Open Access Article
Open Access ArticleSynthesis and antiproliferative activity of 2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazol derivatives as microtubule-destabilizing agents†
Dongjie Fenga,
Yue Wua,
Hao Wanga,
Zhaoshi Baib,
Defa Wanga,
Daiying Zuob,
Kai Bao*c,
Yingliang Wu*b and
Weige Zhang *a
*a
aKey Laboratory of Structure-Based Drug Design and Discovery, Ministry of Education, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenhe District, Shenyang 110016, China. E-mail: zhangweige2000@sina.com
bDepartment of Pharmacology, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenhe District, Shenyang 110016, China. E-mail: yingliang_1016@163.com
cWuya College of Innovation, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenhe District, Shenyang 110016, China. E-mail: baokai@syphu.edu.cn
First published on 5th June 2017
Abstract
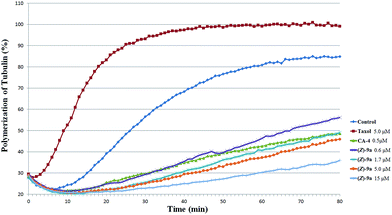
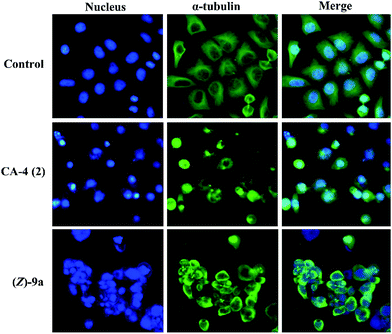
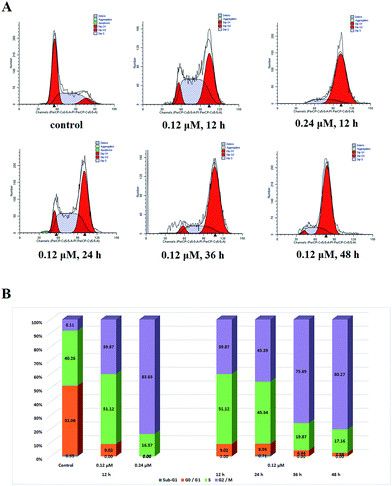
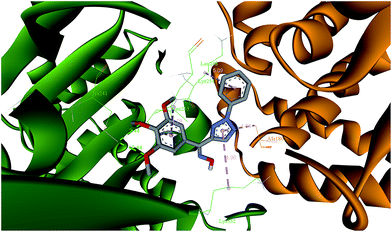
A series of 2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazols were designed as analogs of substituted methoxybenzoyl-aryl-thiazole (SMART) under the consideration of geometric features. The target compounds were synthesized via concise and efficient processes including microwave-assisted cyclization, and were evaluated for their antiproliferative activity against three human cancer cell lines. Most compounds exhibited moderate antiproliferative activity with IC50 values in the micromolar to sub-micromolar range. Tubulin polymerization and immunofluorescence studies demonstrated that (Z)-9a was a potent microtubule-destabilizing agent and disrupted the polymerization dynamics. Moreover, (Z)-9a significantly induced accumulation of cells in the G2/M phase and caused microtubule destabilization. Molecular modeling studies showed that (Z)-9a probably binds to the colchicine site of tubulin.
Introduction
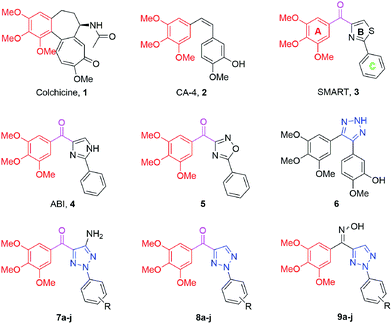
Microtubules, a vital part of the cytoskeleton, play a crucial role in mitosis.1 Disturbing microtubule arrangement and rearrangement, a dynamic equilibrium process, has been proved to be useful in the development of anticancer therapeutics.2 Microtubule targeted agents can be classified into two major types: (1) microtubule-stabilizing agents, including taxanes and epothilones that stimulate tubulin polymerization; (2) microtubule-destabilizing agents, including vinca alkaloids and colchicine (1, Fig. 1) that inhibit tubulin polymerization.3Given the success of the taxanes and vinca alkaloids,4 which have established tubulin as a valid target in cancer therapy, research efforts have been focusing on the development of targeted agents to the colchicine site of microtubules, known as colchicine binding site inhibitors (CBSIs).5 During the past decades, a large number of CBSIs have been reported,6 such as combretastatin A-4 (CA-4 2, Fig. 1), 4-substituted methoxybenzoyl-aryl-thiazole (SMART 3, Fig. 1) and 2-aryl-4-benzoyl-imidazole (ABI 4, Fig. 1).7–9 SMART, a basic three ring (A, B and C) scaffold based on the ring geometry, demonstrates potent antiproliferative activity. Moreover, a range of further structural diversification molecules derived from the three rings has been developed to affect tubulin polymerization.10 In our previous study, a set of SMART analogs (5, Fig. 1) with the oxadiazole moiety as B-ring were reported to exhibit promising antiproliferative activities.11
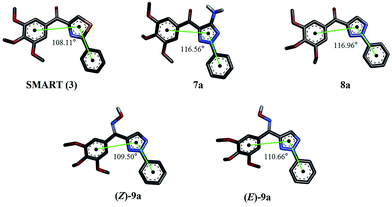 | ||
