Open Access Article
Open Access ArticleFractal analysis of pre-reduced Pt/TiO2 catalysts for formaldehyde oxidation†
Jiaxi Penga,
Xiaoxin Wu*a and
Shudong Wang*b
aDepartment of Polymer Materials and Chemical Engineering, East China Jiaotong University, Nanchang 330013, P. R. China. E-mail: wuxiaoxin@ecjtu.edu.cn
bDalian Institute of Chemical Physics, Dalian National Laboratory for Clean Energy, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: wangsd.@dicp.ac.cn
First published on 28th February 2017
Abstract
The amount of reactive sites, usually dispersed noble atoms, on the top of a catalyst rough surface is characterized within the framework of fractal geometry. The surface fractal dimension dfs was calculated from their nitrogen adsorption isotherms using an A. V. Neimark model. It was found that the catalysts pre-reduced at different temperatures showed different dfs values and distinct formaldehyde oxidation performance. As expected, a good agreement between performance and dfs values was achieved. These results are significant for our understanding of the nature of the catalyst's surface roughness and illustrate the importance of considering difference in surface fractal geometry in analyzing catalytic behavior.
1. Introduction
Some hydrocarbons with a Reid vapor pressure of over 10.3 kPa at normal pressure and temperature are defined as volatile organic compounds (VOCs) and recognized as primary contributors to air pollution, because of their toxic or malodorous nature, or indirectly as ozone precursors and smog precursors. Formaldehyde (HCHO) is considered as the major indoor pollutant emitted from the widely used building and decorative materials in airtight buildings, which is one of the dominating VOCs. Exposure to indoor air containing a low concentration of HCHO for a long time may have adverse effects on human health.1 Thus, great efforts have been made to reduce the indoor emission of HCHO to satisfy the stringent environmental regulations.2–5 More recently, catalytic oxidation of HCHO was achieved over a Pt/TiO2 catalyst even at room temperature.6 Furthermore, we found that the Pt/TiO2 catalyst pre-reduced at different temperatures affected its efficiency significantly,7,8 considered it may be deal with the catalyst surface fractal geometry.Many studies based on theoretical9–11 and computer simulations11–13 have shown that the surface roughness degree of materials distinctly affects the mass transfer in the pores, especially by surface diffusion9,10 and Knudsen diffusion.11,12 Some simulation results have shown that the selectivity, especially in the competing diffusion-limited reactions13,14 could be affected by the degree of heterogeneous catalyst surface geometry. So, it is significant to quantify the degree of surface structural heterogeneity. The concept of fractal may offer a tool by which the rough surface may be both characterized and mathematically simulated. Characterization of catalysts in terms of fractal geometry has been found useful during the last decades.15–21 Many experimental studies22–27 of heterogeneous catalytic processes adapted fractal analysis to show that the fractal nature of the active phase may be responsible for the variation in reactivity. The basic characteristic of fractal systems is their fractal dimension which can be determined with the help of various methods such as adsorption,28,29 small angle scattering (SAXS and SANS),30,31 porometry,32 electron microcopy,33,34 atomic force microscopy,35 etc.
A. V. Neimark et al.36 proposed a simple model based on the nitrogen adsorption isotherm in the region of polymolecular adsorption and corresponds respectively to the scale range from molecular sizes to 300 Å to discriminate the surface geometry. Employment of this method enables to obtain information on a lower limit of possible fractality scales. Such a model is also a complement to testify for or against the fractal properties of adsorbents on larger scales up to 1000 Å. The conclusions based on nitrogen adsorption isotherms are significant for a critical analysis of the results obtained from other non-adsorption methods such as small angle scattering techniques.37
Our previous studies7,8 showed that the Pt/TiO2 catalytic performance is related with many factors, such as the Pt chemical state, TiO2 surface structure, etc. as well mentioned in many studies.38,39 Although the active sites of the catalysts in the reaction are reduced platinum nanoparticles, the mass transfer of reactants in the surface pores is very critical due to the fact that catalytic oxidation of formaldehyde is a diffusion limited intrinsic reaction. In this study, we try to analyze the catalysts surface geometry with A. V. Neimark model36 using N2 adsorption isotherms replacement of atomic force microscopy and other methods, help to understand the role of fractal geometry in mass transfer in catalyst pores, especially in the diffusion-limited catalytic reactions.
