Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effective removal of surface-bound cetyltrimethylammonium ions from thiol-monolayer-protected Au nanorods by treatment with dimethyl sulfoxide/citric acid†
Keisuke Nishida and
Hideya Kawasaki *
*
Faculty of Chemistry, Materials and Bioengineering, Kansai University, 3-3-35 Yamate-cho, Suita 564-8680, Japan. E-mail: hkawa@kansai-u.ac.jp
First published on 24th March 2017
Abstract
Cetyltrimethylammonium bromide (CTAB)-based surfactants are typically used as morphology-directing/stabilising agents for gold nanorods (AuNRs), forming bilayers on their surface. However, the biological applications of AuNRs require the removal of surface-bound CTAB due its high toxicity and the poor colloidal stability of CTAB-covered AuNRs in biological media. Herein, we report a simple and effective strategy for removing surface-bound cetyltrimethylammonium (CTA) cations from poly(ethylene glycol)thiolate-protected AuNRs (PEG-AuNRs) by treatment with dimethyl sulfoxide/citric acid (DMSO/Cit), achieving residual CTA ion levels that cannot be detected by highly sensitive mass spectrometry or X-ray photoelectron spectroscopy (XPS) techniques. The DMSO/Cit treatment is thought to destabilise the Ag–Br–CTA complex on AuNRs, since citric acid forms strongly bound chelate complexes with CTA cations.
1. Introduction
The unique optical properties of gold nanorods (AuNRs) make them a popular research target for applications such as imaging,1,2 biomolecular sensing,3,4 photothermal therapy,5,6 and molecule delivery in biological systems.7,8 Although cetyltrimethylammonium bromide (CTAB)-based surfactants are typically utilised as morphology-directing agents and dispersion stabilisers9–11 in the synthesis of AuNRs, the biological applications of these nanorods require the removal of surface-bound CTAB due to its high toxicity and the poor colloidal stability of CTAB-protected AuNRs (CTAB-AuNRs) in biological media.9–11Several AuNR surface modifications by ligand exchange have been proposed to enhance the biocompatibility and colloidal stability of AuNRs in biological media.12–19 Poly(ethylene glycol)thiol (PEG-SH) is one of the most important ligands used for this purpose, imparting superior biocompatibility and colloidal stability to PEG-S-protected AuNRs (PEG-AuNRs).7,20–24 Fabrication of PEG-AuNRs with subsequent CTAB stripping has been attempted using several ligand exchange strategies such as the use of additives,19 buffers,23 ethanol,22 and extraction with chloroform.24 However, CTAB molecules cannot be easily displaced from the AuNR surface by thiolate ligand exchange, and a certain fraction of CTAB remains bound (particularly on the lateral faces of AuNRs) to PEG-AuNRs.12,24,25
Surface-assisted laser desorption/ionisation mass spectrometry (SALDI-MS) is probably the most sensitive method of detecting residual CTAB on AuNRs, since quaternary ammonium ions have a strong capacity to be desorbed and ionised in LDI-MS.24,25 The ionisation principle of SALDI is similar to that of matrix-assisted laser desorption/ionisation mass spectrometry (MALDI-MS),26–28 with the UV-absorbing organic matrix commonly used in MALDI replaced by UV-absorbing nanomaterials such as gold nanoparticles.28 The organic matrix-free ionisation technique of SALDI-MS can avoid interference caused by organic matrix molecules in the low-mass region (below 500 Da). In particular, SALDI-MS allows highly sensitive detection of analytes adsorbed on the surface of gold nanoparticles via energy transfer from gold to the analyte.26–28 For instance, Liz-Marzán and co-workers demonstrated the presence of CTAB on PEG-AuNRs by SALDI-MS.24 Schulz and co-workers reported that although CTAB could not be identified in purified samples of PEG-AuNRs by nuclear magnetic resonance and X-ray photoelectron spectroscopy (XPS), the presence of residual CTA ions could be confirmed by SALDI-MS.25 Thus, the development of an effective strategy for removing surface-bound CTA cations from PEG-AuNRs is highly challenging.12,24,25
Herein, we have developed an effective strategy for removing surface-bound CTA cations from PEG-AuNRs via simple washing with a dimethyl sulfoxide/citric acid (DMSO/Cit) solution. CTAB removal was confirmed by SALDI-MS and XPS analyses and explained by the destabilisation of the Ag–Br–CTA complex on the AuNR surface via DMSO/Cit treatment.
