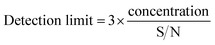Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved Cl2 sensing characteristics of reduced graphene oxide when decorated with copper phthalocyanine nanoflowers
Sanjeev Kumara,
Navdeep Kaura,
Anshul Kumar Sharmaa,
Aman Mahajan *a and
R. K. Bedib
*a and
R. K. Bedib
aMaterial Science Laboratory, Department of Physics, Guru Nanak Dev University, Amritsar-143005, India. E-mail: aman.phy@gndu.ac.in
bSatyam Institute of Engineering and Technology, Ram Tirath Road, Amritsar-143107, India
First published on 10th May 2017
Abstract
A novel gas sensing platform involving a hybrid of reduced graphene oxide (rGO) sheets with unsubstituted copper phthalocyanine (CuPc) nanoflowers has been explored as a room temperature ppb level chemiresistive chlorine (Cl2) sensor with a detection limit as low as 1.97 ppb. The synthesized CuPc/rGO hybrid was characterized by TEM, XRD, FTIR, Raman and UV-Vis spectroscopic and TGA techniques. Improved Cl2 sensing characteristics of rGO on decorating with CuPc nanoflowers is attributed to the synergetic effect of the presence of more Cl2 specific interaction sites in CuPc molecules with nanoflower type morphology along with the conducting network produced by high surface area rGO sheets. A plausible gas sensing mechanism based on Raman and electrochemical impedance spectroscopy (EIS) studies has been proposed.
1. Introduction
Cl2 is a toxic gas with an occupational exposure limit (OEL) of 500 parts per billion (ppb) and is widely used in various industries related with pharmaceuticals, plastics, agrochemicals, water treatment, textiles and household cleaning products.1 Cl2 exposure above the OEL can cause a serious health risk and hence there is a need to monitor Cl2 down to ppb range. So far, several methods like optical,2 thermionic ionization,3 solid state potentiometric,4 opto-chemical,5 calorimetric6,7 and electrochemical8 have been explored to detect Cl2. These methods are complex and cumbersome. Chemiresistors on the other hand are found to be comparatively simple, economical, compact in design and consume less power. Extensive reports have been published over last few years for the detection of Cl2 using various semiconducting oxides,9,10 conducting polymers11 and organic molecules12,13 based nanostructures and thin film at room temperature.Nowadays, the extraordinary mechanical, thermal and electrical properties of 2D monolayer of graphene with large surface area14,15 has made it a potential candidate for detection of various toxic gases like NO2,16 NH3,17 H2S,18 CO2,19 H2,20 SO2,21 and LPG.22 But, high cost involved in large scale production of graphene, has led to the detection of these gases using reduced graphene oxide (rGO) which is a 2D carbon based material with tuneable optical and electrical properties.23,24 Jeong et al.25 reported rGO based NO2 gas sensor with 100 ppm detection limit, however the sensor failed to recover to original baseline on degassing. NO gas sensor with detection limit as low as 420 ppb with response time of 1000 s have been reported by Li et al.26 Inkjet printed rGO based Cl2 sensor exhibited response time of 2–3 minutes and recovery time of 2 hours with detection limit of 100 ppm.27
To overcome the issues of lower sensitivity, slow recovery time and detection limit in rGO based sensors, rGO has been functionalized with different metals,28,29 metal oxides,30–32 organic molecules33,34 and polymers.35,36 Such functionalization of rGO, not only improves the gas sensing parameters but also enhances their selectivity to a particular type of chemical species. Among various functionalizing species, metallo-phthalocyanine (MPc) based materials are demonstrated to be excellent sensing materials with high sensitivity, selectivity and fast response.37–40
Further, the manipulation of central metal ion, substitution of functional groups on phthalocyanine ring, morphology and operating temperature were found to remarkably tune the gas sensing characteristics of phthalocyanine molecules.41–44 We have recently demonstrated the growth of substituted MPcs with nanowire, nanoflowers and nano belts morphology for formation of room temperature Cl2 sensors with detection limit down to ppb level.38–40,45 Very recently Zhao et al.37 reported the growth of single crystal nano column of unsubstituted copper phthalocyanine (CuPc) for room temperature Cl2 sensor with response of 26% for 80 ppb and Kumar et al.13 reported five times enhancement in Cl2 sensing using unsubstituted cobalt phthalocyanine (CoPc) film on porous nanostructured surface on etched glass in comparison to CoPc film on plain glass.
Functionalization of rGO with MPc molecules (MPc/rGO) has attracted remarkable attention for its distinctive selectivity toward hazardous gases due to synergetic effect of specific interaction sites in MPc molecules with conducting network provided by large surface area of rGO.33,34 So far, Zhou et al.34 have reported the functionalization of rGO with substituted metal phthalocyanine and were successful in developing room temperature sensors with a response of 15.4% for 3200 ppm of NH3.
Herein, we report for the first time ppb level Cl2 sensor by growing nanoflowers of CuPc molecules onto rGO sheets. The hypothesis of the work is based upon the fact that large number of gas absorbing sites provided by CuPc nanoflowers with high surface to volume ratio along with the conducting network provided by the large surface area of rGO sheets will induce selective and improved gas sensing characteristics to the hybrid.
