Open Access Article
Open Access ArticleNew alkaloids with unusual spermidine moieties from the seeds of Orychophragmus violaceus and their cytoprotective properties†
Nailiang Zhu‡
 a,
Haifeng Wu‡a,
Ziqian Xub,
Chenqi Liua,
Yu Tiana,
Meigeng Hua,
Zhong Hao Suna,
Pengfei Lia,
Guoxu Ma*a and
Xudong Xu*a
a,
Haifeng Wu‡a,
Ziqian Xub,
Chenqi Liua,
Yu Tiana,
Meigeng Hua,
Zhong Hao Suna,
Pengfei Lia,
Guoxu Ma*a and
Xudong Xu*a
aKey Laboratory of Bioactive Substances and Resource Utilization of Chinese Herbal Medicine, Ministry of Education, Beijing Key Laboratory of Innovative Drug Discovery of Traditional Chinese Medicine (Natural Medicine) and Translational Medicine, Key Laboratory of Efficacy Evaluation of Chinese Medicine against Glycolipid Metabolic Disorders, State Administration of Traditional Chinese Medicine, Institute of Medicinal Plant Development, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100193, China. E-mail: mgxfl8785@163.com; xdxu@implad.ac.cn; Fax: +86 10 57833296; Tel: +86 10 57833296
bSchool Public Health, Peking University, Beijing 100191, China
First published on 25th August 2017
Abstract
The seeds of the plant Orychophragmus violaceus yielded eight new spermidine alkaloids, termed orychophragmuspine A–H (1–8). Their structures were elucidated by extensive spectroscopic analysis and comparison with literature data. Compounds 1–8 are unusual natural alkaloids with spermidine moieties and were tested for cytoprotective properties. The results showed that all compounds displayed mild cytoprotective activities compared with the normal and model groups.
Introduction
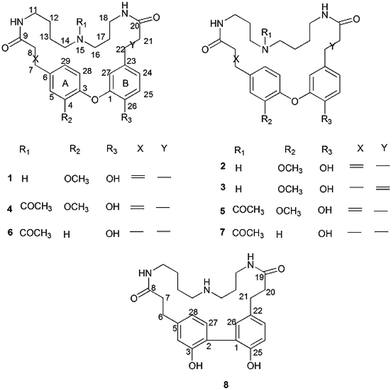
Orychophragmus violaceus (L.) (Brassicae) is mainly distributed in Eastern China and Korea and is cultivated as an ornamental plant in China.1 In addition, it is gathered for its use as a wild vegetable in some regions.2–5 Previous chemical and pharmacological studies on the stems of this plant resulted in the isolation of flavonoids, amino acids, fatty acids and phenolic acids, which showed antioxidative, anti-inflammatory, antidiabetic, antitumor and hepatoprotective effects.6–12Despite their popular use, there have been no chemical or pharmaceutical studies available on O. violaceus seeds until now.11 Our previous bioactivity screening showed that the aqueous extract of O. violaceus seeds displayed significant hepatoprotective activity in vivo, which urged us to further investigate secondary metabolites of seeds.12 In our ongoing search for bioactive constituents, eight new alkaloids with unusual spermidine moieties, orychophragmuspine A–H (1–8) were isolated and characterized (Fig. 1). Herein, the isolation and structural elucidation of the new compounds as well as the evaluation of their cytoprotective properties are described (Fig. 2).