| Fig. 2 Optimized structures of SMART and the target compounds 7a, 8a, (Z)-9a and (E)-9a. Black points represent the geometric centers of A, B and C rings, respectively. | ||
1,2,3-Triazole has gained enormous interest due to their broad spectrum of pharmaceutical and therapeutic applications.12,13 Furthermore, this heterocycle has a high dipole moment and is capable for hydrogen bonding, which could be favorable in the binding to biomolecular targets.14 A 1,2,3-triazol-based CA-4 analog (6, Fig. 1) was found to display potent antiproliferative and antitubulin activities.15
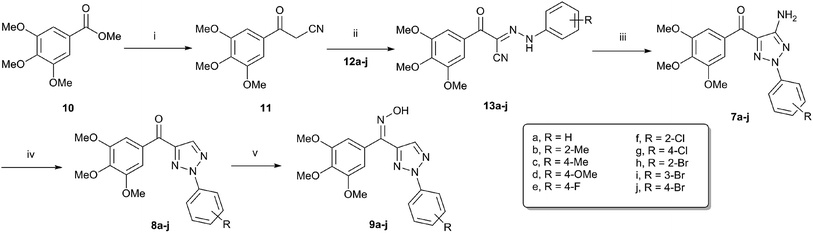
In this study, we envisaged an optimization effort around the bioisosteric B ring of SMART by replacing the thiazole ring with triazole. We hypothesized that the degree of the angle between the geometric centers of A, B and C rings (∠ABC) is important for the activity of SMART and its derivatives (Fig. 2). Thus, to investigate the similarity between the designed compounds and SMART, density functional theory (DFT) calculation was applied in our case for the molecule design.16–18 Calculational results showed the similar values of ∠ABC for (Z)-9a and SMART, which were 109.50° and 108.11°, respectively. Therefore, a series of 2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazole derivatives (7–9) were designed and the effects of substitutions on the C5 position of the triazole ring and changing the carbonyl linker into oxime were evaluated.
Results and discussion
Chemistry
The synthetic routes for 2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazol derivatives (7–9) are outlined in Scheme 1. The starting material methyl 3,4,5-trimethoxybenzoate (10) was converted into the 3-oxo-3-(3,4,5-trimethoxyphenyl)propanenitrile (11) by using sodium hydride and acetonitrile in THF.19 Coupling the diazonium salt of different aromatic amines (12a–j) with compound 11 afforded the corresponding hydrazone derivatives 13a–j.20Under conventional heating conduction, the reaction of 13a with hydroxylamine hydrochloride in the presence of sodium acetate to generate 7a suffered from long reaction time and low yields (entries 1–5).21 As a useful tool in organic synthesis, microwave irradiation could generally accelerate the reaction rate and improve the product's yield.22–24 When microwave irradiation was incorporated into synthesis of 7a, we found that the reaction rate was greatly improved. We systematically screened the influence of reaction temperature and time on the yield of 7a under microwave conditions. As shown in Table 1, the optimized condition was confirmed to be microwave irradiation at 160 °C for 7 min (entry 9). Accordingly, all of the cyclized products 7a–j were obtained smoothly with good to excellent yields 81–93%.
Subsequently, the amino group was removed using isoamyl nitrite in anhydrous THF under reflux conditions to yield the corresponding compounds 8a–j,25 which were then converted into oxime isomers (9a–j) under excess amount of hydroxylamine hydrochloride and sodium acetate by refluxing in anhydrous ethanol for 2 h.26
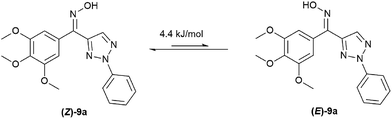
It is worth to note that there are two configurations for compounds 9a–9j (Z and E) and the isomer mixtures were evaluated without separation in our study due to the Z/E tautomerism even at room temperature. Generally, the ratio of two isomers were obtained by HPLC analysis. In addition, DFT calculation of 9a showed that Z-isomer should be the predominant one in the mixture (Fig. 3).