2. Experimental
2.1. Chemicals
Cetyltrimethylammonium bromide (CTAB) (99%), L-ascorbic acid (AA, 99%), and sodium borohydride (NaBH4, 99.9%) were obtained from Sigma-Aldrich. Poly(ethylene glycol)thiol (PEG-SH, Mw = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, ME-20SH) was obtained from NOF Corp., Japan. Polystyrene sulfonate sodium (PSS, Mw = 75
000, ME-20SH) was obtained from NOF Corp., Japan. Polystyrene sulfonate sodium (PSS, Mw = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Polysciences, Inc. HAuCl4·3H2O (99.9%), AgNO3 (99%), citric acid (Cit, Wako Special Grade), dimethyl sulfoxide (DMSO, 99%), sodium chloride, and sodium thiosulfate pentahydrate (Na2S2O3·5H2O, 99%) were obtained from Wako Chemical Co., Japan.
000) was obtained from Polysciences, Inc. HAuCl4·3H2O (99.9%), AgNO3 (99%), citric acid (Cit, Wako Special Grade), dimethyl sulfoxide (DMSO, 99%), sodium chloride, and sodium thiosulfate pentahydrate (Na2S2O3·5H2O, 99%) were obtained from Wako Chemical Co., Japan.
2.2. Synthesis of CTAB-protected AuNRs (CTAB-AuNRs)
CTAB-AuNRs were prepared according to the photomediated method developed by Niidome and co-workers.29 Briefly, acetone (0.1 mL) was added to a mixture of aqueous solutions of CTAB (5 mL, 0.5 M), HAuCl4 (0.5 mL, 24 mM), and AgNO3 (0.5 mL, 10 mM). Upon addition of ascorbic acid solution (0.5 mL, 40 mM), the yellow colour of the reaction mixture vanished, and the resulting solution was irradiated using a high-pressure mercury lamp for 5 min and left to stand for 1 h.2.3. Synthesis of PEG-S-protected AuNRs (PEG-AuNRs)
The as-prepared CTAB-AuNR solution (absorbance = 0.3 at 900 nm; 1 mL) was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, decanted, and re-suspended in 1 mL of water to remove excess CTAB. An aqueous solution of PEG-SH (0.05 wt%/Au atom; 0.01 mL) was added to the AuNR dispersion, and the resulting mixture was stirred for 12 h at 30 °C. Subsequently, AuNRs were collected by centrifugation at 10
000 rpm for 10 min, decanted, and re-suspended in 1 mL of water to remove excess CTAB. An aqueous solution of PEG-SH (0.05 wt%/Au atom; 0.01 mL) was added to the AuNR dispersion, and the resulting mixture was stirred for 12 h at 30 °C. Subsequently, AuNRs were collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 h, decanted, and re-suspended in 1 mL of water, and the process was repeated two more times. Finally, the produced PEG-AuNRs were dialyzed for two days using a dialysis membrane. It is unlikely that some degree of AuNRs fuses during the ligand exchange process, since UV-vis bands were unchanged before and after the ligand exchange (Fig. S1 in ESI†).
000 rpm for 3 h, decanted, and re-suspended in 1 mL of water, and the process was repeated two more times. Finally, the produced PEG-AuNRs were dialyzed for two days using a dialysis membrane. It is unlikely that some degree of AuNRs fuses during the ligand exchange process, since UV-vis bands were unchanged before and after the ligand exchange (Fig. S1 in ESI†).