2. Experimental details
Graphite oxide (GO) was prepared by using Hummer's method.46 Here, 4 g of graphite flakes were treated with 2 g of NaNO3 and 12 g of KMnO4 in 100 ml of concentrated H2SO4 for the oxidation of graphite. The mixture was stirred continuously in an ice bath and H2O was added into the obtained paste. This suspension was maintained at high temperature (98 °C) and H2O2 was added into the suspension for removal of impurities. The pH of solution was maintained around 7 by its several washing and filtration with warm water. Further, obtained material was centrifuged at 5500 rpm for 20 minutes and heated at 80 °C for 24 hours, thus residue as GO in powder form was collected. 10 mg of dried GO was added into 100 ml of DMF in a beaker and sonicated for exfoliating GO. Simultaneously, a solution of 0.5 mg of CuPc powder in 5 ml of DMF was prepared and added to GO solution drop by drop with continuous sonication followed by stirring in the dark for 24 hours. Thereafter, 500 μl of N2H4 was added in the above solution followed by refluxing at 100 °C for 24 hours. After completion of reaction, the solution was filtered and the resultant product, CuPc/rGO hybrid was dried in a vacuum oven for 3 hours at 150 °C. A schematic diagram of synthesized rGO sheets decorated with CuPc nanoflowers for chemiresistive sensor applications is depicted in Fig. 1.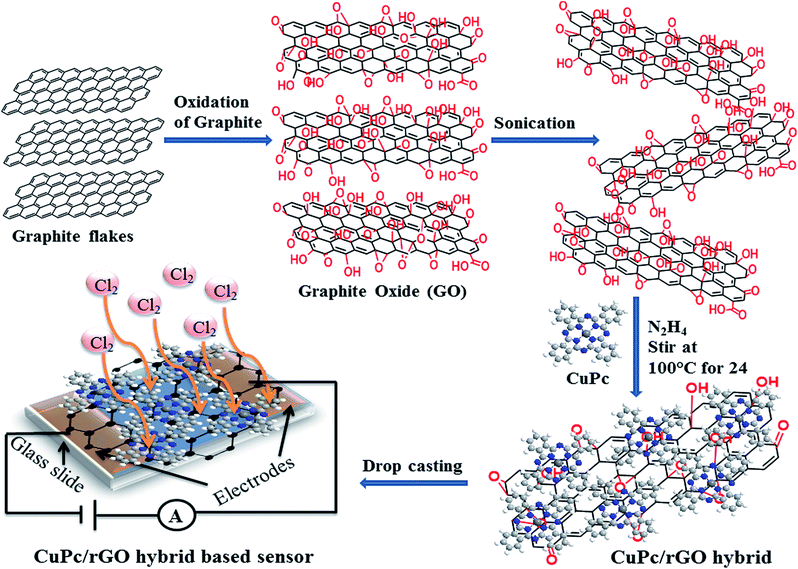 | ||
| Fig. 1 Schematic diagram of synthesized rGO sheets decorated with CuPc nanoflowers for chemiresistive sensor. | ||
The morphology of CuPc/rGO hybrid was characterized by using JEOL JEM-2100 transmission electron microscope (TEM) operating at 200 kV accelerating voltage. The structural characterization was performed using X-ray diffraction D8 FOCUS, Bruker Ettlingen (operated at 30 mA and 40 kV) with Cu Kα radiation (λ = 1.5418 Å) in the range of 5–40°. Fourier transform infrared (FTIR) spectra were recorded on Perkin Elmer Frontier instrument in the range of 400–1800 cm−1. Raman spectroscopy was performed on Renishaw In-Via Reflex micro-Raman system from 1000 to 3200 cm−1 using a 514 nm argon ion laser source. UV-Vis spectroscopy was done using Shimadzu, UV-VIS-NIR 3600. Thermo gravimetric analysis (TGA) was carried out with a Thermogravimetry analyzer (Hitachi STA 7200) under a nitrogen atmosphere from 40 to 900 °C at a scan rate of 10 °C min−1. Electrochemical impedance spectroscopy (EIS) was performed using a frequency response analyser (FRA) attached with Autolab potentiostat galvanostat (PGSTAT302) system. To fabricate gas sensor, two gold electrodes (50 nm thick, dimensions 5 mm × 5 mm and separated by 1 mm) were thermally deposited on glass substrate and CuPc/rGO hybrid solution of 30 μl was drop casted on it and sensor with thickness 1.2 ± 0.2 μm was obtained. The sensing studies were performed using homemade stainless steel static gas sensing instrument at room temperature (25 °C). Sensor was placed in a chamber of 1 litre volume and the temperature of the chamber was controlled by a thermocouple. The electrical conductance of the sensor was measured with Keithley electrometer (model 6517A), by applying a constant DC voltage of 3 V. Cl2, NO2, NO and NH3 gases were commercially procured from Chemtron Science Pvt. Ltd., India in the gas filled canister of volume 0.5 litres with a concentration of 1000 ppm. The sensing measurement was performed by injecting a known quantity of gas using a micro-syringe. On exposure, conductance attained a maximum value and returned to base line on purging and subsequently multiple cycles of dosing and purging were performed. The response of the sensor was calculated by using equation,
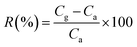 | (1) |
3. Results and discussion
3.1 Structural and spectroscopic studies
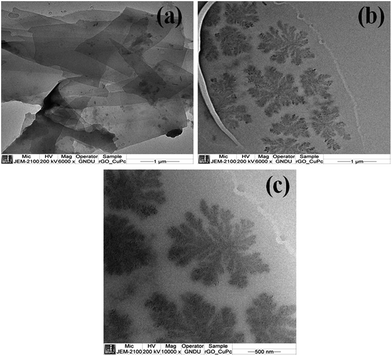
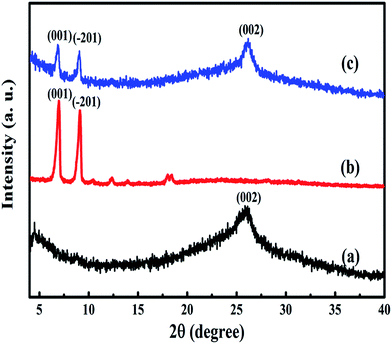
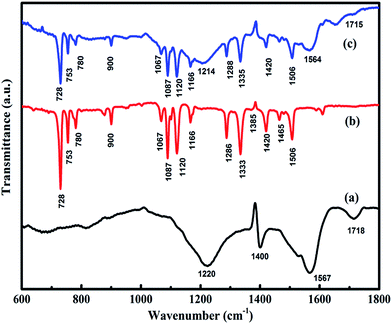