Biological evaluation
| Compound | IC50 (μM) ± SDa | ||
|---|---|---|---|
| SGC-7901 | A549 | HT-1080 | |
| a IC50: concentration of the compound (μM) producing 50% cell growth inhibition after 72 h of drug exposure, as determined by the MTT assay. Each experiment was carried out in triplicate.b Used as a positive control.c Used as mixture without separation. | |||
| 7a | 0.35 ± 0.02 | 0.28 ± 0.04 | 0.70 ± 0.03 |
| 7b | 5.6 ± 0.4 | 8.1 ± 0.9 | 9.3 ± 1.1 |
| 7c | 3.3 ± 0.2 | 0.26 ± 0.02 | 0.41 ± 0.05 |
| 7d | 1.3 ± 0.1 | 3.4 ± 0.4 | 0.38 ± 0.04 |
| 7e | 1.8 ± 0.07 | 2.8 ± 0.2 | 0.28 ± 0.02 |
| 7f | 9.3 ± 0.8 | 5.3 ± 0.4 | 1.5 ± 0.1 |
| 7g | 0.70 ± 0.05 | 1.6 ± 0.1 | 0.24 ± 0.01 |
| 7h | 16.2 ± 1.5 | 10.1 ± 1.2 | 3.2 ± 0.2 |
| 7i | 0.48 ± 0.02 | 1.8 ± 0.2 | 2.4 ± 0.2 |
| 7j | 0.35 ± 0.04 | 0.58 ± 0.06 | 0.14 ± 0.02 |
| 8a | 0.87 ± 0.09 | 7.1 ± 0.6 | 5.2 ± 0.8 |
| 8b | 3.4 ± 0.4 | 8.6 ± 0.6 | 11.4 ± 1.2 |
| 8c | 1.5 ± 0.2 | 7.8 ± 0.7 | 4.3 ± 0.2 |
| 8d | 1.51 ± 0.09 | 6.3 ± 0.5 | 7.9 ± 0.9 |
| 8e | 2.9 ± 0.3 | 6.7 ± 0.8 | 2.4 ± 0.3 |
| 8f | 6.1 ± 0.6 | 19.2 ± 3.1 | 35.8 ± 8.3 |
| 8g | 1.8 ± 0.2 | 3.7 ± 0.4 | 0.68 ± 0.8 |
| 8h | 3.0 ± 0.4 | 11.0 ± 1.0 | 9.8 ± 1.6 |
| 8i | 2.4 ± 0.2 | 10.4 ± 1.1 | 8.2 ± 0.7 |
| 8j | 0.92 ± 0.07 | 2.6 ± 0.2 | 1.8 ± 0.1 |
| (Z)-9a | 0.12 ± 0.02 | 0.52 ± 0.3 | 0.34 ± 0.4 |
| (E)-9a | 0.61 ± 0.05 | 1.23 ± 0.07 | 0.98 ± 0.1 |
| 9ac | 0.19 ± 0.04 | 0.71 ± 0.05 | 0.52 ± 0.06 |
| 9bc | 4.9 ± 0.3 | 7.2 ± 0.7 | 16 ± 1.4 |
| 9cc | 0.32 ± 0.04 | 2.4 ± 0.3 | 0.54 ± 0.05 |
| 9dc | 0.47 ± 0.06 | 0.31 ± 0.03 | 6.1 ± 0.7 |
| 9ec | 0.42 ± 0.04 | 2.1 ± 0.2 | 2.1 ± 0.3 |
| 9fc | 3.1 ± 0.2 | 6.8 ± 0.4 | 37.9 ± 6.2 |
| 9gc | 0.38 ± 0.06 | 1.6 ± 0.09 | 0.49 ± 0.02 |
| 9hc | 1.7 ± 0.1 | 12.1 ± 2.0 | 41.3 ± 6.4 |
| 9ic | 1.3 ± 0.2 | 10.2 ± 0.9 | 8.5 ± 0.7 |
| 9jc | 0.69 ± 0.05 | 0.85 ± 0.06 | 0.83 ± 0.06 |
| SMART (3)b | 0.019 ± 0.008 | 0.029 ± 0.009 | 0.028 ± 0.016 |
| ABI (4)b | 0.81 ± 0.08 | 0.98 ± 0.11 | 0.15 ± 0.05 |
In order to elucidate selectivity of compounds to cancer cell lines and non-cancer cell lines, we further investigated the effect of three compounds with high antiproliferative activity (7a, 7j, (Z)-9a) toward normal fibroblasts L929 cells. Results are presented in Table 3. The selectivity indexes SIA (for SGC-7901 cell line), SIB (for A549 cell line) and SIC (for HT-1080 cell line) were calculated. The higher selectivity index means higher therapeutic safety margin. (Z)-9a showed apparent selectivity to three cancer cell lines and normal fibroblasts L929 cells. Surprisingly, both 7a and 7j displayed very slight effect on L929 cells, which mean these compounds have a higher selectivity than (Z)-9a.