2. Experiments
2.1. Materials preparation
Aqueous ammonia (Shenyang, 12.5%) was drop wisely added to the vigorously stirred solution of titanium sulfate at ambient temperature until its pH reached 7.5. After aging for 16 h, the white hydrous titania was then filtered and washed several times by deionized water until there was no SO42− in the filtration (Ba(NO3)2 test), and then the precipitate was calcined at 600 °C (ramp rate is 10 °C min−1) for 2 h to obtain TiO2 powder. Supported platinum catalysts (0.6 wt%) were prepared by the method of incipient wetness impregnation of as-prepared TiO2 powder, using H2PtCl6 as the precursor. Impregnated samples were dried at 120 °C overnight, then heated to 500 °C in 30 minutes, and calcined for 3 h in air. The catalysts were pre-reduced with 150 ml min−1 hydrogen at 300, 450 and 700 °C, which were denoted as PT300, PT450 and PT700, respectively.2.2. N2 adsorption
N2 adsorption isotherms of the samples were obtained at −196 °C on an ASAP 2010 Micromeritics instrument. The specific surface area was determined by using the linear portion of the Brunauer–Emmett–Teller (BET) model, and the average pore size was calculated by using the Barrett–Joyner–Halenda (BJH) formula from the desorption branch of the N2 adsorption isotherm. Prior to theses measurements, the samples were degassed in vacuum at 300 °C for 2 h.2.3. Catalyst performance test
The pre-reduced catalyst performance for the oxidation of formaldehyde was tested as described in our previous study.73. Model
As well known, low temperature (at 77 K) adsorption isotherms of nitrogen on numerous adsorbents of different origin obey a single empirical law in the region of polymolecular adsorption up to the beginning of capillary condensation. When it takes place, the isotherm measured on a porous solid is proportional to the so called “standard” isotherm or t-curve of de Boer, which corresponds to a nonporous smooth surface.| N(P/P0) = Nmi + SmeNst(P/P0) | (1) |
A low sensitivity of the standard isotherm Nst(P/P0) to the origin of solid surface is the advantage of nitrogen as a probe gas. The universality of Nst(P/P0) implies that the adsorbate–adsorbent interactions as well as the chemical and geometrical surface heterogeneity on molecular level affect the nitrogen adsorption only in the early stages and are reduced during the monolayer filling. A possible explanation is that the quadrupole–quadrupole interactions between adsorbed molecules of nitrogen predominate over the adsorbate–adsorbent interactions. This is one reason why the influence of the surface chemical origin on nitrogen adsorption is not as intrinsic as for other adsorbates and why nitrogen is an ideal adsorbate for an analysis of the geometrical structure of porous solids.
A. V. Neimark36 proposed to use nitrogen adsorption isotherms for the discrimination of surface irregularities, particularly fractal type irregularities. The idea is as follows. The existence of surface irregularities of a size more than the molecular diameter of nitrogen (∼4 Å) should lead to a deviation from the linear relationship (eqn (1)) in the region of polymolecular adsorption. On the other hand, if the adsorption isotherm on an explored surface obeys eqn (1) over a certain range of relative pressures, then it can be concluded that in the corresponding range of scales the given surface is similar to a smooth surface and therefore not fractal. In the region where the experimental nitrogen isotherm does not fit the empirical law (eqn (1)), it is possible to investigate fractal properties using corresponding models. When this occurs, the upper limit of validity of eqn (1) is the lower limit of possible fractality.
To provide a quantitative comparison among surfaces, it is convenient to employ a certain algebraic equation for the standard isotherm. Because of its empirical nature, there is no reliable theoretical equation. The Frenkel–Halsey–Hill (FHH) equation for a standard nitrogen isotherm,
| Nst(P/P0) = K/(ln(P0/P))1/m | (2) |
The deviations from eqn (1) are caused by the capillary condensation in mesopores of size more than ∼10 Å. The fractal properties on the scales more than 10–200 Å can be investigated on the basis of nitrogen adsorption isotherms by means of a thermodynamic method. The thermodynamic method employs the following equation valid for capillary condensation on a fractal dimension dfs:
| log[Slg(P/P0)] = const − (dfs − 2)log[αc(P/P0)] | (3) |
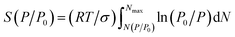 | (4) |
αc(P/P0) = 2συm/RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(P0/P) ln(P0/P)
| (5) |
The combination of the comparative method, based on eqn (1), and the thermodynamic method, based on eqn (3), makes it possible to discriminate the fractal properties of mesoporous materials in the range of scales from ∼4 Å (molecular diameter of nitrogen) to ∼1000 Å on the basis of one experimental nitrogen adsorption isotherm.