The morphology of rGO and CuPc/rGO hybrid were analysed by TEM (Fig. 2). The wrinkled and transparent sheets of rGO with folded edges are seen in Fig. 2(a). CuPc/rGO hybrid depicts the formation of uniformly grown network of nanoflowers of CuPc lying parallel to rGO surface (Fig. 2(b and c)). Fig. 3 shows the X-ray diffraction pattern of rGO, CuPc and CuPc/rGO hybrid. The rGO sample shows a diffraction peak near 25.9° which corresponds to the (002) plane. CuPc shows two diffraction peaks at 6.86° and 9.09°, corresponding to (001) and (−201) crystal planes, respectively. In Fig. 3(c), two peaks at 6.86° and 9.09° with broad spectrum at 25.9° confirms the formation of CuPc/rGO hybrid. The attachment of CuPc molecules onto rGO sheets were investigated by FTIR, Raman and UV-Vis spectroscopic studies. In FTIR spectra of rGO, peaks at 1220, 1400, 1567 and 1718 cm−1 corresponded to C–O–C, O–H deformation vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, respectively (Fig. 4(a)). FTIR peaks of CuPc were assigned in Fig. 4(b) and tabulated in Table 1.47 FTIR spectra of CuPc/rGO hybrid confirmed the presence of all characteristic peaks of rGO and CuPc with comparatively reduced intensities. Moreover, the positions of C–O–C, C
O stretching, respectively (Fig. 4(a)). FTIR peaks of CuPc were assigned in Fig. 4(b) and tabulated in Table 1.47 FTIR spectra of CuPc/rGO hybrid confirmed the presence of all characteristic peaks of rGO and CuPc with comparatively reduced intensities. Moreover, the positions of C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
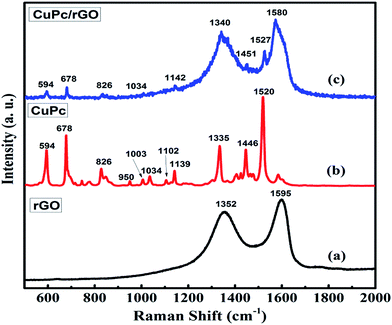
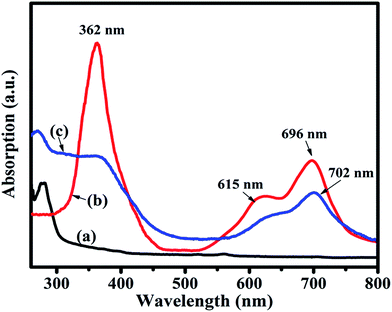
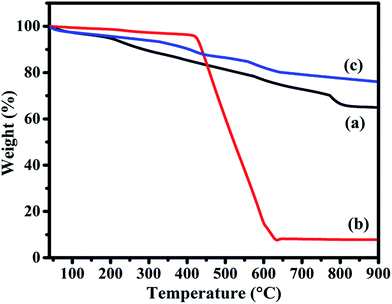
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks of rGO were shifted to 1214, 1564 and 1715 cm−1, respectively (Fig. 4(c)). The observed decrease in intensity and shifting of peaks after hybridization indicated the delocalization of electrons to form non-covalent attachment of CuPc on rGO surface via π–π interactions.48 Raman spectra of rGO, CuPc and CuPc/rGO hybrid are shown in Fig. 5. Raman spectrum of rGO shows two characteristic G and D peaks at 1595 and 1352 cm−1 corresponding to the first order scattering of E2g phonon of sp2 C atoms and breathing mode of k-point phonons of A1g symmetry (Fig. 5(a)).11 Raman spectra of CuPc shows peaks at 594, 678, 826, 1003, 1034 and 1102 cm−1 corresponding to A1g of benzene ring deformation, B1g macrocycle breathing, C–N stretching, isoindole in-plane bending, C–H bending and out of plane C–H bending, respectively. The peaks at 1335, 1446 and 1520 cm−1 corresponds to pyrrole stretching, isoindole ring stretching and displacement of the C–N–C bridge bond related to central copper metal ion of phthalocyanine molecule (Fig. 5(b)).49 In the CuPc/rGO hybrid, Raman spectra peaks at 594, 678, 826, 950, 1034, 1102, 1139, 1142, 1340, 1451 and 1527 cm−1 corresponds to phthalocyanine molecules. The G and D peaks at 1580 and 1340 cm−1 shows downshift by 15 and 12 cm−1 in comparison to rGO spectra (Fig. 5(c)), which confirms the electron transfer interaction between the CuPc molecules and rGO sheets.50 Further, intensity ratio (ID/IG) is used to identify the level of functionalization of C–C bonds. The ID/IG ratio of rGO and CuPc/rGO hybrid is found to be 0.86 and 1.07, respectively. The increase in ID/IG ratio is associated with the decrease in average size of sp2 domains in the hybrid, caused by the non-covalent attachment of CuPc molecules onto rGO sheets.51 Fig. 6 shows the UV-Vis spectra of rGO, CuPc and CuPc/rGO hybrid. The UV-Vis absorption spectra of rGO was found to be featureless52 whereas CuPc exhibited three prominent absorption peaks at 362, 615 and 696 nm. The absorption peaks at 615 and 696 nm correspond to Q band of the dimer and monomer of CuPc molecules, whereas the peak at 362 nm correspond to Soret band of the phthalocyanine ring.53 In CuPc/rGO hybrid, monomer peak of CuPc dominates over the CuPc dimer and Soret band. The monomer absorption peak was comparatively broader and red shifted