Conclusion
A series of 2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazols characterized by a carbonyl/oxime linker, combined with/without amino substituent on the C5 position of the triazole ring, were developed under the strategy of the bioisosteric replacement of B ring of SMART. The target compounds displayed moderate antiproliferative activity against three human carcinoma cell lines. Among them, (Z)-9a exhibited best antiproliferative activity against the SGC-7901 cell lines with IC50 value of 0.12 ± 0.02 μM. Mechanism of action studies confirmed that (Z)-9a was a microtubule-destabilizing agent that induced the accumulation of cells in the G2/M phase and caused cell apoptosis. Structure–activity relationship between 7, 8 and 9 indicated that ∠ABC might be a considerable factor for design of SMART analogs. (Z)-9a was discovered as the most potent compound and further structural optimization and activity test will be reported in the future.Experimental section
Chemistry
All reagents were commercially available and were used without further purification. The silica gel plate (HSGF-254) and silica gel (H, 200–300 mesh) from Yantai Jiangyou silicone Development Co., Ltd. was used for preparative TLC and column chromatography, respectively. Visualization was made with UV light (254 nm and 365 nm). Melting points were tested with Micro-Melting point apparatus (X-4, Shanghai jing science instrument Co., Ltd.) and were uncorrected. Mass spectra (MS) were obtained from Agilent Co. Ltd. on an Agilent 1100-sl mass spectrometer with an electrospray ionization source. 1H-NMR and 13C-NMR spectra were measured in CDCl3 or d6-DMSO with TMS as the internal reference on a Bruker AVANCE spectrometer operating at 400 MHz or 600 MHz (1H at 400 or 600 MHz, 13C at 100 MHz). HPLC (HPLC-LC-20AT, Shimadzu) using SPD-20A UV as the detector, C18 (4.6 × 250 mm, 0.45 μm) as HPLC analysis column. Method A: gradient: 60% methanol in water as mobile elution, flow rate: 1.0 mL min−1; method B: gradient: 80% methanol in water as mobile elution, flow rate: 1.0 mL min−1. The microwave reactions were carried out in a single mode cavity microwave synthesizer (CEM Corporation, NC, USA).General synthetic procedures for 3-oxo-3-(3,4,5-trimethoxyphenyl)propanenitrile (11)
Mixed methyl 3,4,5-trimethoxybenzoate (5.34 g, 23.6 mmol) with NaH (47.2 mmol, 1.62 g) in boiling tetrahydrofuran (20 mL) and then followed by dropwise addition of solution of acetonitrile (23.6 mmol, 0.98 g, 1.4 mL) in tetrahydrofuran (1 mL). After refluxing for 3 h the reaction was entirely finished (TLC control). Then the mixture was cooled to room temperature and diluted by water. The precipitated sodium salt was filtered and washed with diethylether. After the evaporation of the solvent, HCl (1 mol L−1) was added to acidize the compound to pH 2 and the solid was obtained, giving pale yellow solid, yield 84%. The crude 11 was utilized directly for the upcoming step without any extra purification.General synthetic procedures for 3-oxo-3-(3,4,5-trimethoxyphenyl)-2-(phenylhydrazono) propanenitrile (13a–j)
To a solution of the arylamine was added hydrochloric acid (5.8 mmol in 4 mL, 6 M HCl) at 0 °C, a solution of sodium nitrite (6.4 mmol, 0.44 g dissolved in 2 mL water) was added dropwise over a period of 20 min, and stirring at 0 °C for 30 min to form the corresponding aryldiazonium salt (12a–j). Then the resulting solution of aryldiazonium salt was dropwise added to a solution of 3-oxo-3-(3,4,5-trimethoxyphenyl)propanenitrile (1.5 g, 6.4 mmol) in ethanol mixture (10 mL) involving sodium acetate trihydrate (0.95 g, 11.6 mmol) at 0 °C. Then the mixture was stirred at room temperature for 1 h and the resulting solid was collected by filtration, washed with water and ethanol. The crude 13a–j was utilized directly for the upcoming step without any extra purification.General synthetic procedures for 5-amino-2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazol (7a–j)
Independent mixtures of 13a–j (1 mmol) containing hydroxylamine hydrochloride (0.35 g, 5 mmol) and anhydrous sodium acetate (0.41 g, 5 mmol) in DMF (5 mL) were stirred at irradiated in a microwave reactor for 7 min at 160 °C. The reaction mixtures were cooled to room temperature and poured into ice cold water. The formed solids were collected by filtration, washed with water. The residual crude product was purified by column chromatography on silica gel (200–300 mesh) with petroleum ether/AcOEt (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
General synthetic procedures for 2-aryl-4-(3,4,5-trimethoxybenzoyl)-1,2,3-triazol (8a–j)
To a solution of compound 7a–j (1 mmol) in anhydrous THF (5 mL) was added isopropyl nitrite (2 mmol). The original material was completely depleted after one hour refluxing. After the evaporation of the solvent, the residue was purified by column chromatography on silica gel (200–300 mesh) with petroleum ether/AcOEt (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or pure CH2Cl2.
1) or pure CH2Cl2.
General synthetic procedures for compounds (9a–j)
An excess of hydroxylamine hydrochloride (15 mmol) was added to a solution of compound 8a–j (1 mmol) and sodium acetate (15 mmol) in absolute ethanol (5 mL). After reacting at 80 °C for 2 h, the reaction was entirely finished (TLC control). Then the mixture was added to water (20 mL) and extracted with EtOAc (20 mL × 3). The combined organic layers were washed with water, dried over Na2SO4, and the solvent was then evaporated under reduced pressure. The residue was purified by column chromatography (petroleum ether/AcOEt = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent) on silica gel to obtain pure product.