2.4. Synthesis of PSS-protected AuNRs (PSS-AuNRs)
A solution of CTAB-AuNRs (absorbance = 0.3 at 900 nm; 1 mL) was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 10 min, decanted, and re-suspended in 1 mL of water to remove excess CTAB, and the process was repeated two more times. The obtained precipitate of AuNRs was re-suspended in aqueous PSS (1 mL, 0.38 wt%) and the resulting solution was incubated at 30 °C for 1 h, followed by another three precipitation/re-suspension cycles. At the third time, an incubation time of 24 h was used. After incubation, the PSS-AuNR solution was centrifuged at 14
800 rpm for 10 min, decanted, and re-suspended in 1 mL of water to remove excess CTAB, and the process was repeated two more times. The obtained precipitate of AuNRs was re-suspended in aqueous PSS (1 mL, 0.38 wt%) and the resulting solution was incubated at 30 °C for 1 h, followed by another three precipitation/re-suspension cycles. At the third time, an incubation time of 24 h was used. After incubation, the PSS-AuNR solution was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 10 min, decanted, and re-suspended in 1 mL of water. The CTAB-to-PSS ligand exchange on AuNRs was confirmed by the zeta potential change (from +25 mV for CTAB-AuNRs to −41 mV for PSS-AuNRs) in the presence of 1 mM NaCl using a Zetasizer Nano ZSP instrument (Malvern Instruments).
800 rpm for 10 min, decanted, and re-suspended in 1 mL of water. The CTAB-to-PSS ligand exchange on AuNRs was confirmed by the zeta potential change (from +25 mV for CTAB-AuNRs to −41 mV for PSS-AuNRs) in the presence of 1 mM NaCl using a Zetasizer Nano ZSP instrument (Malvern Instruments).
2.5. Synthesis of citric acid-protected AuNRs (Cit-AuNRs)
Cit-AuNRs were prepared according to a previously described method.182.6. CTA removal from PEG-AuNRs by DMSO/Cit treatment
Step 1: a solution of PEG-AuNRs (absorbance = 0.3 at 900 nm; 1 mL) was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 30 min, decanted, and the obtained pellet was re-suspended in 1 mL of water. This process was repeated two more times.
800 rpm for 30 min, decanted, and the obtained pellet was re-suspended in 1 mL of water. This process was repeated two more times.
Step 2: the PEG-AuNR solution obtained above was further centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 30 min, decanted, and the obtained pellet was re-suspended in a solution of citric acid in DMSO (1 mL; 2, 10, or 100 mM). The resulting solution was gently stirred for 2 h at 30 °C, and the above process was repeated two more times.
800 rpm for 30 min, decanted, and the obtained pellet was re-suspended in a solution of citric acid in DMSO (1 mL; 2, 10, or 100 mM). The resulting solution was gently stirred for 2 h at 30 °C, and the above process was repeated two more times.
Step 3: the purified PEG-AuNR solution was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 30 min, and the obtained pellet was re-dispersed in water. The above process was repeated two more times.
800 rpm for 30 min, and the obtained pellet was re-dispersed in water. The above process was repeated two more times.
For comparison, PEG-AuNRs were also treated with DMSO, 10 mM aqueous citric acid, and 10 mM aqueous Na2S2O3, using the same washing procedure as for DMSO/Cit treatment.
2.7. Characterisation
Field-emission scanning electron microscopy (FE-SEM; JSM-6700, JEOL, Japan) imaging was performed at an acceleration voltage of 5–30 kV. Absorption spectra were recorded between 400 and 1100 nm using a V-670 UV/visible/NIR spectrophotometer (JASCO Corporation, Japan). Surface analysis was performed by XPS (Al Kα radiation, 1486.60 eV; PHI 5700 ESCA System, USA).For SALDI-MS analyses, a 1 mL aliquot of PEG-AuNR solution (absorbance = 1.0 at 900 nm) was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 30 min, decanted, and 1 μL of precipitate solution was spotted on the MALDI plate and dried under reduced pressure. SALDI mass spectra were recorded in linear mode using an AXIMA CFR TOF mass spectrometer (Shimadzu, Kyoto, Japan) with a pulsed nitrogen laser (337 nm). One hundred laser shots were used to acquire mass spectra. Analyte ions were accelerated at 20 kV under delayed extraction conditions.
