2 eV, 687.7 eV and 689.2 eV, which may represent various C–F, CF2 and CF3 bonds with or without O.
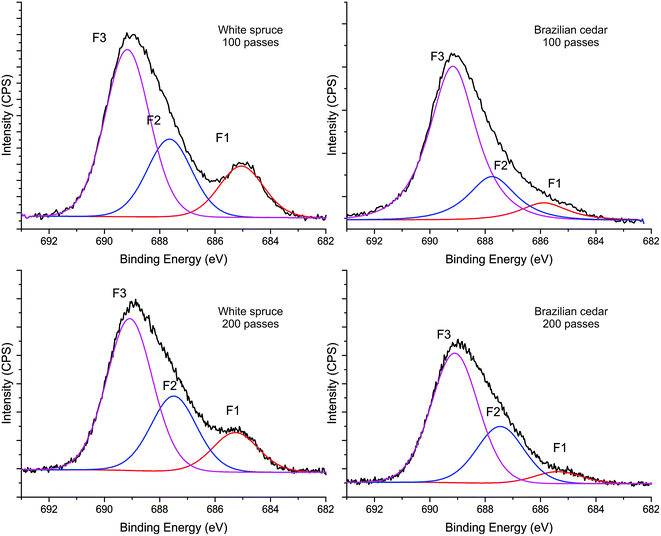 | ||
| Fig. 4 High-resolution F 1s spectra of the woods before and after the Ar/C3F8 DBD afterglow plasma treatments. | ||
Both the surface energy and the surface roughness of a material are important parameters that affect its degree of wettability.47 Previous studies showed that the positive effect of O2 plasma increases the nano/micro roughness of materials,48,49 which helps to generate a hydrophobic or superhydrophobic surface with the subsequent deposition of a low surface energy film.22,50,51 Therefore, this study demonstrates the effect of a pre-treatment with O2 plasma in a DBD afterglow system to improve the wood surface roughness by etching and tries to create a more hydrophobic surface with the subsequent fluorocarbon deposition.
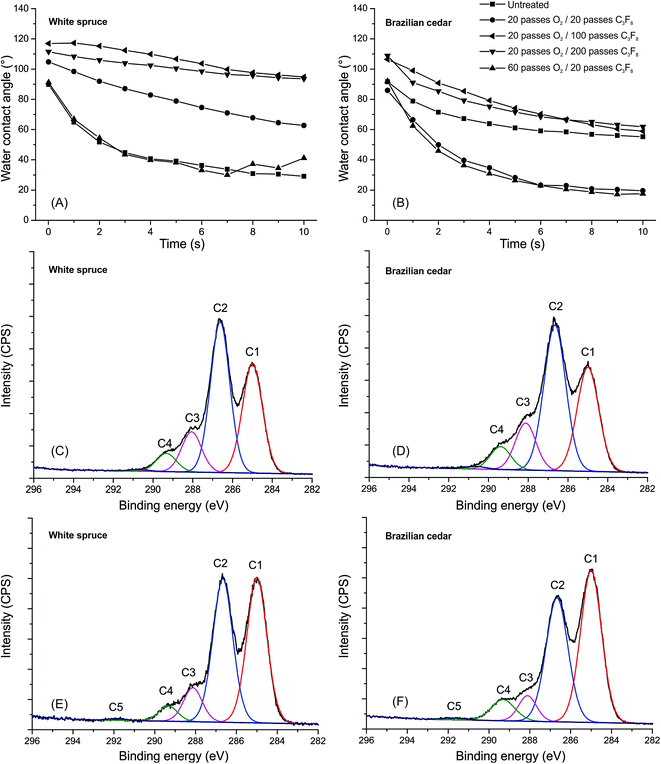
Nevertheless, the addition of a pre-treatment of O2 plasma before the Ar/C3F8 deposition resulted in a negative effect on the water repellency of the wood-coated surfaces (Fig. 5A and B), regardless of the treatment time applied. The WCA of the white spruce and Brazilian cedar coated-woods decreased with the addition of O2 plasma pre-treatment, both with 20 passes (∼6, 6 s) and 60 passes (∼19, 8 s). Besides the decrease in initial WCA, the O2 pre-treatment affected the stabilization of the samples. An O2 pre-treatment with 20 passes was enough to eliminate the WCA stabilization reached with 100 passes and mainly 200 passes of Ar/C3F8, which is very similar to the results obtained for the coating with 20 passes of Ar/C3F8, as displayed in Fig. 2. A larger number of passes in the O2 pre-treatment (60 passes) than in C3F8 deposition (20 passes) resulted in similar or higher wettability to the untreated wood surface, which reinforces the significant effect of the attachment of oxygen molecules on the surface.
