Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Coating of Pd/C catalysts with Lewis-acidic ionic liquids and liquid coordination complexes – SCILL induced activity enhancement in arene hydrogenation†
Martin Lijewskia,
James M. Hoggb,
Małgorzata Swadźba-Kwaśny*b,
Peter Wasserscheida and
Marco Haumann *a
*a
aFriedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Lehrstuhl für Chemische Reaktionstechnik (CRT), Egerlandstr. 3, 91058 Erlangen, Germany. E-mail: marco.haumann@fau.de
bQueen's University Belfast, School of Chemistry and Chemical Engineering, David Keir Building, Stranmillis Road, Belfast, BT9 5AG, Northern Ireland, UK. E-mail: m.swadzba-kwasny@qub.ac.uk
First published on 23rd May 2017
Abstract
New solid catalysts with ionic liquid layer (SCILLs) were prepared by the coating of heterogeneous Pd/C catalysts with a thin film of acidic liquid: a known chloroaluminate ionic liquid (IL), [BMIM]Cl/AlCl3, and a recently developed liquid coordination complex (LCC), urea/AlCl3. Both coatings improved the activity of Pd/C catalyst against toluene hydrogenation; at lower temperatures (40 °C) the IL-SCILL performed better, whereas at 80 °C the LCC-SCILL gave higher conversion. A thin film of ionic liquid proved to be sufficient to increase activity, while larger volumes of ionic liquid resulted in a decrease in activity due to pore blocking and hence mass transport limitations. Kinetic investigations in batch mode revealed a zero order with respect to toluene concentration, a first order in hydrogen pressure, and activation energy of 64.7 kJ mol−1 for the Pd/C catalyst. The SCILL catalysts had slightly lower values of 53.1 kJ mol−1 for [BMIM]Cl/AlCl3 and 61.4 kJ mol−1 for urea/AlCl3, respectively.
Introduction
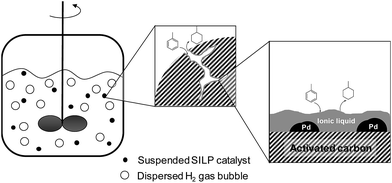
The hydrogenation of aromatic compounds is of major importance for the chemical industry, leading to a plethora of important intermediates and products.1 Amongst them, the total hydrogenation of benzene to cyclohexane is a large-scale application for the production of adipic acid, an important nylon precursor.2 In addition, total hydrogenation of aromatic hydrocarbons allows for efficient storage of hydrogen in liquid organic hydrogen carriers (LOHC).3The majority of heterogeneous hydrogenation catalysts are based on platinum group metals.4 Due to the aromatic nature of the arene compounds, hydrogenation conditions are rather harsh, including high pressures and elevated temperatures. Only a few systems are known that operate at moderate pressures of hydrogen around 10 bar.5 While Pd on activated carbon (Pd/C) shows excellent activation of molecular hydrogen, its hydrogenation activity for aromatics is poor.6 In benzene hydrogenation, hydrogenation activity decreases in the order Rh > Ru > Pt > Ni > Pd.4 In 2008, Song and co-workers demonstrated that the addition of the Lewis-acidic ionic liquid [BMIM]Cl/AlCl3 (xAlCl3 = 0.67) to a finely dispersed Pd/C resulted in full hydrogenation of benzene, naphthalene, anthracene and naphthacene under very mild conditions (room temperature, hydrogen pressure 1–3 bar).7 These authors proposed that, in their batch reaction system, the applied Lewis acidic chloroaluminate ionic liquid activated the aromatic ring as depicted in Fig. 1.
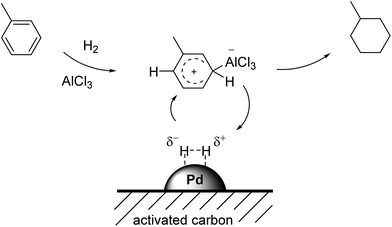 | ||
| Fig. 1 Proposed collaborative mechanism between the Lewis-acidic ionic liquids and the Pd/C catalyst for toluene hydrogenation. Adapted from ref. 8. | ||
It is interesting to note that the ionic liquid was diluted in the work by Song in 1,2-dichloroethane so it was not present directly at the Pd but rather a part of the reaction mixture. Still the observed activation effect was quite significant. Unfortunately, the high amounts of hydrolytically labile ionic liquid used in this work make an industrial relevant scale-up problematic.