800 rpm for 30 min, decanted, and 1 μL of precipitate solution was spotted on the MALDI plate and dried under reduced pressure. SALDI mass spectra were recorded in linear mode using an AXIMA CFR TOF mass spectrometer (Shimadzu, Kyoto, Japan) with a pulsed nitrogen laser (337 nm). One hundred laser shots were used to acquire mass spectra. Analyte ions were accelerated at 20 kV under delayed extraction conditions.
3. Results and discussion
Fig. 1a shows an SEM image of PEG-AuNRs prepared from CTAB-AuNRs by ligand exchange,7,21–24 revealing that the as-synthesised AuNRs exhibited an aspect ratio of ∼5 (length: 45 ± 2 nm, width: 9.0 ± 1 nm). The UV-vis absorbance spectrum of PEG-AuNRs showed bands at 508 and 889 nm, corresponding to transverse and longitudinal surface plasmon resonance of AuNRs, respectively (Fig. 1b). UV-vis bands were unchanged after treating PEG-AuNRs with DMSO/Cit, DMSO, and Cit, indicating that the morphology and size of PEG-AuNRs were preserved.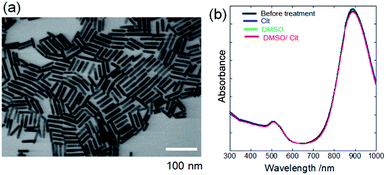 | ||
| Fig. 1 (a) SEM image of PEG-AuNRs prepared from CTAB-AuNRs by ligand exchange. (b) UV-vis absorbance spectra of PEG-AuNRs before and after treating PEG-AuNRs with DMSO/Cit (10 mM), DMSO, and Cit. | ||
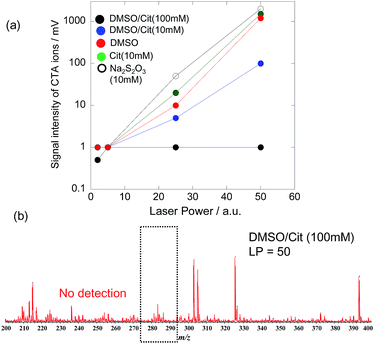
Fig. 2 shows SALDI mass spectra of CTAB-AuNRs, PSS-AuNRs, and Cit-AuNRs obtained at a laser power (LP) of two. For CTAB-AuNRs, a strong CTA cation signal with an intensity of ∼1300 mV was observed at m/z = 284. The intensity of this signal decreased after ligand exchange, equalling ∼260 mV for PSS-AuNRs and ∼70 mV for Cit-AuNRs, and reflecting the decreased amount of CTA cations (Fig. 2a). However, these signals were still observable, indicating the presence of residual CTA cations. The signal of CTA cations (∼50 mV) was also observed in SALDI mass spectra of untreated PEG-AuNRs at an LP of two (Fig. 2b).
To remove residual CTA cations, PEG-AuNRs were treated with DMSO/Cit (10 mM), with other treatments performed for comparison (10 mM Cit, 10 mM Na2S2O3, and DMSO). As a result of these treatments, the SALDI-MS intensities of CTA ion signals dramatically decreased. Since the intensities of these signals increase with increasing LP, we determined them at different LP values, as shown in Fig. 3a. For PEG-AuNRs subjected to DMSO/Cit (100 mM) treatment, these intensities were very low (<1 mV) or not detectable even at a high LP of 50. In contrast, strong signals were observed at LP = 50 for PEG-AuNRs subjected to other treatments. For the DMSO/Cit treatment, the concentration of citric acid is important for efficient CTA ion removal. Thus, treatment with 10 mM citric acid in DMSO proved to be insufficient for removal of CTA cations from PEG-AuNRs, as evidenced by the strong CTA ion signal (Fig. 3a). Treatment with 100 mM citric acid in DMSO was most effective, with no CTA cations observed on treated PEG-AuNRs even at LP = 50 (Fig. 3b).