An analysis of low temperature nitrogen adsorption isotherms provides the capability to determine the surface fractality. The comparative method, based on eqn (1) and (2), gives a definite negative answer or a “soft” positive answer as to whether or not the surface of a given mesoporous material may be considered as a fractal. In the scale range corresponding to the linear relationship between the nitrogen isotherm on a given sample and the standard isotherm on a smooth surface, the fractal properties do not exist definitely. The upper limit of similarity of these isotherms is the lower limit of the region of possible fractality. On the other hand, in the region of nonlinearity of the comparative plot, only additional study can confirm the existence of fractal properties. In this case the thermodynamic method, based on eqn (3)–(5), is useful. This conclusion is also supported by their investigations of such materials as silica gels, porous glasses, natural apatite and coal.32
4. Results and discussion
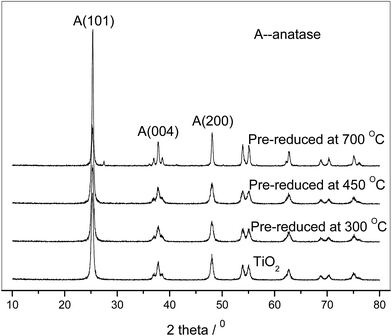
The XRD patterns of the catalysts pre-reduced at different temperatures ranged from 300 °C to 700 °C are shown in Fig. 1. From the patterns, there are no diffraction peaks characteristic of platinum indicating that platinum is well dispersed on TiO2. When pre-reduced below 700 °C, the catalysts show three distinctive peaks at 25.3°, 37.6° and 48.0° as the same to pure TiO2, which corresponding to the anatase (101), (004) and (200) crystal planes (JCPDS 21-1272), indicating that pure anatase phase existed in the catalysts. The physical properties of the Pt/TiO2 catalysts pre-reduced at different temperatures were respectively summarized in Table 1. As we can see from the results in Table 1, all the catalysts have nearly negligible micropore surface area, the mesopore surface area value of Sme does not differ significantly from the BET surface area SBET, which could fit the above model well.| PT300 | PT450 | PT700 | ||
|---|---|---|---|---|
| Surface area m2 g−1 | Micropores Smi | 2.67 | 1.87 | 0.92 |
| Mesopores Sme | 62.16 | 59.66 | 19.57 | |
| BET surface area | 64.83 | 61.53 | 20.49 | |
| Pore volume cm3 g−1 | 0.240 | 0.235 | 0.165 | |
| Pore diameter nm | 12.49 | 11.68 | 28.36 | |
| BET fitted range p/p0 | 0.06–0.20 | 0.06–0.20 | 0.07–0.20 | |
| Correlation coefficient | 0.9999 | 0.9999 | 0.9999 | |
| BET constant C | 102.19 | 101.83 | 99.65 | |
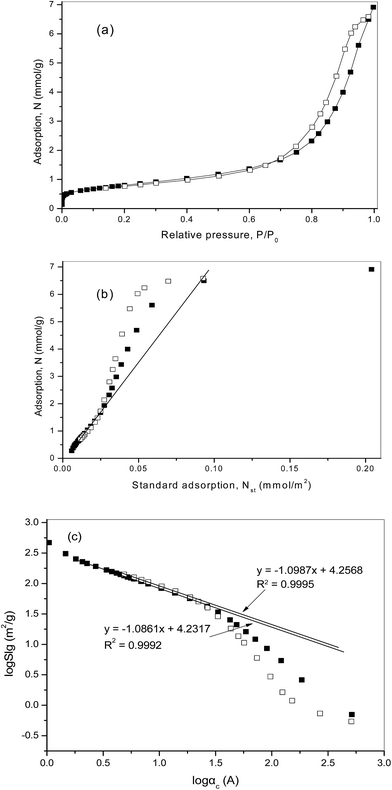
The PT300 catalyst pre-reduced at 300 °C is a mesoporous catalyst, the pore size distribution is relatively narrow with a nominal pore diameter of 120 Å, specific surface area SBET = 64.83 m2 g−1, and pore volume V0 = 0.240 cm3 g−1. The low temperature nitrogen adsorption isotherm on the sample of PT300 employed is shown in Fig. 2a.