The dispersion of thin films of ionic liquids on supports results in supported ionic liquid phase (SILP) materials.8 In case that the support itself is a heterogeneous catalyst, so-called solid catalysts with ionic liquid layer (SCILL) materials are obtained.9,10 In both cases, efficient utilization of the ionic liquid is achieved. In the case of SCILL catalysis, the role of the ionic liquid can be the modification of the catalyst surface, e.g. by blocking active but less selective sites.11 In addition, the ionic liquid can influence the local concentrations on the surface by its solubility properties.
Liquid coordination complexes (LCCs) are a recently developed class of “ionic liquid-like” Lewis acids, synthesized by the addition of organic donor molecules, such as urea (Ur), to a Lewis acidic metal halide, typically AlCl3 or GaCl3.12 They consist of equilibrated mixtures of anionic, cationic and neutral complexes as shown in Scheme 1. LCCs have recently been shown to catalyze oligomerization of 1-decene, Friedel–Crafts alkylation and Diels–Alder cycloaddition.13
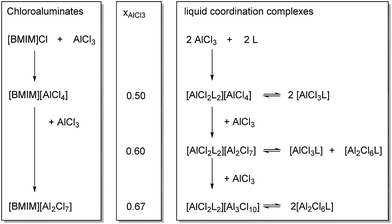 | ||
| Scheme 1 Species in chloroaluminate ionic liquids (left) and liquid coordination complexes LCC (right) at various aluminium chloride loadings (middle); in this work L = urea, CH4N2O. | ||
In this work, we have utilized both chloroaluminate ionic liquids and LCCs as coating materials for the Pd/C catalyzed hydrogenation of toluene (see Fig. 2). In both cases, the coating introduces Lewis acidity, aiming at the activation of the aromatic ring structure in accordance with Fig. 1. We compared the performance of the coated catalysts with the native Pd/C catalyst and varied reaction parameters like temperature, pressure and toluene concentration.
Experimental
Materials
Urea (99.5%), 1-butyl-3-methylimidazolium chloride, [BMIM]Cl (99.5%), AlCl3 (99.999%) and heterogeneous catalyst (10 wt% Pd/C) were purchased from Sigma Aldrich. All chemicals were dried under vacuum (<1 mbar, 115 °C, 12 h) and stored in a glovebox prior to use. Cyclohexane (solvent), toluene (substrate) and decalin (internal standard) were constantly dried over activated 4 Å molecular sieves. Hydrogen (99.99990%) and argon (99.99990%) were purchased from Linde and were used as received.Catalyst preparation
For the Lewis acidic ionic liquid, [BMIM]Cl and AlCl3 were mixed in the desired molar ratio and stirred overnight until a brownish ionic liquid was obtained. For the LCC preparation, AlCl3 was added stepwise to urea, to achieve the desired molar ratio (xAlCl3 = 0.60) and heated (60 °C, 3 h) until a homogenous liquid was obtained. In order to prepare the Pd-SCILL catalyst, the desired amount of Lewis acidic ionic liquid (13 wt% loading) or LCC (10 wt% loading) was added to a round bottom flask with the heterogeneous catalyst Pd/C (10 wt% Pd) and the solvent cyclohexane (7 mL). The solvent was slowly removed using a rotary evaporator (80 °C, 600 mbar). All steps were conducted under inert gas atmosphere. The catalyst was then dried overnight under vacuum (<1 mbar) at 115 °C.Hydrogenation experiments