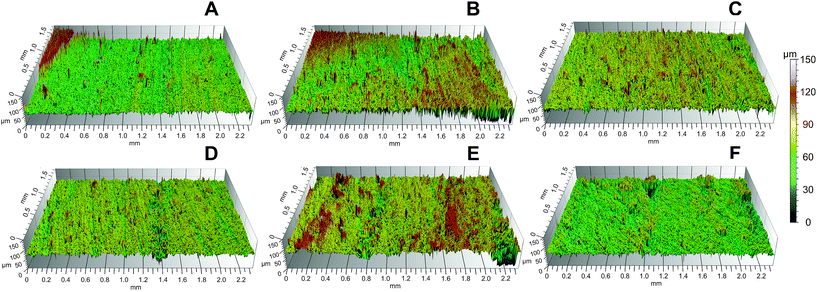
This suggests that the chemical attachment of oxygen molecules is more significant than the physical changes made by the O2 plasma etching on the wood surface. The average values of Spk, Sk and Svk indicate an increase in the surface roughness of both woods due to the physical etching, mainly with 60 passes O2 (Fig. 6 and Table 4). The Sk increased by 33% and 47% in Brazilian cedar and white spruce wood, respectively. These parameters are based on the Abbot-curve and are utilized to separate the processing roughness data from the natural surface irregularities,52 such as wood anatomical structures. For example, deep valleys are associated with the pores of the wood structure and should be removed before determining the wood roughness.53 Subsequently, this type of filter yields better conditions to show the specific changes made by the plasma treatments in this study and avoids misinterpretations. The attachment of oxygen molecules can be observed through the increase in the peaks related to oxygen-containing groups after the O2 plasma treatment (Fig. 5C and D) and after the O2/C3F8 plasma deposition (Fig. 5E and F). The most pronounced modification in both high-resolution C 1s spectra was the increment of C–O groups (C2 peak). According to Klarhöfer et al.,54 oxygen plasma treatments lead to the oxidation of lignin and reduction of cellulose, which are the major chemical components of wood. This incorporation of oxygen-containing groups may be due to the formation of highly unstable free radicals on wood surfaces and their interaction with oxygen from the atmosphere during the atmospheric pressure plasma discharges.45
| Plasma treatment | White spruce | Brazilian cedar | ||||
|---|---|---|---|---|---|---|
| Spk | Sk | Svk | Spk | Sk | Svk | |
| a Average values followed by the same letter in the column are not statistically different by the Tukey test (p ≥ 0.05). Values between parentheses correspond to the standard deviation. | ||||||
| Untreated | 4.69 (1.34) a | 6.07 (0.82) a | 6.77 (1.17) a | 4.30 (1.07) a | 5.13 (0.59) a | 5.51 (0.52) a |
| 20 passes Ar | 5.33 (1.15) ab | 6.82 (0.51) ab | 8.23 (1.17) bc | 4.43 (1.13) ab | 5.27 (0.43) a | 6.36 (0.58) ab |
| 20 passes O2 | 4.54 (1.24) a | 6.14 (1.06) a | 6.74 (1.42) ab | 5.60 (0.75) bc | 6.11 (0.61) ab | 6.33 (0.85) ab |
| 60 passes O2 | 6.10 (1.31) b | 8.91 (1.01) c | 6.98 (3.00) ab | 6.79 (1.23) c | 6.85 (0.59) b | 6.79 (1.52) b |
| 100 passes C3F8 | 6.21 (0.86) b | 8.28 (0.82) bc | 9.15 (1.47) c | 5.17 (1.45) ab | 5.83 (1.07) ab | 6.13 (1.78) ab |
| 200 passes C3F8 | 5.34 (1.09) ab | 7.87 (1.46) bc | 7.79 (1.22) abc | 4.30 (0.76) a | 5.38 (0.51) a | 5.97 (0.56) ab |
The addition of O2 in the gas phase during the plasma treatment at atmospheric pressure and the absence of direct contact between the substrate and the afterglow plasma discharge quench the formation of surface free radicals on the wood surface.36,45 Due to this, even with an increase in wood surface roughness, the attachment of oxygen molecules (as seen in the XPS patterns in Fig. 5) tends to decrease the quantity of chemically active sites available on the wood surface, which are mainly C–C and C–H groups, for the following fluorocarbon deposition. This leads to poor surface conditions for the fluorocarbon deposition, which results in easier interaction between the polar groups on the wood surface and water.