The adsorption and desorption branches form well defined hysteresis loop of type H1 by the IUPAC classification. Such an adsorption–desorption loop with clear boundaries is characteristic of mesoporous solids and indicates a relatively narrow mesoporous size distribution in the sample. The reversible part of the isotherm below the hysteresis corresponds to the mono- and polymolecular adsorption on the surface of mesoporous and to reversible capillary condensation in the surface indentations.
In Fig. 2b, the adsorption isotherm N(P/P0) is replotted by employing standard coordinates of the comparative method as a function of the standard nitrogen adsorption isotherm Nst(P/P0) (mmol m−2). The isotherm is a linear function of the standard isotherm Nst(P/P0) up to the relative pressure P/P0 = 0.60, which is the upper limit of similarity of the adsorption process on the explored sample and on the smooth surface. The slope of the linear part of the plot is related to the value of mesopore surface area Sme = 62.16 m2 g−1, which is in close agreement with the BET specific surface area.
The departures of the comparative plot from linearity are observed long before the beginning of capillary hysteresis, which occurs at P/P0 = 0.70. Such behavior suggests the existence of surface roughness on the scales more than 30 Å, which is quite less than the nominal pore diameter equal to 120 Å. In this region the process of capillary condensation in the surface indentations and pores predominates and to assess the surface fractal properties of the sample we have used the A. V. Neimark model. In Fig. 2c, the two plots, based on the adsorption and desorption branches of the isotherm, respectively, are drawn employing standard coordinates of the model, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Slg versus log
Slg versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αc. The region of fractality corresponds to the linear part of the log
αc. The region of fractality corresponds to the linear part of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Slg − log
Slg − log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αc plot, the plot based on the adsorption branch, testifies the fractal properties over a large scale range, where it can be regarded as linear. The result of linear regression in the range is presented by a straight line. The slope corresponds to the surface fractal dimension dfs = 2.629 ± 0.003. The plot, based on the desorption branch, is linear in a slightly broader scale range, that can be related to the influence of the irreversible desorption from mesopores in the hysteresis region. Linear regression of the desorption plot corresponds the surface fractal dimension dfs = 2.662 ± 0.003. The results obtained from adsorption and desorption branch data are very similarly close.
αc plot, the plot based on the adsorption branch, testifies the fractal properties over a large scale range, where it can be regarded as linear. The result of linear regression in the range is presented by a straight line. The slope corresponds to the surface fractal dimension dfs = 2.629 ± 0.003. The plot, based on the desorption branch, is linear in a slightly broader scale range, that can be related to the influence of the irreversible desorption from mesopores in the hysteresis region. Linear regression of the desorption plot corresponds the surface fractal dimension dfs = 2.662 ± 0.003. The results obtained from adsorption and desorption branch data are very similarly close.
We emphasize that the lower limit of fractality, αmin = 5 Å is related to the value of relative pressure P/P0 = 0.16, which is a little less than the upper limit linearity of the comparative plot. The upper limit of fractality, αmax = 200 Å, is comparable with the mean radius of mesopores. This is characteristic for a porous structure, where the pore network is constituted by channels with a relatively narrow size distribution and, at the same time, with rough walls. In the case, it is obvious that the mean radius of pore channels is the upper limit of possible fractality.