All hydrogenation reactions were carried out in a 300 mL Hastelloy C stainless steel Parr autoclave equipped with hydrogen and argon supply. Details of the reactor setup can be found in the ESI.† For a typical experiment, 70 g of cyclohexane (100 mL), 2.3064 g of toluene (25 mmol) and 0.5766 g of decalin (4 mmol) were put in the reactor, which was stored in an oven at 65 °C. The reactor was then purged with argon and the SCILL/supported LCC (1.065 g of heterogeneous catalyst, i.e. 1 mmol Pd, and 0.1403 g of Lewis acidic IL or 0.1102 g of LCC) were quickly added from the round bottom flask to the reactor. The autoclave was sealed and again purged three times with argon to avoid air and moisture residues. Under stirring at 1000 rpm the reactor was subsequently heated to 60 °C. After that the reactor was pressurized with 15 bar of hydrogen pressure. This marked the starting point of the reaction. The reaction was carried out for 1 h and samples were taken at regular time intervals. All data evaluation was performed with offline GC (Varian GC 3900 with column Varian CP Sil PONA CB). No by-products were detected.Results and discussion
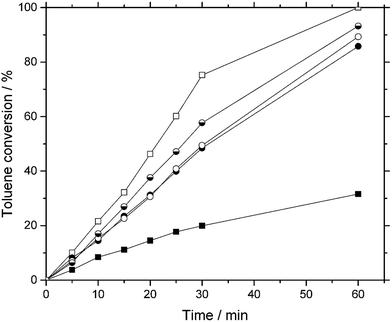
In a first set of experiments, the native Pd/C was tested in the hydrogenation of toluene at 60 °C and 15 bar hydrogen pressure. After 60 min, 30% conversion was obtained (see Fig. 3), while full conversion was achieved after more than 270 min. In agreement with the results from the group of Song, the addition of 330 mg pure AlCl3 to the reaction mixture increased the activity significantly, yielding full conversion after 120 min. Methylcyclohexane was the only product of hydrogenation, and no intermediates were detected in the samples at any times.The same activity was observed for a Pd-SCILL catalyst, but a much smaller amount of 140 mg [BMIM]Cl/AlCl3 coating (Pd-SCILL-1) was required, containing only 12.5% of AlCl3 compared to the addition of pure AlCl3. Three different runs with freshly prepared Pd-SCILL-1 catalyst gave nearly identical conversion profiles (Fig. 3). In an additional experiment, the same AlCl3 content of 13% was adjusted when bulk [BMIM]Cl/AlCl3 was added to the system. Here, a much lower activity, closely resembling the one of the uncoated catalyst, was observed (see Fig. S3 in ESI† for details). This indicates that the local presence of the acidic ionic liquid next to the Pd is better, a fact that can be understood by the proposed mechanism shown in Fig. 1. Activation of the aromatic ring and consecutive hydrogenation are cooperative effects that have to happen in close proximity on the catalyst surface.
For efficient hydrogenation, only a thin film of chloroaluminate ionic liquid seems to be necessary. At ionic liquid loadings of 6.5 and 19.5 wt% the same high activity was observed. When the pore system of the Pd/C was filled with 52 wt% of ionic liquid, severe loss in activity with only 25% conversion was observed, a value even lower than the 30% conversion for the uncoated Pd/C. This effect can be explained by mass transport limitation due to the ionic liquid blocking the transport pores of the catalyst.14,15 The coating of the Pd/C with approx. 6.5 wt% ionic liquid, corresponding to a pore filling degree of 5 vol%, resulted in a significant decrease of surface area: from 920 to 393 m2 g−1 (see Table S1 in ESI† for details). At the same time, the average pore diameter decreased from 4.8 to 2.9 nm, indicative of ionic liquid filling the micropores of the support. It appears that in the Pd-SCILL-1 system using powdered activated carbon as support, there is sufficient number of transport pores to facilitate toluene diffusion into the catalyst.
The acidity of the coating obviously is the crucial factor for the hydrogenation activity. The acidity of chloroaluminate ionic liquid was adjusted by varying the ratio between [BMIM]Cl and AlCl3 (the xAlCl3 value), as shown in Table 1. When no AlCl3 was added to [BMIM]Cl (Lewis basic composition), the Pd-SCILL catalyst gave no conversion within 300 min. Here, the ionic liquid's anion appeared to simply block sites at Pd.