1 as eluent) on silica gel to obtain pure product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 mixture of Z and E isomers; mp 132–135 °C; 1H NMR (400 MHz, CDCl3) (Z isomer): δ 9.23 (s, 1H), 8.57 (s, 1H), 8.10 (d, J = 8.0 Hz, 2H), 7.49 (dd, J = 7.6 Hz, 8.0 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 6.98 (s, 1H), 3.92 (s, 3H), 3.88 (s, 6H) ppm; (E isomer): δ 8.05 (d, J = 7.9 Hz, 2H), 7.96 (s, 1H), 7.45 (dd, J = 7.9 Hz, 2H), 7.35 (m, 1H), 6.86 (s, 1H), 3.94 (s, 3H), 3.86 (s, 6H) ppm; 1H NMR (600 MHz, d6-DMSO) (Z isomer): δ 12.26 (s, 1H), 8.67 (s, 1H), 8.03 (d, J = 7.9 Hz, 2H), 7.59 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 7.1 Hz, 1H), 6.99 (s, 1H), 3.78 (s, 3H), 3.73 (s, 6H) ppm; MS (ESI): [M + H]+ = 355.1, [M + Na]+ = 377.1.
24 mixture of Z and E isomers; mp 132–135 °C; 1H NMR (400 MHz, CDCl3) (Z isomer): δ 9.23 (s, 1H), 8.57 (s, 1H), 8.10 (d, J = 8.0 Hz, 2H), 7.49 (dd, J = 7.6 Hz, 8.0 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 6.98 (s, 1H), 3.92 (s, 3H), 3.88 (s, 6H) ppm; (E isomer): δ 8.05 (d, J = 7.9 Hz, 2H), 7.96 (s, 1H), 7.45 (dd, J = 7.9 Hz, 2H), 7.35 (m, 1H), 6.86 (s, 1H), 3.94 (s, 3H), 3.86 (s, 6H) ppm; 1H NMR (600 MHz, d6-DMSO) (Z isomer): δ 12.26 (s, 1H), 8.67 (s, 1H), 8.03 (d, J = 7.9 Hz, 2H), 7.59 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 7.1 Hz, 1H), 6.99 (s, 1H), 3.78 (s, 3H), 3.73 (s, 6H) ppm; MS (ESI): [M + H]+ = 355.1, [M + Na]+ = 377.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 mixture of Z and E isomers; mp 307–310 °C; 1H NMR (400 MHz, CDCl3) (Z isomer) δ 10.30 (s, 0.6H), 8.60 (s, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.35 (m, 3H), 6.95 (s, 2H), 3.90 (s, 3H), 3.86 (s, 6H), 2.40 (s, 3H) ppm; (E isomer) δ 8.04 (s, 1H), 7.58 (m, 1H), 7.28 (m, 3H), 6.85 (s, 2H), 3.92 (s, 3H), 3.84 (s, 6H), 2.40 (s, 3H) ppm; MS (ESI): [M + H]+ = 369.2.
8 mixture of Z and E isomers; mp 307–310 °C; 1H NMR (400 MHz, CDCl3) (Z isomer) δ 10.30 (s, 0.6H), 8.60 (s, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.35 (m, 3H), 6.95 (s, 2H), 3.90 (s, 3H), 3.86 (s, 6H), 2.40 (s, 3H) ppm; (E isomer) δ 8.04 (s, 1H), 7.58 (m, 1H), 7.28 (m, 3H), 6.85 (s, 2H), 3.92 (s, 3H), 3.84 (s, 6H), 2.40 (s, 3H) ppm; MS (ESI): [M + H]+ = 369.2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 mixture of Z and E isomers; mp 142–145 °C; 1H NMR (400 MHz, CDCl3) (Z isomer) δ 8.39 (s, 1H), 8.02 (d, J = 8.4 Hz, 2H), 7.77 (s, 2H), 7.33 (d, J = 8.4 Hz, 2H), 3.98 (s, 3H), 3.97 (s, 6H), 2.44 (s, 3H) ppm; (E isomer) δ 8.17 (s, 1H), 7.95 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 8.2 Hz, 2H), 6.68 (s, 2H), 3.93 (s, 3H), 3.81 (s, 6H), 2.42 (s, 3H) ppm; MS (ESI): [M + H]+ = 369.1, [M + Na]+ = 391.1.
18 mixture of Z and E isomers; mp 142–145 °C; 1H NMR (400 MHz, CDCl3) (Z isomer) δ 8.39 (s, 1H), 8.02 (d, J = 8.4 Hz, 2H), 7.77 (s, 2H), 7.33 (d, J = 8.4 Hz, 2H), 3.98 (s, 3H), 3.97 (s, 6H), 2.44 (s, 3H) ppm; (E isomer) δ 8.17 (s, 1H), 7.95 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 8.2 Hz, 2H), 6.68 (s, 2H), 3.93 (s, 3H), 3.81 (s, 6H), 2.42 (s, 3H) ppm; MS (ESI): [M + H]+ = 369.1, [M + Na]+ = 391.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 mixture of Z and E isomers; mp 178–180 °C; 1H NMR (400 MHz, CDCl3) (Z isomer) δ 9.80 (s, 1H); 8.57 (s, 1H); 8.08 (d, J = 7.7 Hz, 2H); 7.46 (d, J = 7.7 Hz, 2H); 6.97 (s, 2H); 3.93 (s, 3H); 3.88 (s, 6H), 3.48 (s, 3H) ppm; MS (ESI): [M + H]+ = 385.1, [M + Na]+ = 407.1.