It should be noted that the SALDI-MS of CTA cations on AuNRs lacks quantitative information. The peak intensity of CTA cations would depend on the adsorption states as well as the adsorption amount; we cannot change the adsorption states of CTA cations on AuNRs systematically. As a result, it is difficult to construct the calibration curve of adsorbed CTA cations on AuNRs. Therefore, we examined the detection limit of CTA cations on AuNRs by spiking of CTAB solutions into the AuNRs after DMSO/Cit (100 mM) treatment. The detection limit was 10 ppt (Fig. S2†). Therefore, we speculate that the present method has removed CTA cations to a concentration lower than 10 ppt.
Surface analysis of PEG-AuNRs was performed using XPS,18,19,30–32 which confirmed the removal of residual CTA cations from PEG-AuNRs after DMSO/Cit (100 mM) treatment. Fig. 4 shows a survey XPS spectrum (a) and N 1s (b) and Ag 3d (c) high-resolution (regional) XPS spectra of PEG-AuNRs after DMSO/Cit (100 mM) treatment. For treated PEG-AuNRs, the N 1s (401 eV) peak was absent, supporting the absence of CTA cations indicated by SALDI-MS, whereas Ag 3d peaks at 368 and 373 eV were still observed.
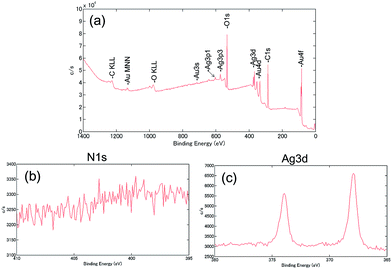 | ||
| Fig. 4 (a) A survey XPS spectrum and (b) N 1s and (c) Ag 3d high-resolution (regional) XPS spectra of PEG-AuNRs after DMSO/Cit (100 mM) treatment. | ||
Several studies have confirmed that CTAB is present on the surface of AuNRs as a bilayer, with electrostatic interactions of the ammonium headgroup and AuNR surface involving metal (Au or Ag)–bromide complexes.33–35 It is proposed that silver provides the aspect ratio control of AuNRs.29,36 According to Hubert et al., complementary XPS and quartz crystal microbalance analyses indicate that the Ag–Br–CTA complex is much stronger adsorbed on the Au surface than pure CTAB and other halide-CTAs.31 DMSO dissolves the AgBr37 and the inhibitory effect of DMSO on micelle formation of CTAB was also reported.38 Citric acid is an effective chelating agent, forming complexes with CTA cations and contributing to the dispersion stability of AuNRs via citrate adsorption on their surface.18 The CTA-citrate complex is believed to dissolve in DMSO, resulting in effective CTA cation removal from PEG-AuNRs. Most likely, therefore, the DMSO/Cit treatment destabilises the Ag–Br–CTA complex on AuNRs.
However, the presence of a small amount of residual Ag on PEG-AuNRs even after DMSO/Cit treatment was demonstrated by XPS, which is possibly explained by the formation of a Au/Ag alloy on the surface. The removal of Ag atoms from PEG-AuNRs was attempted using oxidative etching with sodium thiosulfate. Silver halides (e.g., AgBr) are known to dissolve in aqueous thiosulfate: 2S2O32− + AgBr → [Ag(S2O3)2]3− + Br−.39 However, treatment of PEG-AuNRs with sodium thiosulfate and the resulting oxidative surface etching induced their aggregation. Thus, the DMSO/Cit treatment is more suitable for removing CTA cations, avoiding nanorod aggregation.