Results and discussion
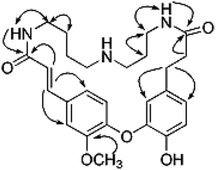
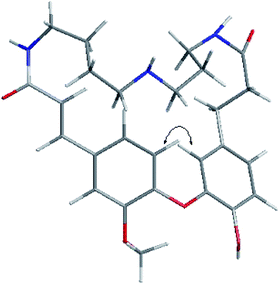
Compound 1 was obtained as an amorphous white solid and reacted positively to Dragendorff's reagent. Its molecular formula was determined to be C26H33N3O5 by HRESIMS. The IR spectrum displayed absorptions at 1652 cm−1 for β-unsaturated amides and 1537 and 1504 cm−1 for aromatic rings. The UV spectrum displayed maximum absorption bands at 233.0 and 287.6 nm. The 1H NMR spectrum of compound 1 (Table S1†) showed two typical ABX systems at δH 6.89 (1H, d, J = 8.4 Hz), 7.38 (1H, d, J = 1.2 Hz), 7.18 (1H, dd, J = 8.4, 1.2 Hz) and δH 6.75 (1H, d, J = 7.8 Hz), 6.08 (1H, d, J = 1.2 Hz), 6.62 (1H, dd, J = 7.8, 1.2 Hz), suggesting the existence of two 1,2,4-trisubstituted benzene rings. The spectrum also contained resonance signals at δH 7.44 (d, 1H, J = 15.6 Hz) and 6.77 (d, 1H, J = 15.6 Hz) which were assigned to a double bond in the E configuration, and resonances at δH 2.62 (2H, m) and 2.09 (m, 2H) due to two methylene protons. The following resonances were also observed: δH 3.80 (3H, s, OCH3), six δH 6.89 (1H, d, J = 8.4 Hz, H-28), δH 6.08 (1H, d, J = 1.2 Hz, H-27), δH 6.62 (1H, d, J = 7.8, 1.2 Hz, H-24), δH 6.75 (d, 1H, J = 7.8 Hz, H-25), δH 7.38 (1H, d, J = 1.2 Hz, H-5), δH 7.18 (1H, dd, J = 8.4, 1.2 Hz, H-29). The 13C APT NMR spectrum of compound 1 displayed 6 carbon resonances including δC 122.7 (C-24), 116.1 (C-25), 114.9 (C-27), 112.3 (C-5), 121.8 (C-28) and 120.7 (C-29). The 1H and 13C NMR spectroscopic data of compound 1 confirmed that the compound was a spermidine alkaloid derivative.13–16 An HMBC experiment was performed to establish the location of functional groups and the full structure of compound 1. Long-range HMBC correlation between δH 7.44 (1H, d, J = 15.6 Hz) and δC 112.3 (C-5) and 120.7 (C-29) suggested the (E)-olefin was attached to the 1,2,4-trisubstituted benzene ring A. HMBC cross-peaks between methylene protons at δH 2.62 (2H, m) and δC 114.9 (C-27) and 122.7 (C-24) confirmed that the methylene was adjacent to the 1,2,4-trisubstituted benzene ring B. HMBC correlation of the OCH3 at δH 3.80 with C-4 (δC 151.3) suggested this OCH3 was attached to C-4. In addition, the HMBC spectra showed that the (E)-olefinic proton at δH 6.77 and the amide proton at δH 8.37 (brs, 1H) were correlated with a carboxyl carbon at δC 165.4 and that methylene protons at δH 2.09 and the amide proton at δH 7.82 (brs, 1H) were correlated with a carboxyl carbon at δC 171.1, allowing the resonances at δH 8.37 (brs, 1H) and 7.82 (brs, 1H) to be assigned to H-10 and H-19, respectively. 1H–1H COSY spectra indicated that the methylene protons at δH 3.28 (2H, m, H-11) were coupled with the amide proton on N-10 at δH 8.37 (1H, brs) as well as with methylene signals at δH 1.57 (2H, m, H-12). Downfield signal at δH 2.80 (2H, m, H-14) coupled with δH 1.60 (2H, m, H-13) indicated that the methylene on C-14 was attached at N-15. In addition, the 1H–1H COSY spectrum indicated that the methylene protons at δH 2.61 (2H, m, H-18) were coupled with the amide proton on N-19 at δH 7.82 (1H, brs) as well as the methylene signal at δH 1.56 (2H, m, H-17). Downfield signal at δH 2.58 (m, 2H, H-16) coupled with δH 1.56 (m, 2H, H-17), indicating that the methylene on C-16 was attached to N-15. The H2-11 was determined to be the terminal methylene group of the (CH2)4 portion of spermidine, attached to N(10)H, and the HMBC correlation of N(10)H with the carboxyl C atom C-9 showed that the C4-unit was connected to ring A. Analogously, the linking of the C3-unit to the carboxyl C atom C-20, starting from C(18)H2 via N(19)H, was likely. The exact attachment of the spermidine moiety to the rest of the molecule was confirmed according to the 1H–1H COSY and HMBC spectra. The relative stereochemistry of compound 1 was resolved by analysis of NOESY spectrum. In the NOESY spectrum, a weak cross peak was found for the protons of δH 6.08 (H-27) and 6.89 (H-28), indicating the proximity of these two groups, which confirmed C(1)–O–C(3) linkages. Based on the above results, the structure of compound 1 was established as shown and named orychophragmuspine A (Fig. 1). Compound 1 is representative of a new alkaloid skeleton with a spermidine moiety between C-9 and C-20 (Fig. 3).Compound 2 was obtained in the form of an amorphous white solid. Its molecular formula C26H33N3O5 was deduced by HRESIMS (m/z 468.2487 [M + H]+). A direct comparison of the NMR data (Table S1†) between compounds 2 and 1 showed that they were isomeric with differences in the spermidine units. Contrary to compound 1, a reversed orientation of the spermidine group was shown in compound 2. C(11)H2, which was correlated to C-9 as above, was shown to belong to the C3-unit by COSY data, while C(18)H2, the terminal methylene group of the (CH2)4 moiety, was linked to the carboxyl C atom C-20. On the basis of the above results, the structure of compound 2 was elucidated and named orychophragmuspine B (Fig. 1).