On the other hand, the fluorocarbon deposition by Ar/C3F8 plasma can change the surface roughness (Table 4). These changes are most evident in the white spruce wood. The Sk parameter increased by 23–27% after the C3F8 deposition, which suggests that fluorine molecules can etch the wood surface at the same time that the fluorocarbon radicals form the hydrophobic film, as previously observed by Hubert et al.35 for Ar–C6F14 plasma deposition at atmospheric pressure. In the case of the white spruce wood, the higher improvement in its roughness may partially explain its higher WCA after the C3F8 deposition, regardless of the quantity of fluorocarbon radicals attached on its wood surface. Based on the negligent effect of ion bombardment at atmospheric pressure, Fanelli et al.55 explained and proved this etching ability of fluoropolymers, which can be called deposition-etching competition.
Considering the best treatments to enhance the water repellency of white spruce and Brazilian cedar woods, the aging effect was investigated (Fig. 7) after exposure in controlled conditions (temperature of 20 °C and 65% relative humidity).
After 40 days of exposure, the hydrophobic character of both wood species slightly changed (Fig. 7A and B). The wood species treated with 100 or 200 passes maintained a similar WCA in the range of 120–140°, which shows the low activity of the wood inactivation phenomenon. On the other hand, the exposure for 40 days resulted in a more hydrophobic surface for the wood treated with 20 passes, which is mainly due to the higher stability of the WCA with the time. This suggests that the insufficient fluorination in short treatments or the absence of fluorine-containing groups in the untreated wood favors the mechanisms of wood inactivation, thus leading to an increase in surface hydrophobicity. Among the many physical and chemical factors, this phenomenon of inactivation can occur due to the surface oxidation, modification of surface hydroxyl bonding sites and/or the migration of extractives on the wood surface.56 Free hydroxyl groups on the cell wall surfaces regulate the level of wettability and glueability of wood. The number of free hydroxyl groups tends to decrease during wood exposure mainly because the adsorption of polar molecules by the wood surface, such as water and volatile organic compounds,57 which results in a less reactive and a more hydrophobic surface. On the other hand, the migration of extractives to the wood surface is controversial in the literature, since a previous study confirmed the influence of this factor on the wettability and/or the reactivity of wood,58 whereas others stated the absence of a significant influence of extractives using wettability, extractive content, fracture and shear strength tests.59
As can be seen in Fig. 7C and D, the thin films deposited on the wood surface present partial defluorination, which occurs mainly because of environmental exposure. Nevertheless, this lower quantity of fluorine-containing groups could also be only in a specific point measured on the wood surface. Even with this partial defluorination, the wood surface of both species retained similar characteristics of water repellency. Therefore, unlike the untreated wood, it is believed that fluorocarbon films deposited on the wood surface may have good barrier properties to avoid or reduce the entire chemical reorganization of the surface during exposure in the environment, especially the potential migration of extractives and/or the accessibility of hydroxyl groups.