4. Conclusions
In this study, we consistently demonstrated a simple and effective strategy for removing surface-bound CTA ions from PEG-AuNRs by DMSO/Cit treatment coupled with advanced purification strategies. The above removal is highly efficient, as proven by the fact that CTA ions could not be unambiguously identified by highly sensitive SALDI-MS and XPS techniques. Most likely, the DMSO/Cit treatment destabilises the Ag–Br–CTA complex on AuNRs, and the formed chelate CTA-citrate complexes dissolve in DMSO, achieving effective removal of CTA ions. Moreover, citric acid also contributes to the stability of AuNR dispersions via citrate ion adsorption on the nanorod surface. If the DMSO/Cit treatment is proven to be effective for other thiol-monolayer-protected AuNRs in future studies, it will enable the synthesis of biocompatible AuNRs with very low toxicity that are potentially applicable in biological/medical imaging and photothermal/photodynamic therapy.Acknowledgements
H. K. wishes to acknowledge the help of Dr Daigou Mizoguchi of Dai Nippon Toryo Co., Ltd. in interpreting the significance of the obtained results and providing samples of CTAB-AuNRs and PEG-AuNRs, and Prof. Yasushi Obora of Kansai University for zeta potential measurements of AuNRs. This work was supported by JSPS KAKENHI (Grant no. 15H03520 and 15H03526) and the “Strategic Project to Support the Formation of Research Bases at Private Universities” with a matching Fund Subsidy from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.Notes and references
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115 CrossRef CAS PubMed.
- N. J. Durr, T. Larson, D. K. Smith, B. A. Korgel, K. Sokolov and A. Ben-Yakar, Nano Lett., 2007, 7, 941 CrossRef CAS PubMed.
- P. K. Sudeep, S. T. Shibu Joseph and K. George Thomas, J. Am. Chem. Soc., 2005, 127, 6516 CrossRef CAS PubMed.
- C. Yu and J. Irudayaraj, Anal. Chem., 2007, 79, 572 CrossRef CAS PubMed.
- E. B. Dickerson, E. C. Dreaden, X. Huang, I. H. El-Sayed, H. Chu, S. Pushpanketh, J. F. McDonald and M. A. El-Sayed, Cancer Lett., 2008, 269, 57 CrossRef CAS PubMed.
- T. B. Huff, L. Tong, Y. Zhao, M. N. Hansen, J.-X. Cheng and A. We, Nanomedicine, 2007, 2, 125 CrossRef CAS PubMed.
- T. Niidome, M. Yamagata, Y. Okamoto, Y. Akiyama, H. Takahashi, T. Kawano, Y. Katayama and Y. Niidome, J. Controlled Release, 2006, 114, 343 CrossRef CAS PubMed.
- A. M. Alkilany, P. K. Nagaria, C. R. Hexel, T. J. Shaw, C. J. Murphy and M. D. Wyatt, Small, 2009, 5, 701 CrossRef CAS PubMed.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- A. Gole and C. J. Murphy, Chem. Mater., 2004, 16, 3633 CrossRef CAS.
- F. Kim, J. H. Song and P. Yang, J. Am. Chem. Soc., 2012, 124, 14316 CrossRef.
- N. D. Burrows, W. Lin, J. G. Hinman, J. M. Dennison, A. M. Vartanian, N. S. Abadeer, E. M. Grzincic, L. M. Jacob, J. Li and C. J. Murphy, Langmuir, 2016, 32, 9905 CrossRef CAS PubMed.
- A. S. D. S. Indrasekara, R. C. Wadams and L. Fabris, Part. Part. Syst. Charact., 2014, 31, 819 CrossRef CAS.
- E. Locatelli, I. Monaco and M. Comes Franchini, RSC Adv., 2015, 5, 21681 RSC.
- J. Wan, J. H. Wang, T. Liu, Z. Xie, X. F. Yu and W. Li, Sci. Rep., 2015, 5, 11398 CrossRef CAS PubMed.
- H. Takahashi, Y. Niidome, T. Niidome, K. Kaneko, H. Kawasaki and S. Yamada, Langmuir, 2006, 22, 2 CrossRef CAS PubMed.