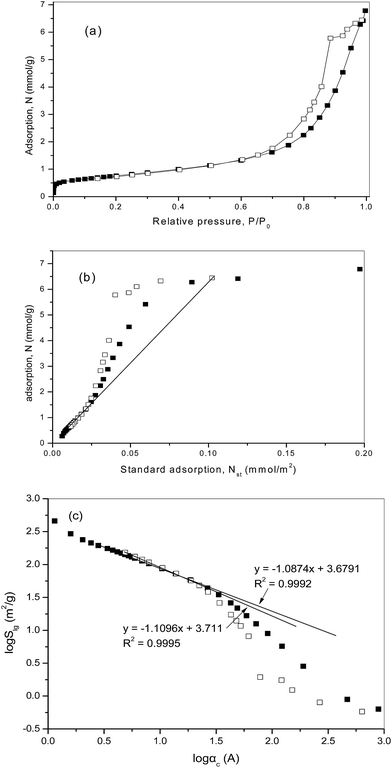
For the considered another sample of PT450, Pt/TiO2 pre-reduced at 450 °C, the specific surface area SBET = 61.53 m2 g−1 and the pore volume V0 = 0.235 cm3 g−1 with a nominal pore diameter of 120 Å. The low temperature nitrogen adsorption isotherm, N, is presented in Fig. 3a as a function of the relative pressure, P/P0, and in Fig. 3b as a function of standard isotherm, Nst(P/P0). The results of fractal analysis by means of the thermodynamic model are presented in Fig. 3c.
These results are quite similar to those for the sample of PT300 (Fig. 2). The comparative plot, Fig. 3b, is linear in the range of relative pressures, P/P0, from 0.05 to 0.80 with a slope related to the value of mesopore surface area Sme = 59.66 m2 g−1 in close agreement with the BET surface area. The low limit of surface roughness is about 5 Å.
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Slg − log
Slg − log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αc plot (Fig. 3c) corresponds the fractal properties over the same scale range with the surface fractal dimension dfs = 2.640 ± 0.002 based on the adsorption branch, and the plot, based on the desorption branch, is linear over the same scale range, and the corresponding value of dfs = 2.720 ± 0.002.
αc plot (Fig. 3c) corresponds the fractal properties over the same scale range with the surface fractal dimension dfs = 2.640 ± 0.002 based on the adsorption branch, and the plot, based on the desorption branch, is linear over the same scale range, and the corresponding value of dfs = 2.720 ± 0.002.
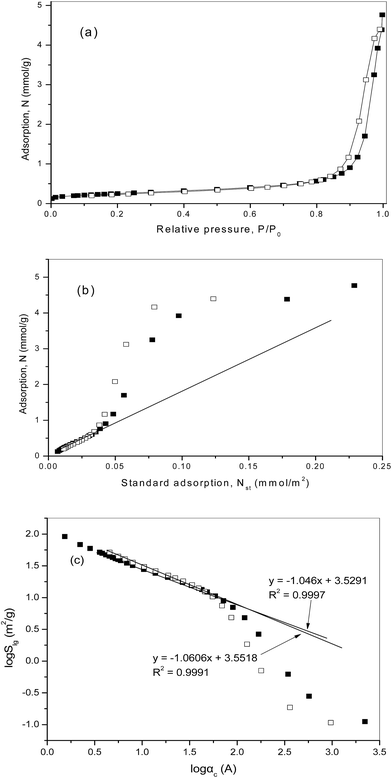
For the considered third sample of PT700, Pt/TiO2 pre-reduced at 700 °C, the specific surface area SBET = 20.49 m2 g−1 and the pore volume V0 = 0.165 cm3 g−1 with a nominal pore diameter of 280 Å. The low temperature nitrogen adsorption isotherm, N, is presented in Fig. 4a as a function of the relative pressure, P/P0, and in Fig. 4b as a function of standard isotherm, Nst(P/P0). The results of fractal analysis by means of the thermodynamic model are presented in Fig. 4c.
These results are quite similar to those for the samples of PT300 and PT450 (Fig. 2 and 3). The comparative plot, Fig. 4b, is linear in the range of relative pressures, P/P0, from 0.01 to 0.50 with a slope related to the value of mesopore surface area Sme = 19.57 m2 g−1 in close agreement with the BET surface area. The low limit of surface roughness is about 5 Å.
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Slg − log
Slg − log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αc plot (Fig. 4c) corresponds the fractal properties over the same scale range with the surface fractal dimension dfs = 2.579 ± 0.005 based on the adsorption branch, and the plot, based on the desorption branch, is linear over the same scale range, corresponding the value of dfs = 2.577 ± 0.005.
αc plot (Fig. 4c) corresponds the fractal properties over the same scale range with the surface fractal dimension dfs = 2.579 ± 0.005 based on the adsorption branch, and the plot, based on the desorption branch, is linear over the same scale range, corresponding the value of dfs = 2.577 ± 0.005.
The Pt/TiO2 catalysts pre-reduced at different temperatures resulted in different catalysts surfaces geometry. The surface is the more complex; the more irregular, fine structure and rumple of the surface exist, which reflects in the fractal dimension being bigger. The fractal surfaces are more efficient than planar ones for diffusion limited reaction processes and therefore, fractal catalyst has an advantage compared to a non fractal one. The fractal dimension, dfs, of the catalysts pre-reduced at different temperatures and the corresponding catalyst efficiency for the catalytic oxidation of formaldehyde are summarized in Table 2. Among all the catalysts, PT450 has the biggest fractal dimension value and in result it shows the highest activity at different temperatures.
| Samples | PT300 | PT450 | PT700 | |
|---|---|---|---|---|
| Fractal dimension dfs | Adsorption branch | 2.629 ± 0.003 | 2.640 ± 0.002 | 2.579 ± 0.005 |
| Desorption branch | 2.662 ± 0.003 | 2.720 ± 0.002 | 2.577 ± 0.005 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
Oxidation conditions: GHSV 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm3 g−1 h−1, HCHO 100 mg m−3 000 cm3 g−1 h−1, HCHO 100 mg m−3 |
||||
| Formaldehyde conversion (%) | 23 °C | 39.4 | 42.1 | 13.2 |
| 40 °C | 76.5 | 78.4 | 19.2 | |
| 60 °C | 88.9 | 94.9 | 41.7 | |
| 90 °C | 95.7 | 99.0 | 89.3 | |
| 120 °C | 97.8 | 99.8 | 96.8 | |
5. Conclusions
A fractal analysis of low temperature nitrogen adsorption isotherms provides the capability to determine the surface geometry. The comparative method, based on eqn (1) and (2), gives a definite negative answer or “soft” positive answer as to whether or not the surface of a given mesoporous catalysts may be considered as a fractal. The thermodynamic model based on eqn (3)–(5) is useful to calculate the fractal properties. Among our presented catalysts, the catalyst pre-reduced at 450 °C has the biggest fractal dimension dfs, and the efficiency for the catalysts used in catalytic oxidation of formaldehyde support the opinion that the bigger of the fractal dimension dfs, the more efficient of the catalyst. The fractal analysis of the catalysts surface geometry could play an important role in the diffusion limited reactions.Acknowledgements
The authors would like to express their gratitude for the financial support for the project by Jiangxi Province Education Department (GJJ150514) and China Sponsorship Council.References
- Y. Sekine, Atmos. Environ., 2002, 36, 5543–5547 CrossRef CAS.
- F. Wang, Q. Zhang, M. Sakurai and H. Kameyama, Catal. Commun., 2007, 8, 2171–2175 CrossRef.
- L. Wang, R. Yunus, J. G. Li, P. L. Li, P. Y. Zhang and J. Kim, Appl. Surf. Sci., 2015, 357, 787–794 CrossRef.
- S. Colussi, M. Boaro, L. Rogatis, A. Pappacena, C. Leitenburg, J. Llorca and A. Trovarelli, Catal. Today, 2015, 253, 163–171 CrossRef CAS.
- F. Qi, B. Cheng, J. G. Yu and W. K. Ho, J. Hazard. Mater., 2016, 301, 522–530 CrossRef PubMed.
- B. Zhang, H. He and K. Tanaka, Catal. Commun., 2005, 6, 211–214 CrossRef.
- X. Peng and S. D. Wang, Appl. Catal., B, 2007, 73, 282–291 CrossRef.
- X. Peng and S. D. Wang, J. Phys. Chem. C, 2007, 111, 9897–9904 Search PubMed.
- P. Rigby, Langmuir, 2002, 18, 1613–1618 CrossRef.
- P. Rigby, Langmuir, 2003, 19, 364–376 CrossRef.
- K. Malek and M. O. Coppens, J. Chem. Phys., 2003, 119, 2801–2811 CrossRef CAS.
- S. Wang, Z. F. Ma, H. Q. Yao and F. S. Liu, Chem. Eng. Sci., 2013, 101, 813–827 CrossRef CAS.
- A. Kriston, T. Y. Xie and B. N. Popov, Electrochim. Acta, 2014, 121, 116–127 CrossRef CAS.
- P. Rigby and L. F. Gladden, J. Catal., 1998, 180, 44–50 CrossRef.