11 mixture of Z and E isomers; mp 178–180 °C; 1H NMR (400 MHz, CDCl3) (Z isomer) δ 9.80 (s, 1H); 8.57 (s, 1H); 8.08 (d, J = 7.7 Hz, 2H); 7.46 (d, J = 7.7 Hz, 2H); 6.97 (s, 2H); 3.93 (s, 3H); 3.88 (s, 6H), 3.48 (s, 3H) ppm; MS (ESI): [M + H]+ = 385.1, [M + Na]+ = 407.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 mixture of Z and E isomers; mp 139–147 °C; 1H NMR (600 MHz, CDCl3) (Z isomer) δ 8.40 (s, 1H), 8.14 (m, 2H), 7.73 (s, 2H), 7.23 (m, 2H), 6.66 (s, 1H), 3.98 (s, 3H), 3.97 (s, 6H) ppm; (E isomer) δ 8.19 (s, 1H), 8.06 (m, 2H), 7.73 (s, 2H), 7.20 (m, 2H), 6.66 (s, 2H), 3.93 (s, 3H), 3.81 (s, 6H). ppm; MS (ESI): [M + H]+ = 373.1.
22 mixture of Z and E isomers; mp 139–147 °C; 1H NMR (600 MHz, CDCl3) (Z isomer) δ 8.40 (s, 1H), 8.14 (m, 2H), 7.73 (s, 2H), 7.23 (m, 2H), 6.66 (s, 1H), 3.98 (s, 3H), 3.97 (s, 6H) ppm; (E isomer) δ 8.19 (s, 1H), 8.06 (m, 2H), 7.73 (s, 2H), 7.20 (m, 2H), 6.66 (s, 2H), 3.93 (s, 3H), 3.81 (s, 6H). ppm; MS (ESI): [M + H]+ = 373.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 mixture of Z and E isomers; mp 215–218 °C; 1H NMR (600 MHz, d6-DMSO) (Z isomer) δ 12.29 (s, 0.2H), 8.75 (s, 1H), 8.14 (m, J = 9.8 Hz, 2H), 7.74 (m, J = 8.0 Hz, 1H), 7.63 (s, 2H), 7.60 (m, 1H), 3.81 (s, 3H), 3.78 (s, 6H); (E isomer) δ 11.89 (s, 0.2H), 8.78 (s, 1H), 8.26 (s, 2H), 8.02 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.58 (m, 1H), 3.90 (s, 9H) ppm; MS (ESI): [M + H]+ = 389.1, [M + Na]+ = 411.1.
16 mixture of Z and E isomers; mp 215–218 °C; 1H NMR (600 MHz, d6-DMSO) (Z isomer) δ 12.29 (s, 0.2H), 8.75 (s, 1H), 8.14 (m, J = 9.8 Hz, 2H), 7.74 (m, J = 8.0 Hz, 1H), 7.63 (s, 2H), 7.60 (m, 1H), 3.81 (s, 3H), 3.78 (s, 6H); (E isomer) δ 11.89 (s, 0.2H), 8.78 (s, 1H), 8.26 (s, 2H), 8.02 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.58 (m, 1H), 3.90 (s, 9H) ppm; MS (ESI): [M + H]+ = 389.1, [M + Na]+ = 411.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 mixture of Z and E isomers; mp 184–189 °C; 1H NMR (600 MHz, CDCl3) (Z isomer) δ 8.55 (s, 1H), 8.03 (d, J = 8.6 Hz, 2H), 7.45 (d, J = 8.6 Hz, 2H), 6.94 (s, 2H), 3.91 (s, 3H), 3.86 (s, 6H) ppm; (E isomer) 8.41 (s, 1H), 8.09 (d, J = 8.8 Hz, 2H), 7.72 (s, 2H), 7.51 (d, J = 8.8 Hz, 2H), 3.98 (s, 3H), 3.96 (s, 6H) ppm; MS (ESI): [M + H]+ = 389.1, [M + Na]+ = 411.1.
12 mixture of Z and E isomers; mp 184–189 °C; 1H NMR (600 MHz, CDCl3) (Z isomer) δ 8.55 (s, 1H), 8.03 (d, J = 8.6 Hz, 2H), 7.45 (d, J = 8.6 Hz, 2H), 6.94 (s, 2H), 3.91 (s, 3H), 3.86 (s, 6H) ppm; (E isomer) 8.41 (s, 1H), 8.09 (d, J = 8.8 Hz, 2H), 7.72 (s, 2H), 7.51 (d, J = 8.8 Hz, 2H), 3.98 (s, 3H), 3.96 (s, 6H) ppm; MS (ESI): [M + H]+ = 389.1, [M + Na]+ = 411.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 mixture of Z and E isomers; mp 195–198 °C; 1H NMR (600 MHz, CDCl3) (Z isomer) δ 8.65 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.56 (d, J = 6.0 Hz, 1H), 7.45 (d, J = 7.4 Hz, 1H), 7.36 (t, J = 7.2 Hz, 1H), 6.96 (s, 2H), 3.88 (s, 3H), 3.86 (s, 6H) ppm; (E isomer) δ 8.09 (s, 1H), 7.72 (s, 1H), 7.55 (s, 1H), 7.42 (d, J = 7.2 Hz, 1H), 7.32 (s, 1H), 6.87 (s, 2H), 3.90 (s, 3H), 3.85 (s, 6H) ppm; MS (ESI): [M + H]+ = 433.0, [M + Na]+ = 455.0.