- J. Wang, B. Dong, B. Chen, S. Xu, S. Zhang, W. Yu, C. Xu and H. Song, Dalton Trans., 2013, 42, 11548 RSC.
- J. G. Mehtala, D. Y. Zemlyanov, J. P. Max, N. Kadasala, S. Zhao and A. Wei, Langmuir, 2014, 30, 13727 CrossRef CAS PubMed.
- K. Liu, Y. Zheng, X. Lu, T. Thai, N. A. Lee, U. Bach and J. J. Gooding, Langmuir, 2015, 31, 4973 CrossRef CAS PubMed.
- M. Tebbe, C. Kuttner, M. Mannel, A. Fery and M. Chanana, ACS Appl. Mater. Interfaces, 2015, 7, 5984 CAS.
- H. Liao and J. H. Hafner, Chem. Mater., 2005, 17, 4636 CrossRef CAS.
- C. Kinnear, H. Dietsch, M. J. D. Clift, C. Endes, B. Rothen-Rutishauser and A. Petri-Fink, Angew. Chem., Int. Ed., 2013, 52, 1934 CrossRef CAS PubMed.
- Z. Zhang and M. Lin, RSC Adv., 2014, 4, 17760 RSC.
- I. Garcia, M. Henriksen-Lacey, A. Sanchez-Iglesias, M. Grzelczak, S. Penades and L. M. Liz-Marzán, J. Phys. Chem. Lett., 2015, 6, 2003 CrossRef CAS PubMed.
- F. Schulz, W. Friedrich, K. Hoppe, T. Vossmeyer, H. Weller and H. Lange, Nanoscale, 2016, 8, 7296 RSC.
- R. Arakawa and H. Kawasaki, Anal. Sci., 2010, 26, 1229 CrossRef CAS PubMed.
- C.-K. Chiang, W.-T. Chen and H.-T. Chang, Chem. Soc. Rev., 2011, 40, 1269 RSC.
- B. Unnikrishnan, C.-Y. Chang, H.-W. Chu, A. Anand and C.-C. Huang, Anal. Methods, 2016, 8, 8123 RSC.
- Y. Niidome, K. Nishioka, H. Kawasaki and S. Yamada, Chem. Commun., 2003, 2376 RSC.
- M. Grzelczak, A. Sánchez-Iglesias, B. Rodríguez-González, R. Alvarez-Puebla, J. Pérez-Juste and L. M. Liz-Marzán, Adv. Funct. Mater., 2008, 18, 3780 CrossRef CAS.
- F. Hubert, F. Testard and O. Spalla, Langmuir, 2008, 24, 9219 CrossRef CAS PubMed.
- Q. Zhang, H. Jing, G. G. Li, Y. Lin, D. A. Blom and H. Wang, Chem. Mater., 2016, 28, 2728 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Langmuir, 2001, 17, 6368 CrossRef CAS.
- J. Gao, C. M. Bender and C. J. Murphy, Langmuir, 2013, 19, 9065 CrossRef.
- Y. Niidome, Y. Nakamura, K. Honda, Y. Akiyama, K. Nishioka, H. Kawasaki and N. Nakashima, Chem. Commun., 2009, 1754 RSC.
- S. R. Jackson, J. R. McBride, S. J. Rosenthal and D. W. Wright, J. Am. Chem. Soc., 2014, 136, 5261 CrossRef CAS PubMed.
- A. Milan, P. Bennema, A. Verbeeck and D. Bollen, J. Cryst. Growth, 1998, 192, 215–224 CrossRef.
- K. L. Mittal, Solution Chemistry of Surfactants, Springer US, 1979, vol. 1, pp. 487–496 Search PubMed.
- D. A. Jones and J. W. Mitchell, Philos. Mag., 1957, 2, 1047 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis spectra and SLADI mass spectra of PEG-AuNRs. See DOI: 10.1039/c7ra02179h |
| This journal is © The Royal Society of Chemistry 2017 |