by 6 nm, indicating transfer of electrons between phthalocyanine ring and rGO. This results in expanded macrocyclic conjugated structure of CuPc and reduced energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) to facilitate π–π interaction of CuPc molecule with rGO sheet.54 In order to check the thermal stability of rGO, CuPc and CuPc/rGO hybrid, TGA was performed under a nitrogen atmosphere (Fig. 7). TGA curve of rGO shows overall weight loss of nearly 35% in the investigated temperature range (40–900 °C) and majority of weight loss is due to water adsorbents in temperature range of 70 to 180 °C. While the TGA curve of CuPc shows major weight loss from 400 to 640 °C, due to the thermal decomposition of CuPc molecules. Similarly, CuPc/rGO hybrid exhibits a weight loss of nearly 10.8% between 390 to 638 °C, due to decomposition of the CuPc on rGO surface. Further, overall weight loss of hybrid is nearly 24% in the entire investigated temperature range, suggesting that only small amount of CuPc is attached to the rGO sheets and CuPc/rGO hybrid has good thermal stability.51,53
O peaks of rGO were shifted to 1214, 1564 and 1715 cm−1, respectively (Fig. 4(c)). The observed decrease in intensity and shifting of peaks after hybridization indicated the delocalization of electrons to form non-covalent attachment of CuPc on rGO surface via π–π interactions.48 Raman spectra of rGO, CuPc and CuPc/rGO hybrid are shown in Fig. 5. Raman spectrum of rGO shows two characteristic G and D peaks at 1595 and 1352 cm−1 corresponding to the first order scattering of E2g phonon of sp2 C atoms and breathing mode of k-point phonons of A1g symmetry (Fig. 5(a)).11 Raman spectra of CuPc shows peaks at 594, 678, 826, 1003, 1034 and 1102 cm−1 corresponding to A1g of benzene ring deformation, B1g macrocycle breathing, C–N stretching, isoindole in-plane bending, C–H bending and out of plane C–H bending, respectively. The peaks at 1335, 1446 and 1520 cm−1 corresponds to pyrrole stretching, isoindole ring stretching and displacement of the C–N–C bridge bond related to central copper metal ion of phthalocyanine molecule (Fig. 5(b)).49 In the CuPc/rGO hybrid, Raman spectra peaks at 594, 678, 826, 950, 1034, 1102, 1139, 1142, 1340, 1451 and 1527 cm−1 corresponds to phthalocyanine molecules. The G and D peaks at 1580 and 1340 cm−1 shows downshift by 15 and 12 cm−1 in comparison to rGO spectra (Fig. 5(c)), which confirms the electron transfer interaction between the CuPc molecules and rGO sheets.50 Further, intensity ratio (ID/IG) is used to identify the level of functionalization of C–C bonds. The ID/IG ratio of rGO and CuPc/rGO hybrid is found to be 0.86 and 1.07, respectively. The increase in ID/IG ratio is associated with the decrease in average size of sp2 domains in the hybrid, caused by the non-covalent attachment of CuPc molecules onto rGO sheets.51 Fig. 6 shows the UV-Vis spectra of rGO, CuPc and CuPc/rGO hybrid. The UV-Vis absorption spectra of rGO was found to be featureless52 whereas CuPc exhibited three prominent absorption peaks at 362, 615 and 696 nm. The absorption peaks at 615 and 696 nm correspond to Q band of the dimer and monomer of CuPc molecules, whereas the peak at 362 nm correspond to Soret band of the phthalocyanine ring.53 In CuPc/rGO hybrid, monomer peak of CuPc dominates over the CuPc dimer and Soret band. The monomer absorption peak was comparatively broader and red shifted by 6 nm, indicating transfer of electrons between phthalocyanine ring and rGO. This results in expanded macrocyclic conjugated structure of CuPc and reduced energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) to facilitate π–π interaction of CuPc molecule with rGO sheet.54 In order to check the thermal stability of rGO, CuPc and CuPc/rGO hybrid, TGA was performed under a nitrogen atmosphere (Fig. 7). TGA curve of rGO shows overall weight loss of nearly 35% in the investigated temperature range (40–900 °C) and majority of weight loss is due to water adsorbents in temperature range of 70 to 180 °C. While the TGA curve of CuPc shows major weight loss from 400 to 640 °C, due to the thermal decomposition of CuPc molecules. Similarly, CuPc/rGO hybrid exhibits a weight loss of nearly 10.8% between 390 to 638 °C, due to decomposition of the CuPc on rGO surface. Further, overall weight loss of hybrid is nearly 24% in the entire investigated temperature range, suggesting that only small amount of CuPc is attached to the rGO sheets and CuPc/rGO hybrid has good thermal stability.51,53