29 mixture of Z and E isomers; mp 195–198 °C; 1H NMR (600 MHz, CDCl3) (Z isomer) δ 8.65 (s, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.56 (d, J = 6.0 Hz, 1H), 7.45 (d, J = 7.4 Hz, 1H), 7.36 (t, J = 7.2 Hz, 1H), 6.96 (s, 2H), 3.88 (s, 3H), 3.86 (s, 6H) ppm; (E isomer) δ 8.09 (s, 1H), 7.72 (s, 1H), 7.55 (s, 1H), 7.42 (d, J = 7.2 Hz, 1H), 7.32 (s, 1H), 6.87 (s, 2H), 3.90 (s, 3H), 3.85 (s, 6H) ppm; MS (ESI): [M + H]+ = 433.0, [M + Na]+ = 455.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 mixture of Z and E isomers; mp 180–185 °C; 1H-NMR (600 MHz, CDCl3): (Z isomer) δ 9.23 (s, 0.6H), 8.63 (s, 1H), 7.61 (dd, J = 1.8 Hz, 7.57 Hz, 1H), 7.6 (dd, J = 1.6 Hz, 7.9 Hz, 1H), 7.43 (dd, J = 1.8 Hz, 7.9 Hz, 1H), 7.41 (dd, J = 1.6 Hz, 7.6 Hz, 1H), 6.96 (s, 2H), 3.88 (s, 3H), 3.87 (s, 6H) ppm; (E isomer) δ 8.49 (s, 1H), 7.79 (s, 2H), 7.70 (dd, J = 1.9 Hz, 7.5 Hz, 1H), 7.63 (d, J = 1.8 Hz, 1H), 7.47 (d, J = 1.9 Hz, 1H), 7.45 (d, J = 1.8 Hz, 1H), 3.95 (s, 3H), 3.94 (s, 6H) ppm; MS (ESI): [M + H]+ = 433.0, [M + Na]+ = 455.1.
18 mixture of Z and E isomers; mp 180–185 °C; 1H-NMR (600 MHz, CDCl3): (Z isomer) δ 9.23 (s, 0.6H), 8.63 (s, 1H), 7.61 (dd, J = 1.8 Hz, 7.57 Hz, 1H), 7.6 (dd, J = 1.6 Hz, 7.9 Hz, 1H), 7.43 (dd, J = 1.8 Hz, 7.9 Hz, 1H), 7.41 (dd, J = 1.6 Hz, 7.6 Hz, 1H), 6.96 (s, 2H), 3.88 (s, 3H), 3.87 (s, 6H) ppm; (E isomer) δ 8.49 (s, 1H), 7.79 (s, 2H), 7.70 (dd, J = 1.9 Hz, 7.5 Hz, 1H), 7.63 (d, J = 1.8 Hz, 1H), 7.47 (d, J = 1.9 Hz, 1H), 7.45 (d, J = 1.8 Hz, 1H), 3.95 (s, 3H), 3.94 (s, 6H) ppm; MS (ESI): [M + H]+ = 433.0, [M + Na]+ = 455.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 mixture of Z and E isomers; mp 201–205 °C; 1H NMR (600 MHz, d6-DMSO) (Z isomer) δ 12.26 (s, 1H), 8.66 (s, 1H), 8.03 (m, 2H), 7.42 (m, 2H), 6.97 (s, 2H), 3.78 (s, 6H), 3.76 (s, 3H) ppm; (E isomer) δ 11.83 (s, 1H), 8.27 (s, 1H), 7.95 (m, 1H), 7.47 (m, 1H), 6.86 (s, 1H), 3.73 (s, 3H), 3.72 (s, 6H) ppm; MS (ESI): [M + H]+ = 433.0.
17 mixture of Z and E isomers; mp 201–205 °C; 1H NMR (600 MHz, d6-DMSO) (Z isomer) δ 12.26 (s, 1H), 8.66 (s, 1H), 8.03 (m, 2H), 7.42 (m, 2H), 6.97 (s, 2H), 3.78 (s, 6H), 3.76 (s, 3H) ppm; (E isomer) δ 11.83 (s, 1H), 8.27 (s, 1H), 7.95 (m, 1H), 7.47 (m, 1H), 6.86 (s, 1H), 3.73 (s, 3H), 3.72 (s, 6H) ppm; MS (ESI): [M + H]+ = 433.0.Biology
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (81673293, 81502932, 81602969) and State Key Laboratory of Natural Medicines and Active Substance (No. GTZK201603) for their generous financial support. This work was also supported by Program for Innovative Research Team of the Ministry of Education and Program for Liaoning Innovative Research Team in University.References
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253 CrossRef CAS PubMed.