Compound 3 was a water-soluble amorphous white solid, possessing the same molecular formula as compound 2, as determined through NMR data and an HRESIMS [M + H]+ ion at m/z 468.2481. A comparison of the NMR spectroscopic data of compound 3 with those of compound 2 (Table S1†) revealed that the main differences between them were the (E)-olefinic protons at δH 6.55 and 7.36 at C-21 and C-22 in compound 3 rather than at C-7 and C-8 in compound 2. In the HMBC spectrum, the correlations from H-21 (6.55) and H-22 (7.36) to δC (C-20) and δC (C-23), together with the 1H–1H COSY spectrum, confirmed these distinctions. As a result, compound 3 was termed orychophragmuspine C (Fig. 1).
Compounds 4 and 5 were isolated as an inseparable mixture of two isomers present in an approximate ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Based on the HRESIMS adduct ion detected at m/z 532.2413 [M + Na]+, the mixture (compounds 4/5) shared the same molecular formula of C28H35N3O6, requiring thirteen degrees of unsaturation. For the NMR data, most of the signals could be assigned to the two compounds on the basis of the relative intensities or integrals of their signals. However, these two compounds could not be completely separated by any chromatographic methods used in the present study. Fortunately, their spectroscopic data were sufficient for the structural elucidation described below. The 1H and 13C NMR data (Table S2†) of compound 4 were similar to those of compound 1 except for the presence of additional acetyl signals at δH 1.94 (3H, s), δC 21.3 and δC 172.4. In the HMBC spectrum, the carbon signal at δC 172.4 had long-range correlations with H-14 (δH 3.07) and H-16 (δH 2.95) suggesting that the acetyl group was at N-15 in compound 4. Compound 5 was an isomeric analogue of compound 4 with the difference that the –NH(CH2)3– unit was located at C-9 and the –NH(CH2)4– unit was located at C-20. In the HMBC spectrum, the terminal protons of the spermidine C(11)H2 and C(18)H2 correlated to the carboxyl carbons C(9) and C(20), respectively, and together with the additional correlations of the amide protons N(10)H to the carbons C(9)/C(11) and N(19)H to the carbons C(18)/C(20), supported this assignment. Thus, compounds 4 and 5 were established as two novel spermidine alkaloids, and named orychophragmuspine D and E, respectively (Fig. 1).
5. Based on the HRESIMS adduct ion detected at m/z 532.2413 [M + Na]+, the mixture (compounds 4/5) shared the same molecular formula of C28H35N3O6, requiring thirteen degrees of unsaturation. For the NMR data, most of the signals could be assigned to the two compounds on the basis of the relative intensities or integrals of their signals. However, these two compounds could not be completely separated by any chromatographic methods used in the present study. Fortunately, their spectroscopic data were sufficient for the structural elucidation described below. The 1H and 13C NMR data (Table S2†) of compound 4 were similar to those of compound 1 except for the presence of additional acetyl signals at δH 1.94 (3H, s), δC 21.3 and δC 172.4. In the HMBC spectrum, the carbon signal at δC 172.4 had long-range correlations with H-14 (δH 3.07) and H-16 (δH 2.95) suggesting that the acetyl group was at N-15 in compound 4. Compound 5 was an isomeric analogue of compound 4 with the difference that the –NH(CH2)3– unit was located at C-9 and the –NH(CH2)4– unit was located at C-20. In the HMBC spectrum, the terminal protons of the spermidine C(11)H2 and C(18)H2 correlated to the carboxyl carbons C(9) and C(20), respectively, and together with the additional correlations of the amide protons N(10)H to the carbons C(9)/C(11) and N(19)H to the carbons C(18)/C(20), supported this assignment. Thus, compounds 4 and 5 were established as two novel spermidine alkaloids, and named orychophragmuspine D and E, respectively (Fig. 1).