Conclusions
This study focused on obtaining a wood surface with higher water repellency via atmospheric pressure plasma deposition of fluorocarbon thin films, and investigated if a pre-treatment of O2 plasma can improve the water repellency of the wood-coated surface. A low surface energy layer was achieved on the wood surface by C3F8 deposition in a DBD afterglow plasma system, which simulated an industrial line. Upon increasing the number of passes, the wood samples became more hydrophobic with a stable water contact angle, mainly for the white spruce. This better water repellency is due to the higher presence of CF functional groups on the surface. The addition of a pre-treatment of O2 did not help to increase the degree of wood surface hydrophobicity since the attachment of oxygen molecules on the surface superimposes the physical changes made by the O2 plasma etching. The fluorocarbon deposition was better suited to increase the wood roughness for both white spruce and Brazilian cedar wood species, which caused the surfaces to be more hydrophobic due to the simultaneous etching and chemical deposition. Furthermore, the fluorocarbon deposition results in a wood surface resistant to aging, especially for longer treatments (>100 passes). The most pronounced improvement in roughness in the white spruce wood was mandatory for a better degree of water repellency since both woods presented a similar quantity of fluorocarbon deposited on their surface.Acknowledgements
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process 202617/2015-7), the Canadian International Scholarship Emerging Leaders in the Americas Program (ELAP) and the Natural Sciences and Engineering Research Council of Canada for the financial support through its IRC and CRD programs (IRCPJ 461745-12 and RDCPJ 445200-12) as well as the industrial partners of the NSERC industrial chair on eco-responsible wood construction (CIRCERB) and FPInnovations for access to the plasma reactor.References
- L. Gao, S. Xiao, W. Gan, X. Zhan and J. Li, RSC Adv., 2015, 5, 98203–98208 RSC.
- A. Kumar, P. Ryparová, A. S. Škapin, M. Humar, M. Pavlič, J. Tywoniak, P. Hajek, J. Žigon and M. Petrič, Cellulose, 2016, 23, 3249–3263 CrossRef CAS.
- Z. Chu and S. Seeger, RSC Adv., 2015, 5, 21999–22004 RSC.
- Z. Tang, L. Xie, D. W. Hess and V. Breedveld, Wood Sci. Technol., 2017, 51, 97–113 CrossRef CAS.
- A. Fridman, A. Chirokov and A. Gutsol, J. Phys. D: Appl. Phys., 2005, 38, R1 CrossRef CAS.
- P. Attri, B. Arora and E. H. Choi, RSC Adv., 2013, 3, 12540–12567 RSC.
- R. Morent, N. De Geyter, J. Verschuren, K. De Clerck, P. Kiekens and C. Leys, Surf. Coat. Technol., 2008, 202, 3427–3449 CrossRef CAS.
- S. Zanini, C. Riccardi, M. Orlandi, V. Fornara, M. Colombini, D. Donato, S. Legnaioli and V. Palleschi, Wood Sci. Technol., 2008, 42, 149–160 CrossRef CAS.
- R. Shishoo, Plasma technologies for textiles, CRC Press LLC, USA, 2007 Search PubMed.
- M. J. Perez-Roldan, D. Debarnot and F. Poncin-Epaillard, RSC Adv., 2014, 4, 64006–64013 RSC.
- S.-C. Jung, K. Lee and B.-H. Kim, Thin Solid Films, 2012, 521, 150–154 CrossRef CAS.
- H.-R. Jiang and D.-C. Chan, Appl. Phys. Lett., 2016, 108, 171603 CrossRef.
- M. R. Chashmejahanbin, A. Salimi and A. Ershad Langroudi, Int. J. Adhes. Adhes., 2014, 49, 44–50 CrossRef CAS.
- H. Peng, G. Zheng, Y. Sun and R. Wang, RSC Adv., 2015, 5, 78172–78179 RSC.
- N. Tenn, N. Follain, K. Fatyeyeva, F. Poncin-Epaillard, C. Labrugere and S. Marais, RSC Adv., 2014, 4, 5626–5637 RSC.
- Y. de Rancourt, B. Couturaud, A. Mas and J. J. Robin, J. Colloid Interface Sci., 2013, 402, 320–326 CrossRef CAS PubMed.
- P. H. G. Cademartori, G. I. B. Muniz and W. L. E. Magalhães, Holzforschung, 2015, 69, 187 Search PubMed.
- B. Hünnekens, F. Peters, G. Avramidis, A. Krause, H. Militz and W. Viöl, J. Appl. Polym. Sci., 2016, 133, 43376 CrossRef.
- I. Novák, A. Popelka, Z. Špitalský, M. Mičušík, M. Omastová, M. Valentin, J. Sedliačik, I. Janigová, A. Kleinová and M. Šlouf, Vacuum, 2015, 119, 88–94 CrossRef.
- P. Král, J. Ráhel’, M. Stupavská, J. Šrajer, P. Klímek, P. Mishra and R. Wimmer, Wood Sci. Technol., 2015, 49, 319–330 CrossRef.
- D. Altgen, M. Bellmann, R. Wascher, W. VioL and C. Mai, Eur. J. Wood Wood Prod., 2015, 73, 219–223 CrossRef CAS.
- L. Xie, Z. Tang, L. Jiang, V. Breedveld and D. W. Hess, Surf. Coat. Technol., 2015, 281, 125–132 CrossRef CAS.
- B. Poaty, B. Riedl, P. Blanchet, V. Blanchard and L. Stafford, Wood Sci. Technol., 2013, 47, 411–422 CrossRef CAS.
- H. T. Sahin, Appl. Surf. Sci., 2013, 265, 564–569 CrossRef CAS.
- W. L. E. Magalhães and M. F. d. Souza, Surf. Coat. Technol., 2002, 155, 11–15 CrossRef.
- A. R. Denes, M. A. Tshabalala, R. Rowell, F. Denes and R. A. Young, Holzforschung, 1999, 53, 318–326 CAS.
- F. Fanelli and F. Fracassi, Plasma Chem. Plasma Process., 2014, 34, 473–487 CrossRef CAS.
- O. Levasseur, L. Stafford, N. Gherardi, N. Naudé, E. Beche, J. Esvan, P. Blanchet, B. Riedl and A. Sarkissian, Surf. Coat. Technol., 2013, 234, 42–47 CrossRef CAS.
- O. Levasseur, L. Stafford, N. Gherardi, N. Naudé, V. Blanchard, P. Blanchet, B. Riedl and A. Sarkissian, Plasma Processes Polym., 2012, 9, 1168–1175 CrossRef CAS.
- G. Avramidis, E. Hauswald, A. Lyapin, H. Militz, W. Viöl and A. Wolkenhauer, Wood Mater. Sci. Eng., 2009, 4, 52–60 CrossRef CAS.
- J. Profili, O. Levasseur, A. Koronai, L. Stafford and N. Gherardi, Surf. Coat. Technol., 2017, 309, 729–737 CrossRef CAS.
- M. Bente, G. Avramidis, S. Förster, E. G. Rohwer and W. Viöl, Holz Roh- Werkst., 2004, 62, 157–163 CrossRef CAS.
- G. Toriz, M. G. Gutiérrez, V. González-Alvarez, A. Wendel, P. Gatenholm and A. d. J. Martínez-Gómez, J. Adhes. Sci. Technol., 2008, 22, 2059–2078 CrossRef CAS.
- F. Busnel, V. Blanchard, J. Prégent, L. Stafford, B. Riedl, P. Blanchet and A. Sarkissian, J. Adhes. Sci. Technol., 2010, 24, 1401–1413 CrossRef.
- J. Hubert, N. Vandencasteele, J. Mertens, P. Viville, T. Dufour, C. Barroo, T. Visart de Bocarmé, R. Lazzaroni and F. Reniers, Plasma Processes Polym., 2015, 12, 1174–1185 CrossRef CAS.
- J. Prégent, L. Vandsburger, V. Blanchard, P. Blanchet, B. Riedl, A. Sarkissian and L. Stafford, Cellulose, 2015, 22, 3397–3408 CrossRef.
- L. Tang, R. Zhang, X. Zhou, M. Pan, M. Chen, X. Yang, P. Zhou and Z. Chen, BioResources, 2012, 7, 3327–3339 CrossRef.
- M. N. Acda, E. E. Devera, R. J. Cabangon and H. J. Ramos, Int. J. Adhes. Adhes., 2012, 32, 70–75 CAS.
- L. Tang, R. Zhang, X. Wang, X. Yang and X. Zhou, Journal, 2015, 69, 193 CAS.
- B. Riedl, C. Angel, J. Prégent, P. Blanchet and L. Stafford, BioResources, 2014, 9, 4908–4923 CrossRef CAS.
- S. Wu, J. Polym. Sci., Part C: Polym. Symp., 1971, 34, 19–30 Search PubMed.
- S. Wu, J. Adhes., 1973, 5, 39–55 CrossRef CAS.
- K. K. Samanta, A. G. Joshi, M. Jassal and A. K. Agrawal, Surf. Coat. Technol., 2012, 213, 65–76 CrossRef CAS.
- M. Moreno-Couranjou, N. D. Boscher, D. Duday, R. Maurau, E. Lecoq and P. Choquet, in Atmospheric Pressure Plasma Treatment of Polymers: Relevance to Adhesion, ed. M. Thomas and K. L. Mittal, Wiley-Scrivener, 2013, vol. 1 Search PubMed.
- J.-M. Hardy, O. Levasseur, M. Vlad, L. Stafford and B. Riedl, Appl. Surf. Sci., 2015, 359, 137–142 CrossRef CAS.
- S. R. Coulson, I. S. Woodward, J. P. S. Badyal, S. A. Brewer and C. Willis, Chem. Mater., 2000, 12, 2031–2038 CrossRef CAS.
- A. Quade, K. Schröder, A. Ohl and K.-D. Weltmann, Plasma Processes Polym., 2011, 8, 1165–1173 CrossRef CAS.
- Z. Liu, P. Chen, X. Zhang, Q. Yu, K. Ma and Z. Ding, Appl. Surf. Sci., 2013, 283, 38–45 CrossRef CAS.
- D. Liu, P. Chen, J. Mu, Q. Yu and C. Lu, Appl. Surf. Sci., 2011, 257, 6935–6940 CrossRef CAS.
- M. N. Mirvakili, S. G. Hatzikiriakos and P. Englezos, ACS Appl. Mater. Interfaces, 2013, 5, 9057–9066 CAS.
- L. Li, V. Breedveld and D. W. Hess, ACS Appl. Mater. Interfaces, 2013, 5, 5381–5386 CAS.
- L. Gurau, Doctoral thesis, Brunel University, 2004.
- Y. Fujiwara, Y. Fujii, Y. Sawada and S. Okumura, J. Wood Sci., 2004, 50, 35–40 CrossRef.
- L. Klarhöfer, W. Viöl and W. Maus-Friedrichs, Journal, 2010, 64, 331 Search PubMed.
- F. Fanelli, F. Fracassi and R. D'Agostino, Surf. Coat. Technol., 2010, 204, 1779–1784 CrossRef CAS.
- A. W. Christiansen, Wood Fiber Sci., 1991, 23, 69–84 CAS.
- C. Piao, J. E. Winandy and T. F. Shupe, Wood Fiber Sci., 2010, 42, 490–510 CAS.
- P. Nzokou and D. P. Kamdem, Wood Fiber Sci., 2004, 36, 483–492 CAS.
- R. M. Nussbaum and M. Sterley, Wood Fiber Sci., 2002, 34, 57–71 CAS.
| This journal is © The Royal Society of Chemistry 2017 |