- C. Dumontet and M. A. Jordan, Nat. Rev. Drug Discovery, 2010, 9, 790 CrossRef CAS PubMed.
- E. A. Perez, Mol. Cancer Ther., 2009, 8, 2086 CrossRef CAS PubMed.
- Y. Lu, J. Chen, M. Xiao, W. Li and D. D. Miller, Pharm. Res., 2012, 29, 2943 CrossRef CAS PubMed.
- M. N. Islam and M. N. Iskander, Mini-Rev. Med. Chem., 2004, 4, 1077 CrossRef CAS PubMed.
- G. G. Dark, S. A. Hill, V. E. Prise, G. M. Tozer, G. R. Pettit and D. J. Chaplin, Cancer Res., 1997, 57, 1829 CAS.
- G. R. Pettit, B. Toki, D. L. Herald, P. V. Pinard, M. R. Boyd, E. Hamel and R. K. Pettit, J. Med. Chem., 1998, 41, 1688 CrossRef CAS PubMed.
- Y. Lu, C.-M. Li, Z. Wang, C. R. Ross II, J. Chen, J. T. Dalton, W. Li and D. D. Miller, J. Med. Chem., 2009, 52, 1701 CrossRef CAS PubMed.
- J. Chen, Z. Wang, C. M. Li, Y. Lu, P. K. Vaddady, B. Meibohm, J. T. Dalton, D. D. Miller and W. Li, J. Med. Chem., 2010, 53, 7414 CrossRef CAS PubMed.
- J. Chen, S. Ahn, J. Wang, Y. Lu, J. T. Dalton, D. D. Miller and W. Li, J. Med. Chem., 2012, 55, 7285 CrossRef CAS PubMed.
- Q. Guan, D. Feng, Z. Bai, Y. H. Cui, D. Zuo, M. Zhai, X. Jiang, W. Zhou, K. Bao, Y. Wu and W. Zhang, MedChemComm, 2015, 6, 1484 RSC.
- H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS PubMed.
- V. D. Bock, D. Speijer, H. Hiemstra and J. H. V. Maarseveen, Org. Biomol. Chem., 2007, 5, 971 CAS.
- Y. C. Duan, Y. C. Ma, E. Zhang, X. J. Shi, M. M. Wang, X. W. Ye and H. M. Liu, Eur. J. Med. Chem., 2013, 62, 11 CrossRef CAS PubMed.
- N. R. Madadi, N. R. Penthala, K. Howk, A. Ketkar, R. L. Eoff, M. J. Borrelli and P. A. Crooks, Eur. J. Med. Chem., 2015, 103, 123 CrossRef CAS PubMed.
- A. D. Baecke, J. Chem. Phys., 1993, 98, 5648 CrossRef.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, J. C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- S. Wang, Y. Yin, Y. Zhang, S. Mi, M. Zhao, P. Lv, B. Wang and H. Zhu, Eur. J. Med. Chem., 2015, 93, 291 CrossRef CAS PubMed.
- H. Behbehani, H. M. Ibrahim and S. Makhseed, Heterocycles, 2009, 78, 3081 CrossRef CAS.
- L. Wu, S. Guo, X. Wang, Z. Guo, G. Yao, Q. Lin and M. Wu, Tetrahedron Lett., 2015, 56, 2145 CrossRef CAS.
- C. O. Kappe and D. Dallinger, Mol. Diversity, 2009, 13, 171 CrossRef PubMed.
- A. Hoz, A. Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164 RSC.
- Z. Wen, J. Xu, Z. Wang, H. Qi, Q. Xu, Z. Bai, Q. Zhang, K. Bao, Y. Wu and W. Zhang, Eur. J. Med. Chem., 2015, 90, 184 CrossRef CAS PubMed.
- T. Yang, G. Chen, Z. Sang, Y. Liu, X. Yang, Y. Chang, H. Long, W. Ang, J. Tang, Z. Wang, G. Li, S. Yang, J. Zhang, Y. Wei and Y. Luo, J. Med. Chem., 2015, 58, 6389 CrossRef CAS PubMed.
- Y. Lu, C.-M. Li, Z. Wang, J. Chen, M. L. Mohler, W. Li, J. T. Dalton and D. D. Miller, J. Med. Chem., 2011, 54, 4678 CrossRef CAS PubMed.
- R. B. Ravelli, B. Gigant, P. A. Curmi, I. Jourdain, S. Lachkar, A. Sobel and M. Knossow, Nature, 2004, 428, 198 CrossRef CAS PubMed.
- A. Massarotti, A. Coluccia, R. Silvestri, G. Sorba and A. Brancale, ChemMedChem, 2012, 7, 33 CrossRef CAS PubMed.
- Q. Xu, Y. Wang, J. Xu, M. Sun, H. Tian, D. Zuo, Q. Guan, K. Bao, Y. Wu and W. Zhang, ACS Med. Chem. Lett., 2016, 7, 1202 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c7ra02720f |
| This journal is © The Royal Society of Chemistry 2017 |