Similar to compounds 4 and 5, compounds 6 and 7 were also an inseparable mixture of two isomers (ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The 1H and 13C NMR data (Table S3†) of compounds 6 and 7 were similar to those of compounds 4 and 5, except for the disappearance of an OCH3 group at C-4 and (E)-olefinic proton signals at C-7 and C-8 in compounds 6 and 7. In the HMBC spectrum, the additional methylene signals at (H2-7) and (H2-8) had long-range correlations with C-6 (135.5) and C-9 (173.9). Together with the molecular formula deduced from HRESIMS, this supported the missing functional groups. As a result, the structures of compounds 6 and 7 were elucidated as shown and they were named orychophragmuspine F and G (Fig. 1).
3). The 1H and 13C NMR data (Table S3†) of compounds 6 and 7 were similar to those of compounds 4 and 5, except for the disappearance of an OCH3 group at C-4 and (E)-olefinic proton signals at C-7 and C-8 in compounds 6 and 7. In the HMBC spectrum, the additional methylene signals at (H2-7) and (H2-8) had long-range correlations with C-6 (135.5) and C-9 (173.9). Together with the molecular formula deduced from HRESIMS, this supported the missing functional groups. As a result, the structures of compounds 6 and 7 were elucidated as shown and they were named orychophragmuspine F and G (Fig. 1).
Compound 8 was obtained as an amorphous white solid. The 1H and 13C NMR spectra were closely related to those of compound 1 except for the disappearance of a trans double bond at C-6/C-7 and an ether bond between C-1 and C-2. In the HMBC spectrum, the correlations from aliphatic methylene protons at (H2-6) and (H2-7) to C-5 (131.2) and C-8 (171.9) indicated that the double bond at C-6/C-7 was hydrogenated. The upfield shifted C-1 (127.0) and C-2 (126.8) in compound 8, in contrast to the C-1 (144.8) and C-3 (145.4) in compound 1, as well as the HMBC correlations from H-26 (δH 6.97) to C-2, suggested a direct linkage between C-1 and C-2. The molecular formula (C25H33O3N4), together with the upfield carbon signal at (C-3), was evidence for a hydroxyl group at C-3 (154.2) in compound 8 instead of a methoxyl group at C-4 (151.3) in compound 1. Therefore, compound 8 was established as a novel spermidine alkaloid and named orychophragmuspine H (Fig. 1).
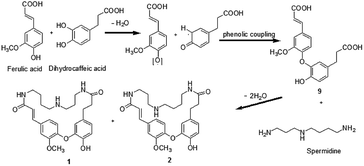
A plausible biosynthetic pathway of orychophragmuspine A and B was proposed (Scheme 1). The key intermediate product 9 was produced from ferulic acid and dihydrocaffeic acid by dehydration and a phenolic coupling reaction. Then, the intermediate product 9 and spermidine unit were cyclized through a dehydration reaction with N atom as linkage.
Cytoprotective effects of compounds 1–8 against hydrogen peroxide (H2O2)-induced cell death in HepG2 cell lines were evaluated using MTT method. The results of their cytoprotective properties are shown in Tables S4 and S5 (see ESI†). Overall, compounds 1–8 exhibited moderate cytoprotective effects at 100 μg ml−1 against cytotoxicity induced by H2O2. The structure–activity relationship need to be further researched.
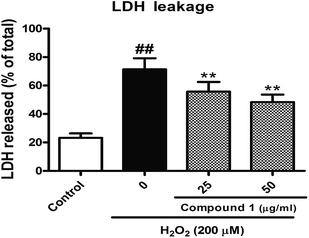
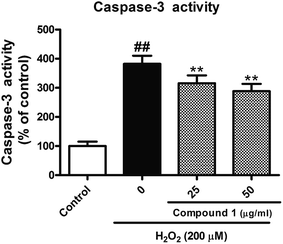
To confirm and evaluate the anti-apoptotic effects of these compounds, we selected compound 1 (C1) as test molecular and determined the C1 induced neuroprotection with the LDH release assay. As depicted in Fig. 4, exposure to indicated concentration C1 reversed H2O2-induced elevation of the levels of LDH release, which demonstrated C1 attenuated H2O2-caused cell damage. Simultaneously, we took the caspase-3 activity assay to further confirm the anti-apoptotic effects of C1 in H2O2-damaged HepG2 cells. As shown in Fig. 5, H2O2 significantly increased the caspase-3 activity, while C1 reversed this tendency and showed the dose-dependent manner. The down-regulation of LDH release confirmed the dramatic neuroprotective activity of C1, and the attenuated caspase-3 activity suggested the pro-apoptotic effect of C1.