| Wavenumber (cm−1) | Assignment | Wavenumber (cm−1) | Assignment |
|---|---|---|---|
| 728 | Out of plane C–H | 1120 | C–C stretching |
| 753 | Out of plane C–H deformation | 1166 | In plane C–N–C bending |
| 780 | Out of plane C–H deformation | 1286 | C–O stretching |
| 876 | Benzene wagging | 1333 | C–H bending |
| 1021 | Skeletal mode of central ring | 1385 | Isoindole stretching |
| 1067 | In plane C–H deformation | 1420 | In plane pyrrole stretching |
| 1087 | Cu–N stretching | 1506 | Pyrrole stretching |
3.2 Gas sensing characteristics
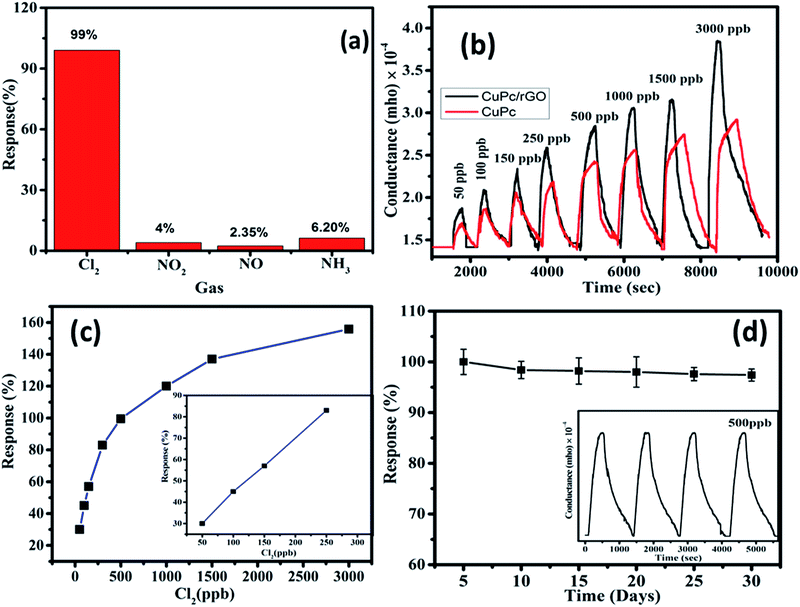
To study the gas sensing characteristics of CuPc/rGO hybrid sensor, we exposed the sensor to 500 ppb of Cl2, NO2, NO and NH3 at room temperature to check its selectivity (Fig. 8(a)). It was noted that sensor exhibited a response of 99% for Cl2 whereas, the response for NO2, NO and NH3 gases were sluggish and found to be 4%, 2.35% and 6.20%, respectively. Thus, CuPc/rGO hybrid sensor is found to be highly selective towards Cl2 as compared to other exposed gases. The conductance–time plots of CuPc and CuPc/rGO hybrid sensor for different doses of Cl2 have been shown in Fig. 8(b). It demonstrates that there was an increase in the sensor conductance after exposure to Cl2 and it gets saturated after some time and after purging air, sensor conductance reaches to its original baseline, thus showing good reversibility of sensor. While in case of CuPc sensor, the sensor response is lesser in comparison to CuPc/rGO hybrid sensor. For 3000 ppb of Cl2, hybrid sensor exhibits response as large as 156% with a response/recovery time of 190/545 s, respectively. The faster response time, reversible and reproducible response is due to large number of Cl2 selective sensing sites provided by CuPc nanoflowers which are further non-covalently attached to large surface area rGO sheets in CuPc/rGO hybrid. Fig. 8(c) shows that the response of hybrid sensor increases from 30% to 156% with an increase in Cl2 concentration (50–3000 ppb). The linear region observed in lower concentration range (50–250 ppb) has been shown in the inset of Fig. 8(c). This behaviour can be understood on the basis of effective occupancy of active sites. As seen from TEM images of the hybrid, nanoflower structures of CuPc molecules onto rGO sheets provide large number of active sites. At low concentration, abundant active sites are available and response increases with increase in concentration. To check the reproducibility of the response, CuPc/rGO sensor is repeatedly exposed to 500 ppb of Cl2 at room temperature and it is observed that response of sensor remains nearly same (inset of Fig. 8(d)) without any drift in the baseline conductance and hence sensor is reproducible. We have also investigated the repeatability and stability of CuPc/rGO hybrid sensor devices. Out of different fabricated sensors, set of randomly selected five sensors were checked for Cl2 sensing at room temperature. A small deviation in sensor response from its average value indicates high degree of repeatability of fabricated devices. The response of the sensors remains almost same even after 30 days (Fig. 8(d)), which depicts that the sensors have a long-term stability.The lowest detectable concentration, being reported here, is dependent on the experimental set up used. However, the minimum detection limit of sensor can be calculable from signal to noise ratio (S/N) using the formula,55,56
3.3 Gas sensing mechanism