| Catalyst | xAlCl3/— | X30 min/% | X60 min/% |
|---|---|---|---|
| a Reaction conditions: T = 60 °C, phydrogen = 15 bar, ctoluene = 0.25 mol L−1, 100 mL cyclohexane as solvent, mcat = 1 g, wPd = 10 wt%, stirring speed at 1000 min−1, ionic liquid coating at 13 wt%. | |||
| — | — | 20.0 | 31.6 |
| [BMIM]Cl | — | <1 | <1 |
| [BMIM]Cl/AlCl3 | 0.33 | 22.1 | 33.8 |
| [BMIM]Cl/AlCl3 | 0.50 | 44.3 | 78.2 |
| [BMIM]Cl/AlCl3 | 0.55 | 54.0 | 84.5 |
| [BMIM]Cl/AlCl3 | 0.60 | 39.7 | 82.9 |
| [BMIM]Cl/AlCl3 | 0.67 | 48.4 | 85.8 |
When sub-stoichiometric amount of AlCl3 was used (xAlCl3 = 0.33), therefore creating a Lewis basic coating, but with some [AlCl4]− present, the activity resembled that of the uncoated Pd/C catalyst. The use of ‘neutral’ chloroaluminate ionic liquid (xAlCl3 = 0.50) resulted in a strong increase in activity with 78% conversion within 60 min. This result is striking, since in this system no Lewis acidity is found and the only anion present is [AlCl4]−.16 We are currently investigating this effect in more detail. Further increase in AlCl3 content, up to xAlCl3 = 0.67, did improve the hydrogenation activity only marginally.
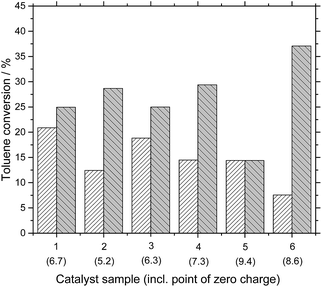
The beneficial influence of the ionic liquid coating on the activity of the Pd/C catalyst was tested by using different commercially available catalysts. Two different loadings, namely 5 and 10 wt% Pd on carbon, were tested. All supports resembled fine powders with particle sizes around 20 μm. From the results in Fig. 4 it becomes obvious that the activity increase is a general trend for all Pd/C catalysts. Except for the 5 wt% Pd/C on activated carbon from Sigma-Aldrich (SA 75992), all catalysts showed an activity increase when coated with the acidic ionic liquid. We suspect that the reason for this stems from the fact that this catalyst support material had the most basic point of zero charge (PZC) of 9.4 of all support materials. It is therefore likely that the acidic character of the ionic liquid coating is partly (or completely) lost due to interaction of the acidic ionic liquid with the basic surface groups. This assumption seems valid since the support materials with lower PZC resulted in increase in activity. However, the PZC cannot be solely responsible for the observed activity increase, since catalyst sample 6, having a PZC of 8.6, showed also significant boost in activity upon coating. It seems obvious that for a comprehensive understanding of this behavior more detailed studies are required.
In a next step, the acidic coating was achieved by the use of liquid coordination complexes. Considering substantial discrepancy in molecular weight between the ionic liquid and the LCC, the quantity of supported LCC was adjusted to contain equimolar amounts of AlCl3, when compared to [BMIM]Cl/AlCl3 (xAlCl3 = 0.67). Under the standard reaction conditions, the Ur/AlCl3 (xAlCl3 = 0.60) LCC coating (Pd-SCILL-2) resulted in a slightly improved activity over uncoated Pd/C catalyst, with 47% conversion (Table 2) after 60 min, but was inferior when compared to Pd-SCILL-1.
| T/°C | Pd/C | Pd-SCILL-1 | Pd-SCILL-2 | |||
|---|---|---|---|---|---|---|
| X60/% | keff/mol L−1 s−1 | X60/% | keff/mol L−1 s−1 | X60/% | keff/mol L−1 s−1 | |
| a Reaction conditions: phydrogen = 15 bar, ctoluene = 0.25 mol L−1, solvent = 100 mL cyclohexane, mcat = 1 g, wPd = 10 wt%, stirring speed at 1000 min−1, ionic liquid coating at 13 wt%. | ||||||
| 40 | 6.2 | 0.4 × 10−5 | 13.8 | 0.9 × 10−5 | 11.3 | 0.9 × 10−5 |
| 50 | 13.7 | 0.9 × 10−5 | 26.4 | 1.5 × 10−5 | 24.4 | 1.7 × 10−5 |
| 60 | 19.8 | 1.8 × 10−5 | 41.7 | 3.2 × 10−5 | 47.5 | 3.3 × 10−5 |
| 70 | 53.4 | 4.1 × 10−5 | 55.4 | 4.6 × 10−5 | 83.1 | 5.8 × 10−5 |
| 80 | 89.9 | 6.7 × 10−5 | 69.2 | 5.9 × 10−5 | 99.9 | 13.8 × 10−5 |
| 90 | — | — | — | — | 99.9 | 21.6 × 10−5 |
Reaction temperature was varied between 40 and 80 °C to study the influence of mass transport (Table 2). When using the uncoated Pd/C catalysts the toluene conversion increased significantly with temperature (detailed conversion plots can be found in Fig. S4–S6 the ESI†).