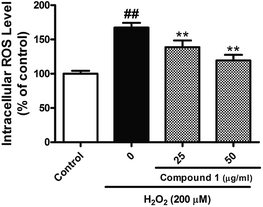
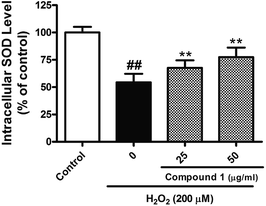
Excessive ROS levels could initiate the perturbation of cellular redox balance and induce damage to major macromolecules in cells. In order to elaborate the close connection between the H2O2 induced injury and oxidative stress, we measured both oxidant and antioxidant parameters. Our results suggest that treated HepG2 cells with H2O2 could significantly increase the level of ROS with accompanying decrease in the activities of SOD. However, C1 could significantly alleviate such situation by dropping out the level of ROS (Fig. 6), as well as enhancing the activities of SOD (Fig. 7). It was suggested that C1 suppressed oxidative stress induced by H2O2 via both direct and indirect antioxidant actions.
Conclusions
In summary, eight new spermidine alkaloids (1–8) with moderate cytoprotective properties were isolated from the seeds of O. violaceus. The cellular mechanism for cytoprotection were the pro-apoptotic effect and antioxidant actions. Future studies are needed to focus on the cytoprotective activities of spermidine alkaloids from O. violaceus and clarify their structure–activity relationship.Acknowledgements
The work was financially supported by the Technological Large Platform for Comprehensive Research and Development of New Drugs in the CAMS Innovation Fund for Medical Science (CIFMS) (Grant No. 2016-I2M-1-012), the Twelfth Five-Year “Significant New Drugs Created” Science and Technology Major Projects (No. 2012ZX09301-002-001-026), the National Science and Technology Support Program (No. 2012BA127B06), the Beijing Natural Science Foundation (No. 7164281), and the PUMC Youth Fund (No. 3332015141).Notes and references
- T. Y. Zhou, K. J. Guan and R. L. Guo, Science Press, 1987, 33, 40–43 Search PubMed.
- L. Xiao and P. Luo, Acta Bot. Boreali-Occident. Sin., 1994, 14, 237–241 Search PubMed.
- P. Luo, B. Q. Huang, J. M. Yin, Z. L. Chen, Y. H. Chen and Z. Q. Lan, Genet. Resour. Crop Evol., 1998, 45, 491–494 CrossRef.
- Q. J. Ren, J. P. Yu and G. L. Zhang, Chinese Wild Plant Resources, 1998, 2, 24–25 Search PubMed.
- D. B. Weng and X. F. Huang, Acta Bot. Boreali-Occident. Sin., 2001, 21, 673–677 CAS.
- P. Luo, Z. Q. Lan and Z. Y. Li, Plant Breed., 1994, 113, 83–85 CrossRef.
- Y. Y. Wu, A study of the inorganic nutrition mechanism of Orychophragmus violaceus's adaptability to karst, in Comprehensive Studies on Plants of Adaptability to Karst -Orychophragmus violaceus, ed. Y. Y. Wu, Guizhou Sci. Publ. House, Guiyang, 1997, pp. 29–35 Search PubMed.
- D. B. Weng, H. F. Wang and J. Y. Weng, Chinese Wild Plant Resources, 2000, 19, 13–15 Search PubMed.
- Y. Y. Wu, Anticancer substance glucoraphanin new resources-Orychophragmus violaceus, in 2002 Innovative Excellent Achievement of Medicine in the World, Académie Européenne de Sciences Naturelles, Engelhardt-Ng Verlag, Bonn, 2002, pp. 62–63 Search PubMed.
- M. X. MA and Y. Mei, J. Anhui Agric. Sci., 2012, 40, 5109–5113 CAS.
- T. T. Zhang, G. X. Ma, F. Q. Xu, J. N. Wu, X. P. Zhang and J. S. Yang, Chin. Pharm. J., 2014, 49, 2165–2167 CAS.
- Y. W. Zhan, Z. Q. Xu, X. H. Guo, R. J. Li, J. P. Shen, X. D. Xu and B. X. Zhang, Chin. J. Pharmacol. Toxicol., 2016, 30, 101–106 Search PubMed.
- R. W. Doskotch, A. B. Ray and J. L. Beal, Chem. Commun., 1971, 300–301 RSC.
- A. B. Ray, W. Kubelka, E. H. Fairchild, C. D. Hufford and J. L. Beal, Tetrahedron, 1974, 30, 3229–3236 CrossRef.
- M. Khatib, G. Pieraccini, M. Innocenti, F. Melani and N. Mulinacci, J. Pharm. Biomed. Anal., 2016, 123, 53–62 CrossRef CAS PubMed.
- Y. Forster, A. Ghaffar and S. Bienz, Phytochemistry, 2016, 128, 50–59 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02951a |
| ‡ These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2017 |