The underlying mechanism of CuPc/rGO hybrid sensor for Cl2 has been investigated using Raman and EIS. Raman spectra of CuPc/rGO hybrid sensor with exposure to 1000 ppb of Cl2 (Fig. 9) shows a change in position and intensity of peaks w.r.t. unexposed sensor. The peaks at 594, 678, 831 and 1143 cm−1 corresponding to the region of macrocyclic vibrations of CuPc molecules45,55 shows a downshift of 2 cm−1 on Cl2 exposure (Fig. 9(b)). A significant shift of 5 and 6 cm−1 is observed in the peaks at 1451 and 1527 cm−1 corresponding to isoindole ring vibration and displacement of the C–N–C bridge bond related to central copper metal ions.56 This confirms that interaction of Cl2 most probably occurs at copper metal ions of CuPc/rGO hybrid. After purging air, peaks again recover to their original position indicating reversibility of fabricated sensor (Fig. 9(c)). EIS has been used to understand the contributions of bulk resistance of rGO sheets (R0), resistance and capacitance across the grain boundaries between CuPc/rGO grains (R1 and C1), contact resistance and capacitance between hybrid and metal electrode (R2 and C2) towards sensor response. Fig. 10(A) shows the Nyquist plot of CuPc/rGO hybrid plotted between real component (Z′ (Ω)) and imaginary component (−Z′′ (Ω)) from low (0.1 Hz) to high frequency (1 MHz). The impedance measurement has been carried out in the presence of air and after exposure to 1000 ppb of Cl2. A single semicircle is observed before and after exposure to Cl2 and is fitted (solid line) using equivalent circuit consisting of R0 in series with two RC networks as shown in Fig. 10(B). It is observed that, after exposure of Cl2 the diameter of semicircle is reduced leading to decrease in R0, R1 and R2 and corresponding increase in C1 and C2 in comparison to unexposed Cl2 (Table 2). The prominent change in R1 and C1 suggests that incoming Cl2 gas molecules physiosorbed onto grain boundaries separating grains of CuPc molecules and rGO sheets, which are the dominating sites for physio-sorption. This physio-sorption improves conductivity of the sensor through electron charge transfer from CuPc to rGO sheets, which acts as the conducting network between them.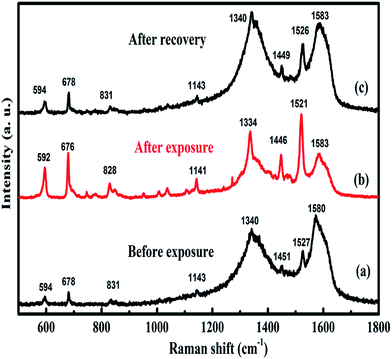 | ||
| Fig. 9 Raman spectra of CuPc/rGO hybrid recorded (a) before exposure, (b) after exposure and (c) after recovery to 1000 ppb of Cl2. | ||
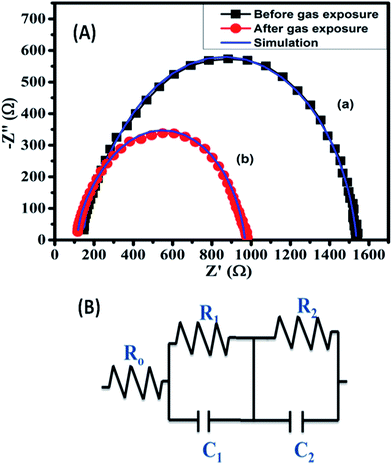 | ||
| Fig. 10 (A) Impedance spectra of CuPc/rGO hybrid (a) before and (b) after exposure to 1000 ppb of Cl2 and (B) equivalent circuit to interpret the impedance spectroscopy data. | ||
| R0 (Ω) | R1 (Ω) | R2 (Ω) | C1 (nF) | C2 (nF) | |
|---|---|---|---|---|---|
| Unexposed gas | 149 | 1150 | 216 | 9.78 | 7.71 |
| Exposed gas | 119 | 653 | 180 | 13.5 | 9.16 |
4. Conclusions
We have demonstrated that small organic semiconducting molecules with controlled nanostructured morphology on combining with conducting platform can be utilized as selective and highly sensitive sensor. The illustration has been made by combining the properties of CuPc and rGO to produce Cl2 selective room temperature chemiresistor with minimum detection limit of 1.97 ppb. Structural and spectroscopic studies confirmed that CuPc nanoflower structures are non-covalently attached on rGO sheets. The physio-sorption of incoming Cl2 molecules on central metal ion present on the grains of CuPc decorated rGO sheets is responsible for selective Cl2 detection. Moreover, nanoflower morphology of CuPc provides large number of physio-sorption sites and conducting network of large surface area of rGO sheets improve the Cl2 sensing of present sensor.Acknowledgements
The authors would like to acknowledge DAE-BRNS, Mumbai, India for providing financial assistance to carry out this research work. One of the authors, Sanjeev Kumar is grateful to UGC-RGNF New Delhi, India for awarding senior research fellowship.References
- A. K. Saroha, J. Chem. Health Saf., 2006, 13, 5–11 CrossRef CAS.