Already at 40 °C the LCC-coated Pd-SCILL-2 catalyst and the chloroaluminate-based Pd-SCILL-1 catalyst showed twice the activity of the Pd/C one. Best fitting results for the reaction rate constant k were obtained by a zero order dependency with respect to toluene. However, with increasing temperature, the Pd/C catalyst became as active as the Pd-SCILL-1 catalyst and around 80 °C, a 89.9% conversion was obtained while the Pd-SCILL-1 catalyst yielded only 69.2%.
The LCC-based Pd-SCILL-2 catalyst showed the strongest dependency with temperature and yielded full conversion at 80 °C, while at 90 °C all toluene reacted after 25 min already. This strong activity increase at higher temperatures cannot stem from changes in the viscosity of the LCC coating but might be a result of a change in speciation within the LCC. Here, more insight into this behavior is required to support this hypothesis.
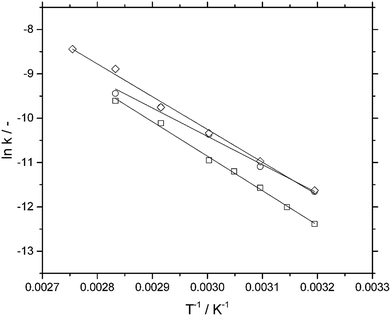
For all catalysts, the effective reaction rate constants keff were calculated from the slope of the conversion-time plots (see Fig. S7–S9 in the ESI†). As mentioned above, best fits were obtained for all systems when assuming a zero order dependency in toluene concentration. From an Arrhenius-type diagram (Fig. 5), an apparent activation energy of 64.7 kJ mol−1 was calculated for the Pd/C catalyst. From experiments with different agitation speeds (see Fig. S10 in ESI†) it can be concluded that the gas–liquid transfer of hydrogen does not play a role for these powdered catalysts. The Pd-SCILL-1 catalyst revealed a lower temperature dependency, indicated by the apparent activation energy of 53.1 kJ mol−1.
The lowering of the activation energy by 11.6 kJ mol−1 is a result of the interaction between the AlCl3 and the aromatic ring and is in agreement with literature data on e.g. the hydrogenation of borazine in the presence of AlCl3.17 A slightly higher apparent activation energy was found for the LCC coated Pd-SCILL-2 with 61.4 kJ mol−1. Since both activation energies of the coated catalysts were in the same range to the one obtained for Pd/C, pore diffusion did not seem to be the limiting factor in these systems either. This result is not surprising taking into account the rather small particle size of approx. 20 μm.
A pressure variation study was carried out between 5 and 25 bar of hydrogen using all three catalyst systems, and the resulting effective reaction rate constants keff are summarized in Table 3. For all catalysts, the best data fitting was again obtained for a zero order in toluene substrate concentration. For the uncoated catalyst, a strong dependency of hydrogenation activity with hydrogen partial pressure was observed, exemplified by the apparent reaction order of 1.4. Taking into account the zero order with respect to toluene, it can be assumed that the rate-determining step (RDS) on the pure Pd surface requires the presence of significant amounts of hydrogen.
| phydrogen/bar | keff/10−5 mol L−1 s−1 | ||
|---|---|---|---|
| Pd/C | Pd-SCILL-1 | Pd-SCILL-2 | |
| a Reaction conditions: T = 60 °C, phydrogen = 5–25 bar, ctoluene = 0.25 mol L−1, solvent = 100 mL cyclohexane, mcat = 1 g, wPd = 10 wt%, stirring speed at 1000 min−1, ionic liquid coating at 13 wt%. | |||
| 5 | 0.9 | 3.1 | 1.5 |
| 10 | 1.5 | 3.9 | 1.9 |
| 15 | 3.0 | 6.2 | 3.3 |
| 20 | 5.8 | 5.5 | 4.1 |
| 25 | 7.6 | 5.8 | 3.7 |
| neff,H2/— | 1.4 | 0.4 | 0.6 |
This situation changes when acidic coatings are present. For both Pd-SCILL catalysts lower apparent orders in hydrogen were found: 0.4 for Pd-SCILL-1 and 0.6 for Pd-SCILL-2. Beside a postulated activation of the aromatic ring the acidic ionic liquid on the Pd surface might facilitate the heterolytic cleavage of H2 into surface bound Pd-hydride and additional protons. As a result, the surface concentration of hydrogen becomes less important for the RDS. This RDS has a slightly lower activation energy, the result being the increased hydrogenation rates for Pd-SCILL systems at low temperatures. Such a postulated activation of the aromatic ring by both Lewis acidity (chloroaluminates or LCCs) and Brønsted acidity (protons) certainly has to be verified by additional experiments and DFT calculations.