- M. Wang and S. F. Li, Ind. Eng. Chem. Res., 1997, 36, 1598–1602 CrossRef.
- K. Lee, Ind. Eng. Chem. Res., 1998, 37, 3939–3942 CrossRef.
- A. Smith, A. Zoelle, Y. Yang, R. M. Rioux, N. G. Hamilton, K. Amakawa, P. K. Nielsen and A. Trunschke, J. Catal., 2014, 312, 170–178 CrossRef.
- Z. Jelčič, Z. M. Samardžić and S. Zrnčević, Appl. Catal., A, 2013, 456, 30–43 CrossRef.
- M. He, N. T. Tung, Y. Adachi and K. Kobayashi, Catal. Today, 2016, 271, 91–101 CrossRef.
- I. Trypolskyi, T. M. Gurnyk and P. E. Strizhak, Catal. Commun., 2011, 12, 766–771 CrossRef.
- R. Larraz, Chem. Eng. J., 2002, 86, 309–317 CrossRef CAS.
- O. Coppens and G. F. Froment, Chem. Eng. Sci., 1996, 51, 2283–2292 CrossRef.
- F. Galindo-Hernández, J. M. Domínguez and B. Portales, J. Power Sources, 2015, 287, 13–24 CrossRef.
- A. Montesinos-Castellanos, E. Lima, J. A. de los Reyes and H. V. Lara, J. Phys. Chem. C, 2007, 111, 13898–13904 CAS.
- H. Abu Bakar, M. M. Bettahar, M. Abu Bakar, S. Monteverdi, J. Ismail and M. Alnot, J. Catal., 2009, 265, 63–71 CrossRef.
- B. Jefferson, P. Jarvis, G. K. Bhagianathan, H. Smith, O. Autin, E. H. Goslan, J. MacAdam and I. Carra, Chem. Eng. J., 2016, 288, 359–367 CrossRef CAS.
- B. Guan, H. Lin, L. Zhu, B. Tian and Z. Huang, Chem. Eng. J., 2012, 181–182, 307–322 CrossRef CAS.
- Y. Zhang, H. Y. Cui, Z. Z. He, L. Su and D. G. Fu, RSC Adv., 2016, 6, 8639–8643 RSC.
- F. Galindo-Hernández, J. A. Wang, L. F. Chen and X. Bokhimi, J. Mater. Res., 2013, 28, 3297–3309 Search PubMed.
- K. Sinko, V. Torma and A. Kovacs, J. Non-Cryst. Solids, 2008, 354, 5466–5474 CrossRef CAS.
- X. Guo, K. Gao, A. Gutsche, M. Seipenbusch and H. Nirschl, Powder Technol., 2015, 272, 23–33 CrossRef CAS.
- M. Cuerda-Correa, A. Macias-Garcia, M. A. Diaz-Diaz and A. L. Ortiz, Microporous Mesoporous Mater., 2008, 111, 523–529 CrossRef.
- S. Talu, S. Solaymani, M. Bramowicz, N. Naseri, S. Kulesza and A. Ghaderi, RSC Adv., 2016, 6, 27228–27234 RSC.
- N. Sahoo and B. Kandasubramanian, RSC Adv., 2014, 4, 11331–11342 RSC.
- S. Tălu, M. Bramowicz, S. Kulesza, A. Shafiekhani, A. Ghaderi, F. Mashayekhi and S. Solaymani, Ind. Eng. Chem. Res., 2015, 54, 8212–8218 CrossRef.
- V. Neimark and K. K. Unger, J. Colloid Interface Sci., 1993, 158, 412–419 CrossRef.
- A. Hohr, H. B. Neumann, P. W. Schmidt, P. Pfeifer and D. Avnir, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 1462–1467 CrossRef.
- A. Somorjai and J. Y. Park, Angew. Chem., Int. Ed., 2008, 47, 9212–9228 CrossRef PubMed.
- Y. Oh, K. Qadir and J. Y. Park, Catal. Lett., 2017, 147, 39–45 CrossRef.
- J. Krim and E. T. Watts, A.I.Ch.E., New York, 1991 Search PubMed.
- M. Dubinin, L. I. Kataeva and V. I. Ulin, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1977, 26, 459–463 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02161e |
| This journal is © The Royal Society of Chemistry 2017 |