- S. Usha, S. Mishra and B. Gupta, Materials, 2015, 8, 2204 CrossRef.
- C. F. Poole, J. Chromatogr. A, 2015, 1421, 137–153 CrossRef CAS PubMed.
- H. Zhang, J. Li, H. Zhang, X. Liang, C. Yin, Q. Diao, J. Zheng and G. Lu, Sens. Actuators, B, 2013, 180, 66–70 CrossRef CAS.
- M. Ralfs and J. Heinze, Sens. Actuators, B, 1997, 44, 257–261 CrossRef CAS.
- L. Huixia, L. Yong, T. Yanni, L. Lanlan, Z. Qing, L. Kun and T. Hanchun, New J. Chem., 2015, 39, 3865–3874 RSC.
- Y. Xiong, J. Tan, C. Wang, J. Wu, Q. Wang, J. Chen, S. Fang and M. Duan, Sens. Actuators, B, 2017, 245, 674–682 CrossRef CAS.
- K. Senthilkumar and J.-M. Zen, Electrochem. Commun., 2014, 46, 87–90 CrossRef CAS.
- S. M. Ingole, S. T. Navale, Y. H. Navale, D. K. Bandgar, F. J. Stadler, R. S. Mane, N. S. Ramgir, S. K. Gupta, D. K. Aswal and V. B. Patil, J. Colloid Interface Sci., 2017, 493, 162–170 CrossRef CAS PubMed.
- S. T. Navale, V. V. Jadhav, K. K. Tehare, R. U. R. Sagar, C. S. Biswas, M. Galluzzi, W. Liang, V. B. Patil, R. S. Mane and F. J. Stadler, Sens. Actuators, B, 2017, 238, 1102–1110 CrossRef CAS.
- D. R. Kumar, S. Kesavan, T. T. Nguyen, J. Hwang, C. Lamiel and J.-J. Shim, Sens. Actuators, B, 2017, 240, 818–828 CrossRef CAS.
- A. Singh, A. Kumar, A. Kumar, S. Samanta, N. Joshi, V. Balouria, A. K. Debnath, R. Prasad, Z. Salmi, M. M. Chehimi, D. K. Aswal and S. K. Gupta, Appl. Phys. Lett., 2013, 102, 132107 CrossRef.
- A. Kumar, S. Samanta, S. Latha, A. K. Debnath, A. Singh, K. P. Muthe and H. C. Barshilia, RSC Adv., 2017, 7, 4135–4143 RSC.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- F. Yavari, E. Castillo, H. Gullapalli, P. M. Ajayan and N. Koratkar, Appl. Phys. Lett., 2012, 100, 203120 CrossRef.
- M. Gautam and A. H. Jayatissa, J. Appl. Phys., 2012, 112, 064304 CrossRef.
- B. Paulus and K. Rosciszewski, Int. J. Quantum Chem., 2009, 109, 3055–3062 CrossRef CAS.
- H. J. Yoon, D. H. Jun, J. H. Yang, Z. Zhou, S. S. Yang and M. M.-C. Cheng, Sens. Actuators, B, 2011, 157, 310–313 CrossRef CAS.
- R. C. Ehemann, P. S. Krstić, J. Dadras, P. R. Kent and J. Jakowski, Nanoscale Res. Lett., 2012, 7, 198 CrossRef PubMed.
- Y. Ren, C. Zhu, W. Cai, H. Li, H. Ji, I. Kholmanov, Y. Wu, R. D. Piner and R. S. Ruoff, Appl. Phys. Lett., 2012, 100, 163114 CrossRef.
- K. R. Nemade and S. A. Waghuley, J. Electron. Mater., 2013, 42, 2857–2866 CrossRef CAS.
- L. Ganhua, E. O. Leonidas and C. Junhong, Nanotechnology, 2009, 20, 445502 CrossRef PubMed.
- R. K. Joshi, H. Gomez, F. Alvi and A. Kumar, J. Phys. Chem. C, 2010, 114, 6610–6613 CAS.
- H. Y. Jeong, D.-S. Lee, H. K. Choi, D. H. Lee, J.-E. Kim, J. Y. Lee, W. J. Lee, S. O. Kim and S.-Y. Choi, Appl. Phys. Lett., 2010, 96, 213105 CrossRef.
- W. Li, X. Geng, Y. Guo, J. Rong, Y. Gong, L. Wu, X. Zhang, P. Li, J. Xu, G. Cheng, M. Sun and L. Liu, ACS Nano, 2011, 5, 6955–6961 CrossRef CAS PubMed.
- V. Dua, S. P. Surwade, S. Ammu, S. R. Agnihotra, S. Jain, K. E. Roberts, S. Park, R. S. Ruoff and S. K. Manohar, Angew. Chem., Int. Ed. Engl., 2010, 49, 2154–2157 CrossRef CAS PubMed.