Both SCILL-type catalysts were tested with respect to their recyclability. After the end of the reaction, the solution was released via the bottom valve and fresh reaction mixture was introduced by opening the lid of the reactor. As shown in Fig. 6, the chloroaluminate-based Pd-SCILL-1 catalyst lost activity with each recycle, while the LCC-based Pd-SCILL-2 catalyst gave identical activity in the second run. However, the third and fourth runs yielded slightly lower conversion levels. The reason for the loss in activity for Pd-SCILL-1 probably stems from the contact of the catalysts with air and moisture.15 This problem can, however, be solved in a continuous hydrogenation process with the SCILL catalyst in a fixed-bed reactor. Another possible route for catalyst deactivation is the leaching of Pd and Al-species from the catalysts. Pd leaching was analyzed by means of ICP and the results indicate a negligible loss of Pd during the course of reaction (see Fig. S11 in ESI†). Loss of Al would reduce the acidity required for high activity. ICP analysis revealed no clear trend (see Fig. S11 in ESI†), but it can be assumed that the Al leaching was more pronounced than the Pd leaching. Al leaching from chloroaluminates has been reported for continuous alkylation reaction. Here, the leaching stopped after a few hours once the most labile Al-species leached from the ionic liquid.18 Whether a similar stabilization process occurs in the Pd-SCILL system under hydrogenation conditions is in the focus of ongoing studies. Different approaches can be envisioned to improve the retention of the Al-species including e.g. the use of quaternary ammonium based ionic liquids, grafting of IL fragments onto the carbon support or cross-linking of the ionic liquid.
Conclusions
Lewis acidity has previously been shown to be beneficial for arene hydrogenation by adding lump amounts of chloroaluminate ionic liquids to a suspension containing a heterogeneous Pd/C catalyst. In this work, we have incorporated the Lewis acidity in a well-defined form as thin film liquid coatings on standard Pd/C catalysts, yielding solid catalysts with ionic liquid layer (SCILL). In addition to the previously reported chloroaluminate ionic liquids, we have tested novel liquid coordination complexes (LCC). Both coatings increased the activity of the resulting Pd-SCILL catalysts against toluene hydrogenation and yielded methylcyclohexane as the only hydrogenation product. At lower temperatures (40 °C) the LCC-coated Pd-SCILL-2 catalyst was only slightly more active than the Pd/C one, whereas the chloroaluminate-based Pd-SCILL-1 catalyst showed three times the activity of the Pd/C one. However, with increasing temperature, the Pd-SCILL-2 catalyst, showing the higher apparent activation energy, outperformed the Pd-SCILL-1 catalyst at around 80 °C, yielding full conversion in less than 50 min. The uncoated Pd/C catalyst showed a zero order dependency in toluene and a strong dependency in hydrogen partial pressure of 1.4, indicating hydrogen surface coverage being important in the rate-determining step (RDS). Coated catalysts also gave zero order with respect to substrate concentration, the hydrogen dependency became less pronounced, with only 0.6 (Pd-SCILL-1) and 0.4 (Pd-SCILL-2). The acidity of the coating film might, by activating the aromatic ring, reduce the importance of high hydrogen surface coverage in the RDS.The recyclability of the Pd-SCILL catalysts in consecutive batch runs yielded similar results with a gradual decrease in conversion with each cycle. Giving the high potential of these Lewis acidic SCILL catalysts for industrial arene hydrogenation, continuous processes can be envisaged, in which contact of the SCILL catalyst with air and moisture can be excluded during operation times. Beside hydrogenation other industrially important reactions where acidity is crucial can benefit from the use of such SCILL catalysts in the future.
Acknowledgements
The authors gratefully acknowledge the funding of the German Research Council (DFG), which, within the framework of its ‘Excellence Initiative’ supports the Cluster of Excellence ‘Engineering of Advanced Materials’ (http://www.eam.fau.de) at the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). M. L. is especially grateful for a PhD scholarship supplied by EAM. J. H. is thankful to the travel grant received via the EU COST-1206 STSM.Notes and references
- H.-J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 5th edn, 2010 Search PubMed.
- S. van de Vyver and Y. Román-Leshkov, Catal. Sci. Technol., 2013, 3, 1465 CAS.
- D. Teichmann, W. Arlt and P. Wasserscheid, Int. J. Hydrogen Energy, 2012, 37, 18118 CrossRef CAS.
- R. L. Augustine, Heterogeneous Catalysis for the Synthetic Chemist, Marcel Dekker, New York, 1996 Search PubMed.
- T. Maegawa, A. Akashi, K. Yaguchi, Y. Iwasaki, M. Shigetsura, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2009, 15, 6953 CrossRef CAS PubMed.
- C. Bianchini, V. Dal Santo, A. Meli, S. Moneti, M. Moreno, W. Oberhauser, R. Psaro, L. Sordelli and F. Vizza, Angew. Chem., Int. Ed., 2003, 42, 2636 CrossRef CAS PubMed.
- R. R. Deshmukh, J. W. Lee, U. S. Shin, J. Y. Lee and C. E. Song, Angew. Chem., Int. Ed., 2008, 47, 8615 CrossRef CAS PubMed.
- R. Fehrmann, A. Riisager and M. Haumann, Supported Ionic Liquids – Fundamentals and Applications, Wiley-VCH, Weinheim, 2014, and references therein Search PubMed.
- (a) U. Kernchen, B. Etzold, W. Korth and A. Jess, Chem. Eng. Technol., 2007, 30, 985 CrossRef CAS; (b) A. Jess, C. Kern and W. Korth, Oil, Gas, 2012, 38, OG38 CAS.
- (a) J. Arras, M. Steffan, Y. Shayeghi, D. Ruppert and P. Claus, Green Chem., 2009, 11, 716 RSC; (b) J. Arras, E. Paki, C. Roth, J. Radnik, M. Lucas and P. Claus, J. Phys. Chem. C, 2010, 114, 10520 CrossRef CAS.
- (a) T. Cremer, M. Stark, A. Deyko, H.-P. Steinrück and F. Maier, Langmuir, 2011, 27, 3662 CrossRef CAS PubMed; (b) H.-P. Steinrück, J. Libuda, P. Wasserscheid, T. Cremer, C. Kolbeck, M. Laurin, F. Maier, M. Sobota, P. S. Schulz and M. Stark, Adv. Mater., 2001, 23, 2571 CrossRef PubMed.
- F. Coleman, G. Srinivasan and M. Swadźba-Kwaśny, Angew. Chem., Int. Ed., 2013, 52, 12582 CrossRef CAS PubMed.
- (a) J. M. Hogg, F. Coleman, A. Ferrer-Ugalde, M. P. Atkins and M. Swadźba-Kwaśny, Green Chem., 2015, 17, 1831 RSC; (b) K. Matuszek, A. Chrobok, J. Hogg, F. Coleman and M. Swadźba-Kwaśny, Green Chem., 2015, 17, 4255 RSC.
- P. R. Rony, J. Catal., 1969, 14, 142 CrossRef CAS.
- (a) A. Riisager, R. Fehrmann, M. Haumann and P. Wasserscheid, Eur. J. Inorg. Chem., 2006, 695 CrossRef CAS; (b) S. Werner, N. Szesni, M. Kaiser, M. Haumann and P. Wasserscheid, Chem. Eng. Technol., 2012, 35, 1962 CrossRef CAS.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS; (b) J. Estager, J. D. Holbrey and M. Swadźba-Kwaśny, Chem. Soc. Rev., 2014, 43, 847 RSC.
- A. S. Lisovenko and A. Y. Timoshkin, Russ. Chem. Bull., 2012, 61, 897 CrossRef CAS.
- V. Ladnak, N. Hofmann, N. Brausch and P. Wasserscheid, Adv. Synth. Catal., 2007, 349, 719 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03295a |
| This journal is © The Royal Society of Chemistry 2017 |