- L. Huang, Z. Wang, J. Zhang, J. Pu, Y. Lin, S. Xu, L. Shen, Q. Chen and W. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 7426–7433 CAS.
- R. Furue, E. P. Koveke, S. Sugimoto, Y. Shudo, S. Hayami, S.-I. Ohira and K. Toda, Sens. Actuators, B, 2017, 240, 657–663 CrossRef CAS.
- Q. Feng, X. Li, J. Wang and A. M. Gaskov, Sens. Actuators, B, 2016, 222, 864–870 CrossRef CAS.
- U. Yaqoob, A. S. M. I. Uddin and G.-S. Chung, Sens. Actuators, B, 2016, 224, 738–746 CrossRef CAS.
- S. Liu, Z. Wang, Y. Zhang, C. Zhang and T. Zhang, Sens. Actuators, B, 2015, 211, 318–324 CrossRef CAS.
- X. Li, B. Wang, X. Wang, X. Zhou, Z. Chen, C. He, Z. Yu and Y. Wu, Nanoscale Res. Lett., 2015, 10, 373 CrossRef PubMed.
- X. Zhou, X. Wang, B. Wang, Z. Chen, C. He and Y. Wu, Sens. Actuators, B, 2014, 193, 340–348 CrossRef CAS.
- L. Al-Mashat, K. Shin, K. Kalantar-zadeh, J. D. Plessis, S. H. Han, R. W. Kojima, R. B. Kaner, D. Li, X. Gou, S. J. Ippolito and W. Wlodarski, J. Phys. Chem. C, 2010, 114, 16168–16173 CAS.
- Y. Zhao, X.-g. Li, X. Zhou and Y.-n. Zhang, Sens. Actuators, B, 2016, 231, 324–340 CrossRef CAS.
- J. Zhao, Z. Qiao, Y. Zhang, T. Zou, L. Yu, L. Luo, X. Wang, Y. Yang, H. Wang and L. Tang, AIP Adv., 2016, 6, 095303 CrossRef.
- R. Saini, A. Mahajan, R. K. Bedi, D. K. Aswal and A. K. Debnath, Sens. Actuators, B, 2014, 203, 17–24 CrossRef CAS.
- R. Saini, A. Mahajan, R. K. Bedi, D. K. Aswal and A. K. Debnath, Sens. Actuators, B, 2014, 198, 164–172 CrossRef CAS.
- R. Saini, A. Mahajan, R. K. Bedi, D. K. Aswal and A. K. Debnath, RSC Adv., 2014, 4, 15945–15951 RSC.
- F. I. Bohrer, C. N. Colesniuc, J. Park, M. E. Ruidiaz, I. K. Schuller, A. C. Kummel and W. C. Trogler, J. Am. Chem. Soc., 2009, 131, 478–485 CrossRef CAS PubMed.
- R. D. Yang, J. Park, C. N. Colesniuc, I. K. Schuller, J. E. Royer, W. C. Trogler and A. C. Kummel, J. Chem. Phys., 2009, 130, 164703 CrossRef PubMed.
- X. Ding, H. Xu, L. Zhang, D. Jiang and A. Lu, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1999, 337, 481–484 CrossRef CAS.
- B. Wang, X. Zhou, Y. Wu, Z. Chen, C. He and X. Zuo, Sens. Actuators, B, 2012, 161, 498–503 CrossRef CAS.
- G. S. S. Saini, S. Sukhwinder, K. Sarvpreet, K. Ranjan, S. Vasant and S. K. Tripathi, J. Phys.: Condens. Matter, 2009, 21, 225006 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- T. V. Basova, I. V. Jushina, A. G. Gürek, D. Atilla and V. Ahsen, Dyes Pigm., 2009, 80, 67–72 CrossRef CAS.
- B. Wang, Y. Wu, X. Wang, Z. Chen and C. He, Sens. Actuators, B, 2014, 190, 157–164 CrossRef CAS.
- R. Prabakaran, R. Kesavamoorthy, G. L. N. Reddy and F. P. Xavier, Phys. Status Solidi B, 2002, 229, 1175–1186 CrossRef CAS.
- A. Chunder, T. Pal, S. I. Khondaker and L. Zhai, J. Phys. Chem. C, 2010, 114, 15129–15135 CAS.
- J.-P. Zhong, Y.-J. Fan, H. Wang, R.-X. Wang, L.-L. Fan, X.-C. Shen and Z.-J. Shi, J. Power Sources, 2013, 242, 208–215 CrossRef CAS.
- H. Zhao, Y. Zhang, B. Zhao, Y. Chang and Z. Li, Environ. Sci. Technol., 2012, 46, 5198–5204 CrossRef CAS PubMed.
- B.-P. Jiang, L.-F. Hu, D.-J. Wang, S.-C. Ji, X.-C. Shen and H. Liang, J. Mater. Chem. B, 2014, 2, 7141–7148 RSC.
- S. A. Mamuru, K. I. Ozoemena, T. Fukuda, N. Kobayashi and T. Nyokong, Electrochim. Acta, 2010, 55, 6367–6375 CrossRef CAS.
- L. L. Gladkov, V. V. Gromak and V. K. Konstantinova, J. Appl. Spectrosc., 2007, 74, 328–332 CrossRef CAS.
- M. Szybowicz and J. Makowiecki, J. Mater. Sci., 2012, 47, 1522–1530 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |