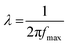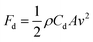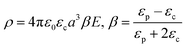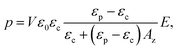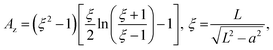Open Access Article
Open Access ArticleFabrication of a silica/titania hollow nanorod and its electroresponsive activity†
Chang-Min Yoon,
Jungchul Noh,
Yoonsun Jang and
Jyongsik Jang *
*
School of Chemical and Biological Engineering, College of Engineering, Seoul National University (SNU), Seoul, Korea. E-mail: jsjang@plaza.snu.ac.kr; Tel: +82 2 880 7069
First published on 4th April 2017
Abstract
In this study, a 1D oriented hollow SiO2/TiO2 (HST) rod-like material was successfully fabricated via a sequential combination of sol–gel use, TiO2 incorporation, and a sonication-mediated etching and redeposition method. This carefully manipulated new material has numerous advantageous physical and intrinsic properties, such as increased surface area, pore volume, interfacial polarization, and dielectric properties introduced from each synthetic step. The synthesized HST rod was adopted as an electrorheological (ER) material for practical examination of these characteristics. The HST rod materials exhibited 1.5- and 3-fold higher ER performance than a non-metal SiO2 rod and a non-hollow SiO2/TiO2 core/shell (ST/CS) rod, which are interim synthetic steps. Moreover, the HST rod exhibited remarkable 6-fold increased ER efficiency relative to a sphere-shaped hollow SiO2/TiO2 particle synthesized using a similar experimental method. These notable enhancements in ER performance are attributed to incorporation of the experimentally designed characteristics of the HST rod: 1D structure, metal oxide incorporation, and creation of a hollow cavity. For future study, we expect that these versatile HST rod materials can be applied in a range of fields including drug delivery, photo-catalysis, and as building blocks.
1. Introduction
One-dimensional (1D) inorganic nanomaterials (rod, tube, and wire) have recently drawn significant attention owing to their unique properties compared with high-dimensional 2D and 3D materials.1–3 Because of their dimensionality and vertical structure, 1D inorganic nanomaterials are regarded as an emerging candidate for photonics, electronics, and nanodevices.4–6 By manipulation of various 1D inorganic nanomaterials, physical properties such as electron transportation, mechanical strength, and photoconductivity can be tailored to a desired application.7,8 Therefore, significant efforts have been devoted to the development of synthetic methods for 1D inorganic nanomaterials using materials such as carbon fibers, metals, metal oxides, silicon, and their hybrids.9–12Among these inorganic materials, metal oxides are of great interest because they are among the most abundant minerals on Earth.13 Metal oxides are ionic compounds consisting of a positive metal component and a negative oxygen ion, resulting in a chemically and thermally stable molecule. However, most of the distinct properties of metal oxides arise from the unfilled d-orbital and unpaired electrons; these result in metal oxides (e.g. ZnO, Cu2O, NiO, V2O5, Fe2O3, and TiO2) having unique properties such as photoconduction, catalytic activity, a wide bandgap, and a high dielectric constant.14–16 Nevertheless, controlling the anisotropic growth of metal oxides and manipulating a target 1D structure remain major challenges. One method for resolving difficulties in the synthetic process and taking advantage of metal oxides is to incorporate metal oxides into nonmetal-template materials such as a carbon substrate or SiO2.17,18
Among metal oxides, titania (TiO2) nanoparticles have been widely studied owing to their excellent characteristics, including high stability, corrosion resistance, dielectric properties, and photocatalytic activity.19–21 Various methods for the fabrication of TiO2 nanoparticles have been developed, including co-precipitation, electrospray deposition, spray pyrolysis, and sol–gel methods.22–24 Moreover, TiO2 materials can be easily composited with hard templated SiO2 to attain additional advantages, such as easy dimension control, improved light scattering, and feasibility of mass production.25–27 With further modification of a SiO2 template, SiO2/TiO2 composite materials can achieve additional physical characteristics such as hollow spaces and increased porosity.28,29
Synergistic effects from the methods discussed so far can be achieved through a combination of controlling the templated structure, adding metal oxides, and introducing porosity. Although, there is few studies addressing the fabrication of hollow SiO2 rod-like material or hollow TiO2 fibers, but there were drawbacks of complex experimental procedures and unevenly structured final products. Therefore, there is a necessity for well-constructed hollow 1D rod-like nanomaterial with tunable aspect ratio (L/D) by facile method is arising. Our group has previously reported the mass scale synthesis of well-defined SiO2 rod with controllable L/D. Also, we have reported the fabrication of uniformly ordered various hollow metal oxide-composited nanospheres by simple procedure using sonication-mediated etching and redeposition (SMER) method.30–32 Particularly, detailed experimental conditions such as types of etchants (e.g. NH4OH, NaOH, alkaline earth metal hydroxides) and sonication times are varied with size of nanospheres and intended final products (e.g. hollow SiO2/TiO2, TiO2, alkaline earth metal-doped SiO2/TiO2).33–35 However, none of previous studies including our group have reported the fabrication of hollow metal oxide-composited rod-like nanomaterial with tunable L/D. Accordingly, the objective of this study was to fabricate a material combining all of the advantages of a metal oxide, a 1D orientation, and high porosity by combining well-known simple (sol–gel, modified Stöber, and SMER) methods from other and our groups with some inventive modifications according to our experimental condition.
Electrorheology (ER) is the rheological study of materials dispersed in an insulating medium under the influence of an electric field.36,37 Under an applied electric field, randomly dispersed particles form fibril-like chains to exert a mechanical change, which can be measured in terms of shear stress (Pa) and viscosity (Pa s).38,39 According to previous studies, ER performance is greatly affected by the characteristics of the dispersed material and other factors including temperature, electric field strength, and the dispersed medium.40–43 In the last few decades, most studies have focused on the fabrication of new ER materials and controlling material properties to obtain enhanced ER performance. Thus, numerous materials with different compositions, structures, sizes, and properties have been adopted as ER materials to find the maximum efficiency.44–46 Interestingly, positive ER effects require material characteristics such as a large active surface area, low particle density, high porosity, polarizability, and high electrical conductivity,47–50 and these properties have potential for utilization in other applications such as drug delivery, electrochemical studies, and photovoltaic applications.34,51,52 Thus, ER applications are effective methods for investigating these newly designed materials.
In this study, we report a successful method for fabricating a hollow SiO2/TiO2 (HST) composite rod-like material for the first time. The SiO2 rod with tunable L/D fabricated by modified Stöber method reported in our previous studies were used as core material. Afterwards, a porous TiO2 shell was introduced to obtain a SiO2/TiO2 core/shell (ST/CS) rod. A hollow structure was obtained using the sonication-mediated etching and redeposition (SMER) method reported in our previous study. The core SiO2 rod was completely dissolved in the etchant by the high sonication energy and then some was redeposited onto the TiO2 shell to create a SiO2/TiO2 composite. Also, the aspect ratio (L/D) of HST rod was adjustable up to ca. 6 by changing the length of SiO2 template. The resulting HST rod exhibited various advantages, such as a hollow morphology, 1D orientation, high surface area, high pore volume, large dielectric constant, and feasibility for mass production. Furthermore, we investigated the electrorheological activity of the HST rod to elucidate the unique properties of the material. This newly designed 1D HST rod can be applied across a range of fields for future study.
2. Experimental
2.1. Materials
Tetraethyl orthosilicate (TEOS), titanium isopropoxide (TTIP, 97.0%), cetyltrimethylammonium bromide (CTAB), and silicone oil [poly(methylphenylsiloxane), 100 cST] were purchased from Aldrich Chemical Co. Hydrochloric acid (HCl, 35.0–37.0%) and ammonium hydroxide solution (NH4OH, 28.0–32.0%) were purchased from Samchun Chemical Co. (Korea). Absolute ethanol (ethyl alcohol, 99.5) was obtained from Fisher Chemical Co. All chemicals were used as received.2.2. Synthesis of SiO2/TiO2 core/shell (ST/CS) rod
Core SiO2 rod template (L/D = 3) was synthesized according to the modified Stöber method.31 In a typical synthesis of SiO2 rod, absolute ethanol (6 mL), ammonia solution (2.1 mL), and DI water (140 mL) were mixed by magnetic stirring for 5 min. Then CTAB (0.62 g) was dissolved into the resulting mixture and stirred for 20 min. Subsequently TEOS (1.2 mL) was injected into the as-prepared solution and the reaction proceeded for 3 h at room temperature. SiO2 rod particles were collected by centrifugation. To remove the remaining organic species, SiO2 rods were washed by mixture of HCl (3 mL) and ethanol (20 mL) for 6 h (70 °C). Washed SiO2 rod particles were centrifuged (6500 × g) with ethanol for several times and re-dispersed into the absolute ethanol (45 mL) by vigorous stirring. A mixture of acetonitrile (15 mL) and TTIP (9 mL) was slowly added to the colloidal suspension of SiO2 rod using a burette. The condensation reaction proceeded for 12 h at 4 °C and resulting white solution was centrifuged to obtain SiO2/TiO2 core/shell (ST/CS) rod particles. Obtained ST/CS rod was dried in oven for overnight (100 °C). The aspect ratio (fixed diameter of ca. 65 nm, varying length) of core SiO2 was controlled by changing the amount of DI water in above process, resulting in fabrication of ST/CS materials with varying aspect ratio. Sphere SiO2 (L/D = 1) and two SiO2 rods (L/D = 2 and 6) were synthesized using 25 mL, 40 mL, and 120 mL of DI water, respectively.2.3. Fabrication of hollow SiO2/TiO2 (HST) rod via SMER method
HST rod particles were fabricated via sonication-mediated etching and re-deposition (SMER) method.30 Firstly, dried ST/CS rod (0.5 g) was well-dispersed in DI water (20 mL) by vigorous stirring for 6 h. And then, NH4OH (0.2 M, 40 mL) solution was added to the ST/CS dispersion and placed in the sonicator for 5 h. Resulting white cloudy solution was centrifuged (6500 × g) with DI water and ethanol for several times to remove the etchant solution and residues. Finally, collected HST rod was dried in oven for overnight (100 °C). HST materials with different aspect ratio were fabricated by same synthetic method except using ST/CS materials with different lengths. The yield of HST rod was ca. 0.4 g, by following the described procedure. Under our experimental condition, the etching process can be scaled up to 20 times to obtain 8.0 grams of HST rods in one process by increasing the amount of ST/CS rod, DI water, and NH4OH solution.2.4. Characterization
The morphologies of SiO2 rod, ST/CS rod, and HST rods were analyzed by transmission electron microscope (JEM-200CX, JEOL) and field-emission scanning electron microscope (JEOL-6700, JEOL) equipped with an EDS spectrometer (Inca). Elemental mapping of materials were provided with STEM (Tecnai F20, FEI) equipped with image filter (Gatan, Inc.). Additional molecular information of materials were obtained by Fourier transform infrared spectroscopy (Cary-600, Agilent). The crystalline phase of materials were investigated by X-ray diffraction analysis by M18XHF-SRA (MAC Science Co.) and AXIS-His spectroscopy (KRATOS). The pore size and surface area of materials were determined by BJH and BET calculation associated with N2-sorption curves (ASAP-2010, Micrometrics). Dispersion stability of materials was determined by dispersing particles into silicone oil and calculating the sedimentation ratio (R). Dielectric properties of particles were measured by impedance spectroscope (Solatron-1260) coupled with interface analyzer (Solartron-1296).2.5. Electro-response (ER activity) of HST rod-based ER fluid
Electro-response of SiO2 rod, ST/CS rod, and HST rods were studied by preparing samples as an electrorheological (ER) fluid. To prepare ER fluids, dried materials (0.3 g) were well-dispersed into silicone oil (10 mL) by three steps. Firstly, particles were finely grinded using a mortal and pestle. And then, grinded particles were dissolved into the silicone oil by vigorous stirring for 12 h. Finally, ER fluids were sonicated for additional 6 h to attain homogeneous solution. Practical ER measurements were carried out using rheometer and accessory sets (AR 2000, TA instruments). In specific, rheometer was equipped with an insulating cup (d = 30.0 mm, h = 30.0 mm), cylinder geometry (d = 28.0 mm, h = 30.0 mm), and a high voltage generator (Trek 677B). To start a measurement, as-prepared ER fluids were poured into a cup and cylinder geometry was inserted into the cup. The gap distance between the cup and geometry was fixed to 1.00 mm. Subsequently, pre-shear (3.0 s−1) was applied for 10 min to attain equilibrium state. Finally, DC voltage was applied to examine the electro-response of SiO2 rod-, ST/CS rod-, and HST rod-based ER fluids.3. Results and discussion
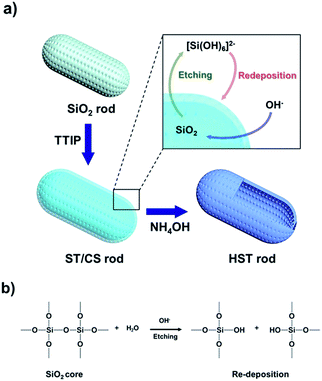
3.1. Fabrication of HST rod material
The strategic method for fabrication of the HST material is shown in Fig. 1a. First, the core SiO2 template rod was synthesized using a simple sol–gel method (modified Stöber method) with CTAB as the surfactant. After the reaction remaining organic residue was completely removed by ethanolic acid wash for uniform coating of TiO2. And then, the TTIP precursor was slowly added to the core SiO2 rod to synthesize the porous TiO2 shell-coated SiO2 rod, denoted a SiO2/TiO2 core/shell (ST/CS) rod, via a TiO2 condensation reaction. Finally, the TiO2-embedded ST/CS rod was etched using a NH4OH etchant with sonication to synthesize a hollow HST rod material via the SMER method. The mechanism for the SiO2 core etching process and redeposition of silica species is shown in Fig. 1b. The SiO2 core was partially dissolved in a solution of NH4OH etchant under basic conditions. The SiO2 core was then sequentially etched further and then released into the etchant solution as a silicic ion form of [Si(OH)6]2− with strong intensity driven by the sonication. The dissolved silicic species reacted with hydroxide ions (OH−) from the etchant solution and was redeposited as SiO2 and SiO3 onto the TiO2 shell through the process of Ostwald ripening. In addition, some TiO2 also dissolved in the NH4OH solution and was redeposited onto the final HST rod. However, owing to the chemical stability of TiO2, the redeposition of TiO2 was generally negligible compared with the completely dissolved SiO2 core material.30Transmission electron microscope (TEM) analysis was carried out to investigate the morphology of the materials (Fig. 2). The diameters of the SiO2, ST/CS, and HST rods were determined to be ca. 60, 66 ± 4, and 72 ± 4 nm, and the lengths of the SiO2, ST/CS, and HST rods were found to be ca. 200, 210, and 230 nm, respectively. Hence, the aspect ratio (L/D) of three materials were determined to be ca. 3. The particle sizes of the ST/CS and HST rods were larger than that of the SiO2 rod owing to the formation of a porous TiO2 shell and redeposition of SiO2. The thickness of the porous TiO2 shell was determined to be ca. 3 to 5 nm and the redeposited composite shell thickness was found to be ca. 3 to 5 nm. Thus, the combined shell thickness of the HST rod was ca. 8 to 10 nm, and a well-defined internal hollow space beneath the shell was clearly observed. SEM observations verified fairly uniform morphologies for all three materials (Fig. S1†), and an internal hollow space created within the HST rod was clearly observed from the ruptured structure (Fig. S1d†). During the fabrication process, it is important to remove the remaining CTAB and organic residues by HCl treatment. Without HCl treatment, leftover organic residues on SiO2 rod can cause aggregation of final HST rods and formation of random TiO2 particles. Also, calcination of SiO2 rod caused aggregation of SiO2 rod and resulting HST rods. HCl untreated and calcined SiO2 rods and resulting HST rods are presented in Fig. S2.† Hence, ethanolic acid wash of SiO2 rod is essential step to attain well-constructed final HST rods. Also, the shell thicknesses of HST rods were controlled by changing the amount of added TTIP solution (Fig. S3†). However, HST rods with thin and thick shells had problems like aggregation, structural deformation, and blockage of etchant pathway. Moreover, the HST materials with various aspect ratios were fabricated by controlling the anisotropic growth of the core SiO2 template. Particularly, the core SiO2 materials were synthesized with fixed diameter of ca. 60 nm and only lengths were varied by changing the amount of added reagents. As a result, the HST materials from sphere-shaped (L/D = 1) to long rod (L/D = 6) were prepared by same fabrication methods (Fig. S4†). However, long HST rod (L/D = 6) showed relatively aggregated state and slight structural deformation compared to well-dispersed HST material with low aspect ratio (Fig. S5†). The particle aggregation of long HST rod is due to the increased amount of redeposited SiO2 species from elongated SiO2 template. Also, elongated structure of long HST rod could not withstand the energy driven from the sonication during the etching process resulting in some deformation of structure. Thus, the HST rod with L/D of 3 is the longest rod-like material without forming particle aggregation or distorted structure under our experimental conditions. In this regard, the HST rod discussed in further context is regarded to HST rod with (L/D = 3). Detailed physical parameters of various HST materials are listed in Table S1.†
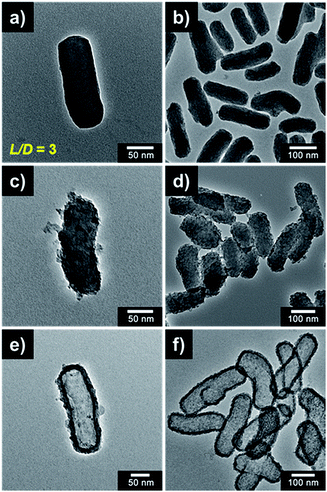 | ||
| Fig. 2 TEM micrographs of (a, b) SiO2 rod, (c, d) ST/CS rod, and (e, f) HST rod, respectively (a, b, c = high magnified images, b, d, f = low magnified images). | ||
N2-sorption curves and associated Brunauer–Emmett–Teller (BET) surface area and Barrett–Joyner–Halenda (BJH) pore distributions were calculated to investigate the porosity, pore volume, and surface area of the materials, as shown in Fig. 3. The pore sizes of the SiO2, ST/CS, and HST rods were found to be 3.3, 4.0, and 3.8 nm, respectively. The pore size of the HST rod was diminished compared with that of the ST/CS rod due to the redeposition of silicic species. On the other hand, the pore volume of the HST rod was significantly larger at 0.87 cm3 g−1 than those of the ST/CS rod (0.56 cm3 g−1) and the SiO2 rod (0.41 cm3 g−1). The increase in pore volume suggested that the core SiO2 core template was successfully etched and an internal hollow space was created within the HST rod. The N2-sorption curves of the materials confirmed the porous nature of the HST rod. While the core SiO2 rod showed a type III isotherm, the ST/CS and HST rods exhibited typical type IV isotherms of porous materials. In particular, the hysteresis loop of the HST rod was larger than that of the ST/CS rod, indicating higher porosity. As a result, the surface area of the HST rod material was significantly larger than those of the ST/CS and core SiO2 rods owing to the creation of an internal cavity and a porous shell. Detailed physical characteristics of the rods are listed in Table S2.†
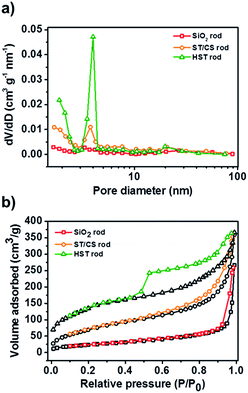 | ||
| Fig. 3 (a) BJH pore distribution curves of SiO2 rod, ST/CS rod, and HST rod. (b) N2-sorption curves of SiO2 rod, ST/CS rod, and HST rod. | ||
The atomic compositions of the materials were determined by EDS analysis, as shown in Table S3.† In particular, the core SiO2 rods contained only Si and O elements. In contrast, Ti was detected in the ST/CS and HST rods, indicating that the SiO2 core had been successfully coated with a TiO2 shell. Notably, the Si atomic composition of the HST rod was significantly lower than that of the ST/CS rod after the core etching process; however, some Si atoms remained and participated in the redeposition process to obtain the final SiO2/TiO2 composited material. Scanning transmission electron microscope (STEM) analysis was carried out on each material (Fig. 4), and the STEM image of the SiO2 rod confirmed that the material was composed of Si elements without Ti (Fig. S6†). In the case of the ST/CS rod, Ti elements were well distributed in the outer shell and the Si remained inside the TiO2 shell. Interestingly, the elemental mapping image of the HST rod showed that the shell was composed of both Ti and ample amounts of Si elements distributed with the Ti elements to produce the composite state of the HST rod. The internal hollow space of the HST rod was clearly observed inside the Si/Ti composite shell. To further investigate the molecular composition of materials, Fourier transform infrared (FT-IR) analysis were carried out (Fig. S7a†). Particularly, all three samples showed characteristics peaks of SiO2, Si–O stretching vibration was located near 1045 cm−1 and Si–O bending at 940 cm−1. Also, peaks for water and organic species from synthetic process were detected at 1635 cm−1 for H–O–H bending and 3800 cm−1 for O–H stretching. Notably, TiO2 related peaks were found from the ST/CS rod and HST rod. Two prominent TiO2 peaks were located near 1430 cm−1 and 550 cm−1, corresponding to stretch vibration of Ti–O–Ti and Ti–O, respectively.53 Also, Si–O–Ti bonds were detected from the ST/CS and HST rod at 952 cm−1 and 947 cm−1 to indicate the successful formation of composite of SiO2 and TiO2. Additionally, FT-IR spectra of acid washed and untreated SiO2 rod is shown in Fig. S8.† Particularly, the CTAB and organic residues of untreated SiO2 rod were successfully removed by ethanolic acid washing supported from diminished intensity of carbon related peaks at 2980 cm−1 (C–H stretching), 2920 cm−1 (CH2 scissoring mode), 2850 cm−1 (symmetric vibration of methylene chain), and 1450 cm−1 (symmetric vibration of methylene chain). As mentioned previously, ethanolic acid wash helped the uniform coating of TiO2 shell by removing the organic residues. Moreover, phase of materials was determined by X-ray diffraction (XRD) analysis (Fig. S7b†). Only one broad peak was detected for all materials between 20° and 30°, indicating the amorphous phase of sol–gel fabricated SiO2, TiO2, and its composite.33,54 These results confirmed that the HST rod was successfully fabricated as a 1D, hollow, metal oxide composite material.
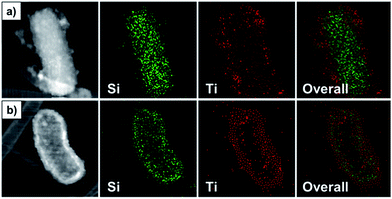 | ||
| Fig. 4 STEM-HADDF image (left) and elemental mapping images of (a) ST/CS rod and (b) HST rod (detected elements: Si and Ti). | ||
3.2. Various intrinsic advantages of HST material
A range of intrinsic properties were measured to confirm the advantageous characteristics of the HST rod. First, the dispersion stability (which is associated with pore volume and hollow spaces) of the SiO2, ST/CS, and HST rods was investigated (Fig. 5) ground materials were dispersed in silicone oil (viscosity = 100 cST) at a concentration of 3.0 wt% and the sedimentation ratio (R) was determined. The dispersed materials gradually subsided and reached an equilibrium state after 90 h. At first, the three materials showed similar sedimentation rates; however, after 10 h had passed, the core SiO2 rod and ST/CS rod particles settled much more quickly than the HST rod material. After 90 h, the HST material maintained an excellent sedimentation ratio of 0.9, meaning that about 90% of the materials were still dispersed in the medium, while the ST/CS rod showed a sedimentation ratio of 0.77 and the core SiO2 rod showed 0.81. This can be attributed to the enhanced porosity and pore volume of the HST material. Since HST materials are synthesized with large internal cavity, dispersion stability may have increased due to the enhanced pore volume. In contrast, the ST/CS rod showed the lowest value of R due to the presence of both the SiO2 core and the TiO2 shell, which increased the particle density and mass. The actual particle densities of the three rod-like materials were determined using a pycnometer. The measured particle densities of the core SiO2, ST/CS, and HST rods were 2.77, 3.01, and 2.71 g cm−3, respectively. The sedimentation results were in good agreement with the measured particle densities. According to previous studies, a decrease in particle density can enhance the dispersion stability of a material and the particle mobility in a dispersing medium.50,55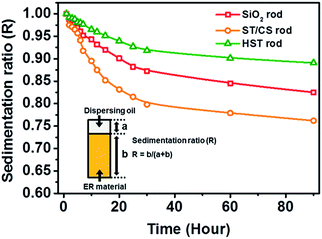 | ||
| Fig. 5 Dispersion stability of SiO2 rod, ST/CS rod, and HST rod dispersed in silicone oil medium (inset: definition of sedimentation ratio). | ||
The dielectric properties of the HST rod were investigated to confirm the advantages of the TiO2 (metal oxide) coating effect. The dielectric constants (or permittivity) (ε′) and loss factors (ε′′) of the three materials dispersed in silicone oil were measured as a function of electric field frequency (f), as shown in Fig. 6. The dielectric constant of material is closely related to the polarizability of the material under an electric field; specifically, the achievable polarizability (Δε) and relaxation time (λ) can be determined from the dielectric constant (ε′) and loss factor (ε′′) to investigate the polarization tendencies of a material.56 The achievable polarizability (magnitude of the polarization tendency) is derived from the difference between the fictitious (ε0) and static (ε∞) dielectric constants. The former is measured in the low electric field frequency region (f → 0) and the latter is determined from the high frequency region (f → ∞). The measured achievable polarizabilities of the SiO2, ST/CS, and HST rods were 1.27, 1.98, and 3.05, respectively. The differences in achievable polarizability of the three rod-like materials were attributed to the combined effect of the TiO2 coating and increased surface area. In particular, the TiO2 coating increased the charge accumulation capacity and electrical conductivity and narrowed the band gap of the ST/CS and HST rods compared with those of the SiO2 rod, owing to the intrinsic and physical properties of TiO2. Moreover, the increased surface area of the HST rod also contributed to the increased charge accumulation capacity. The relaxation times (λ) of the three samples were measured to estimate the interfacial polarization efficiency of the materials using the following equation57
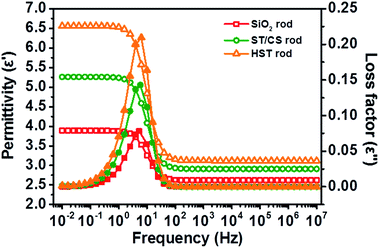 | ||
| Fig. 6 Permittivity (ε′, open symbol) and loss factor (ε′′, closed symbol) of SiO2 rod, ST/CS rod, and HST rod as a function of electric field frequency (f). | ||
| ε0 | ε∞ | Δε = (ε0 − ε∞) | fmaxb (Hz) | Λc (s) | |
|---|---|---|---|---|---|
| a Dielectric parameters were acquired by impedance analyzer (Solatron 1260) coupled with dielectric interface (Solatron 1296).b The fmax values of ER fluids were determined by nonlinear regression using OriginPro program.c The relaxation time was determined using λ = 1/(2πfmax) equation. | |||||
| SiO2 rod | 2.62 | 3.89 | 1.27 | 5.30 | 0.03 |
| ST/CS rod | 2.91 | 4.89 | 1.98 | 5.89 | 0.027 |
| HST rod | 3.12 | 6.17 | 3.05 | 6.32 | 0.025 |
Considering these results, the HST rod was successfully fabricated by incorporation of the as-mentioned components to achieve various intrinsic and physical advantages, such as enhanced dielectric properties, dispersion stability, high porosity, and increased surface area. According to previous studies, each integrated component can provide useful properties for practical applications. For example, incorporation of the TiO2 composite provides photocatalytic activity, a rapid electron transportation layer for DSSC applications, and inhibition of bacterial growth.21,23,59 In the case of hollow structures, possible applications are drug delivery, nanocages for encapsulation, and nanoreactors for material synthesis.29,60,61 The 1D metal oxide structure can be applied as a building block for nanodevices, provide mechanical strength by the overlapping of materials, and act as an electrical pathway.4,6,62
3.3. Electro-response of HST rod-based ER fluid
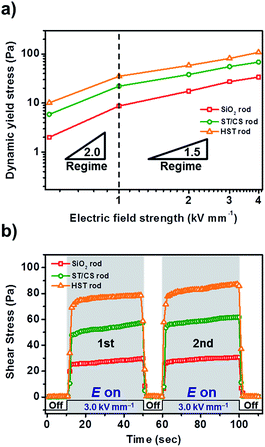
In this study, we chose ER application to investigate the advantages of the as-synthesized HST rod. Generally, ER performance is affected by numerous factors, including applied electric field strength, temperature, electrode pattern, frequency of electric field, and properties of the material itself.40–43 In recent decades, a huge number of materials with different compositions, sizes, and characteristics have been applied in ER studies to maximize performance.44–46 Among various characteristics, the most important factors for ER materials are dispersion stability (or anti-sedimentation property) and the dielectric property, which directly affects the ER activity.33 Thus, ER materials with a low particle mass and density are necessary to enhance dispersion stability. As mentioned previously, the HST rods were fabricated as a hollow material with a low particle density compared with non-hollow SiO2 and ST/CS rods, to attain enhanced dispersion stability. Moreover, the dielectric properties of the HST rod were greatly improved compared with those of the SiO2 and ST/CS rods owing to the synergistic effect of a high tailored surface area and TiO2 decoration. Previous studies also reported that geometric effects arising from 1D rod-like materials can improve ER performance. For instance, Lee et al. reported that rod-like GO-coated SiO2 materials exhibited superior ER efficiency compared with spherical GO-coated SiO2.63 Moreover, Hong et al. provided a graphical explanation of the enhanced ER performance of TiO2 rod materials relative to that of TiO2 spheres.62 Specifically, the 1D rod-like material was able to exert excellent mechanical strength owing to the compact overlapping of materials. In this regard, our expectation was that the HST rod may be a good candidate for a ER material, combining the aforementioned advantages and fulfilling the requirements for a positive ER effect.The shear stress curves of the SiO2, ST/CS, and HST rod-based ER fluids were examined as a function of shear rate (τ′) under an applied electric field of 3.0 kV mm−1 (Fig. 7). By applying the electric field, all ER fluids manifested immediate shear stress curves due to the creation of fibril-like structures in response to the electrostatic force. In the low shear rate region, all three materials showed plateau curves representing Bingham-like behavior due to competition (and balancing) between the formation of fibril-like structures from the interfacial polarization and deformation caused by the hydrodynamic force. After passing the critical shear rate (τcrit) near 100 s−1, Newtonian fluid behavior of proportional shear stress to shear rate was observed for all ER fluids. This was ascribed to the hydrodynamic force overcoming the electrostatic force, resulting in a sudden increase in shear stress. From this set of curves, it was clearly observed that the HST rod-based ER fluid demonstrated the best ER performance, followed by the ST/CS rod and then the SiO2 rod. In detail, the ST/CS rod exhibited higher ER activity than the SiO2 rod, even though the dispersion stability was slightly decreased by the TiO2 coating process. As discussed earlier, this can be attributed to the increased dielectric property of materials incorporating the TiO2 shell. It was clear that synergistic contributions from the incorporation of TiO2 metal oxide and the increase in surface area effectively enhanced the interfacial polarizability of the ST/CS and HST rod materials. After the core SiO2 etching process, the ER activity of the HST rod was further improved compared with that of the ST/CS rod due to enhanced dispersion stability and particle mobility originating from the large internal space created within the TiO2 shell. In general, ER materials with high dispersion stability can create more fibril-like structures to exert better ER performance by minimizing the sedimentation problem.50 Hence, the optimal ER performance of the HST rod-based ER fluids can be understood to originate from low particle density, high porosity, and the presence of the hollow space, combined with the enhanced dielectric property of the material. Also, ER activities of HST rods with different L/D were measured to investigate the influence of geometry on ER performance (Fig. S9†). Notably, ER performance of HST nanomaterials increased with increasing aspect ratio, but HST rod with L/D of 5 showed decreased ER activity compared to rod with L/D of 3 due to the aggregated states of material, as shown in Fig. S5.†
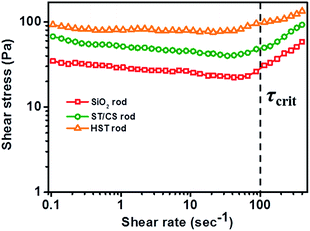 | ||
| Fig. 7 Shear stress of SiO2 rod-, ST/CS rod-, and HST rod-based ER fluids as a function of shear rate (τ′) under 3.0 kV mm−1 of electric field strength (3.0 wt% in silicone oil). | ||
In addition, the rod-like geometric (1D orientation) effect of the HST rod on ER activity was assessed by direct comparison with spherical particles. For a precise comparison, spherical hollow SiO2/TiO2 (HST sphere) particles were synthesized using exactly the same method as that used for the HST rod, except SiO2 spheres were used as a core template material. Specifically, an HST sphere was fabricated with a diameter of ca. 75 nm (Fig. S5a†) composed of the same elements (Si, Ti, and O) as the HST rod material (Table S4†). Therefore, the HST spheres possessed similar characteristics and advantages to the HST rod materials, such as a hollow structure, high porosity, and a TiO2 shell; the only difference between the two materials was in their geometrical structures. The ER activities of the HST sphere and the HST rod were examined and compared, as shown in Fig. S9.† Noticeably, the HST sphere-based ER fluids exhibited maximum shear stress of ca. 16 Pa, which was a 6-fold lower than that of the HST rod-based ER fluid, with maximum shear stress of ca. 93 Pa. This result can be explained by various geometric effects originating from the 1D rod-like structure of the HST rod: flow resistance, mechanical stability, and polarization ability. First, the resistance of the HST rod to hydrodynamic force was greatly improved, owing to the rod-like structure of the material. The relationship between the flow resistance and the geometric structure of the material can be described in terms of dynamic drag (Fd) as follows:64
Finally, the polarization ability of a material is greatly influenced by the particle geometry. According to previous studies, 1D oriented (or rod-like) materials exhibited higher polarizability than spherical particles owing to the increased induced dipole moment.32,62,63,65 The magnitude of the dipole moment of spherical and ellipsoidal materials can be theoretically exploited by theories provided by Stratton and Dukhin. For a spherical particle, the dipole moment is determined by Stratton's equation:66,67
where p is the dipole moment, V is the volume of the elongated material (V = 4/3πa3L/a), and Az is the depolarization factor of material defined by the following equation:
where ξ is the geometrical factor, L is the radius of the major axis of the material, and a is the minor axis of the material. From these sets of equations, it can be deduced that the higher polarization ability of a 1D material originates from the increase in induced dipole moment. To support this theoretical explanation, the dielectric properties of HST spheres were also examined and compared with those of HST rods, and the practical polarization ability of the materials was verified (Fig. S10†). Furthermore, the achievable polarizability (Δε) and relaxation time (λ) of the HST sphere were determined as 1.39 and 0.03 s, respectively. From these results, the HST rod material with larger Δε (3.05) and shorter λ (0.025 s) has a better polarization ability and response than an HST sphere due to the increased dipole moment induced by the 1D structure. These experimental results confirm the advantage of rod-like materials over spherical ones in ER activity, originating from enhanced flow resistance, mechanical strength, and dielectric properties.
3.4. Various stability and suitability test of HST-based ER fluid
To investigate the stability and suitability of HST rod-based ER fluids, various characterizations were undertaken: ER activity as a function of electric field strength, electric field on-off test, and optical microscope analysis. The stability of the HST rod against an electric field was measured by varying the applied electric field strength at a fixed shear rate of 0.1 s−1, as shown in Fig. 8a. The applied electrical field strength was increased to 4.0 kV mm−1 in increments of 1.0 kV mm−1. The three rod materials exhibited stable yield stresses without any fluctuation up to 4.0 kV mm−1, verifying their stability as ER fluids. In particular, the yield stress of materials increased in proportion to the square of the electric field strength in the low electric field region (<1.0 kV mm−1). In contrast, yield stresses increased with a 1.5 power of electric field strength in the high electric field region (>1.0 kV mm−1). From these results, ER fluids composed of HST rod and intermediate rod materials possess sufficient sustainability to withstand high electric field strengths without electrical shorting.The continuousity, reversibility, and reproducibility of the materials were examined using an electric field on-off test (Fig. 8b). The applied electric field strength (3.0 kV mm−1) and shear rate (0.1 s−1) were completely fixed during this investigation; hence, the ER activity was solely controlled by the presence of the electric field. Under the applied electric field, ER fluids exerted immediate shear stress curves. When the electric field was turned off, the shear stresses of all samples decreased almost immediately to their original state. Similar shear stress curves were observed in the second cycle, showing the reproducibility of ER fluids even after the application of large electric fields. Furthermore, the real-time structural changes of HST rod-based ER fluids were visualized using an optical microscope (OM) (Fig. S11†). First, well-dispersed ER fluids were placed between the electrodes to show randomly distributed particles. With the application of an electric field (1.0 kV mm−1), large numbers of rigid fibril-like structures were suddenly constructed within a few milliseconds to provide practical observation of ER activity. These results successfully demonstrated the stability, high reversibility, and reproducibility of HST rod materials for ER application.
4. Conclusions
A 1D oriented hollow SiO2/TiO2 (HST) rod was successfully synthesized via sequential steps of sol–gel use, shell coating, and the SMER method. The resulting HST rod particles manifested various advantages arising from the creation of an internal hollow space, incorporation of a TiO2 shell, and a rod-like structure. In particular, the hollow structure contributed to high pore volume, low density, and increased surface area. The TiO2 shell coating enhanced the dielectric property of the material through increased interfacial polarization and charge accumulation capacity. To obtain deeper insight into the synthesized material, the HST rod was adopted as an ER fluid, which requires various parameters for high performance. The HST rod exhibited 3.0- and 1.5-fold enhanced ER performance compared with the preliminary fabricated SiO2 and ST/CS rods due to the combined contributions of these properties. Moreover, HST rod-based ER fluids exhibited sixfold higher ER activity than similarly fabricated HST spheres due to enhanced mechanical strength, flow resistance, and dielectric property originating from the 1D structure. The ER application clearly demonstrated the numerous positive characteristics of HST rods, indicating that these versatile materials can be employed in various applications for future study.Acknowledgements
This work was supported by Development Fund of Seoul National University funded by Dongjin Semichem Co., Korea (0458-20130066).Notes and references
- R. S. Devan, R. A. Patil, J.-H. Lin and Y.-R. Ma, Adv. Funct. Mater., 2012, 22, 3326 CrossRef CAS.
- T. Zhai, L. Li, Y. Ma, M. Liao, X. Wang, X. Fang, J. Yao, Y. Bando and D. Golberg, Chem. Soc. Rev., 2011, 40, 2986 RSC.
- S. M. Liu, L. M. Gan, L. H. Liu, W. D. Zhang and H. C. Zeng, Chem. Mater., 2002, 14, 1391 CrossRef CAS.
- X. Duan, Y. Huang, Y. Cul, J. Wang and C. M. Liever, Nature, 2001, 409, 66 CrossRef CAS PubMed.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and H. Q. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- C. N. R. Rao and A. Govindaraj, Adv. Mater., 2009, 21, 4208 CrossRef CAS.
- K. Lee, W. S. Seo and J. T. Park, J. Am. Chem. Soc., 2003, 125, 3408 CrossRef CAS PubMed.
- X. Fang, Y. Bando, U. K. Gautam, C. Ye and D. Golberg, J. Mater. Chem., 2008, 18, 509 RSC.
- S. Hu and X. Wang, J. Am. Chem. Soc., 2008, 130, 8126 CrossRef CAS PubMed.
- K. R. Joshi and J. J. Schneider, Chem. Soc. Rev., 2012, 41, 5285 RSC.
- Z. R. Dai, Z. W. Pan and Z. L. Wang, Adv. Funct. Mater., 2003, 13, 9 CrossRef CAS.
- Q. Zhang, H.-Y. Wang, X. Jia, B. Liu and Y. Yang, Nanoscale, 2013, 5, 7175 RSC.
- T. Zhai, X. Fang, M. Liao, X. Xu, H. Zeng, B. Yoshino and D. Golberg, Sensors, 2009, 9, 6504 CrossRef CAS PubMed.
- A. Dev, J. P. Richters, J. Sartor, H. Kalt, J. Gutowski and T. Voss, Appl. Phys. Lett., 2011, 98, 131111 CrossRef.
- S. V. N. T. Kuchibhatla, A. S. Karakoti, D. Bera and S. Seal, Prog. Mater. Sci., 2007, 52, 699 CrossRef CAS.
- M. Gutowski, J. E. Jaffe, C.-L. Liu, M. Stoker, R. I. Hedge, R. S. Rai and P. J. Tobin, Appl. Phys. Lett., 2002, 80, 1897 CrossRef CAS.
- X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- B. C. Satishkumar, A. Govindaraj, M. Nath and C. N. R. Rao, J. Mater. Chem., 2000, 10, 2115 RSC.
- A. I. Abdulagatov, Y. Yan, J. R. Cooper, Y. Zhang, Z. M. Gibbs, A. S. Cavanagh, R. G. Yang, Y. C. Lee and S. M. George, ACS Appl. Mater. Interfaces, 2011, 3, 4593 CAS.
- J. Robertson, Eur. Phys. J.: Appl. Phys., 2004, 28, 265 CrossRef CAS.
- N. Wu, J. Wang, D. N. Tafen, H. Wang, J.-G. Zheng, J. P. Lewis, X. Liu, S. S. Leonard and A. Manivannan, J. Am. Chem. Soc., 2010, 132, 6679 CrossRef CAS PubMed.
- Z. Miao, D. Xu, J. Ouyang, G. Guo, X. Zhao and Y. Tang, Nano Lett., 2002, 2, 717 CrossRef CAS.
- D. Hwang, H. Lee, S.-Y. Jang, S. M. Jo, D. Kim, Y. Seo and D. Y. Kim, ACS Appl. Mater. Interfaces, 2011, 3, 2719 CAS.
- K. M. Parida and B. Naik, J. Colloid Interface Sci., 2009, 333, 269 CrossRef CAS PubMed.
- W. Li and D. Zhao, Adv. Mater., 2013, 25, 142 CrossRef CAS PubMed.
- S. Son, S. H. Hwang, C. Kim, J. Yun and J. Jang, ACS Appl. Mater. Interfaces, 2013, 4, 4815 Search PubMed.
- J.-L. Hu, H.-S. Qian, J.-J. Li, Y. Hu, Z.-Q. Li and S.-H. Yu, Part. Part. Syst. Charact., 2013, 30, 306 CrossRef CAS.
- J. Lee, S. H. Hwang, J. Yun and J. Jang, ACS Appl. Mater. Interfaces, 2014, 6, 15420 CAS.
- Y. Jang, S. Kim, W.-K. Oh, C. Kim, I. Lee and J. Jang, Chem. Commun., 2014, 50, 15345 RSC.
- M. Choi, C. Kim, S. O. Jeon, K. S. Yook, J. Y. Lee and J. Jang, Chem. Commun., 2011, 47, 7092 RSC.
- K. Lee, C.-M. Yoon, J. Noh and J. Jang, Chem. Commun., 2016, 52, 4231 RSC.
- C.-M. Yoon, K. Lee, J. Noh, S. Lee and J. Jang, J. Mater. Chem. C, 2016, 4, 1713 RSC.
- C.-M. Yoon, S. Lee, O. J. Cheong and J. Jang, ACS Appl. Mater. Interfaces, 2015, 7, 18977 CAS.
- S. H. Hwang, D. H. Shin, J. Yun, C. Kim, M. Choi and J. Jang, Chem.–Eur. J., 2014, 20, 4439 CrossRef CAS PubMed.
- J. Yun, S. H. Hwang and J. Jang, ACS Appl. Mater. Interfaces, 2015, 7, 2055 CAS.
- J. B. Yin and X. P. Zhao, Chem. Mater., 2004, 16, 321 CrossRef CAS.
- M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Marzán, ACS Nano, 2010, 4, 3591 CrossRef CAS PubMed.
- S. Y. Oh, M. K. Oh and T. J. Kang, Colloids Surf., A, 2013, 436, 354 CrossRef CAS.
- J.-Y. Hong and J. Jang, Soft Matter, 2012, 8, 7348 RSC.
- T. Hao, Adv. Mater., 2001, 13, 1847 CrossRef CAS.
- J. B. Yin and X. P. Zhao, Chem. Mater., 2002, 14, 4633 CrossRef CAS.
- J. Yin and X. Zhao, J. Phys. D: Appl. Phys., 2001, 34, 2063 CrossRef CAS.
- P. Gonon and J.-N. Foulc, J. Appl. Phys., 2000, 87, 3563 CrossRef CAS.
- C. McIntyre, H. Yang and P. F. Green, ACS Appl. Mater. Interfaces, 2013, 5, 8925 CAS.
- Y. D. Liu, X. Quan, B. Hwang, Y. K. Kwon and H. J. Choi, Langmuir, 2014, 30, 1729 CrossRef CAS PubMed.
- S. D. Kim, W. L. Zhang and H. J. Choi, J. Mater. Chem. C, 2014, 2, 7541 RSC.
- T. Hao, A. Kawai and F. Ikazaki, Langmuir, 1998, 14, 1256 CrossRef CAS.
- S. H. Kwon, S. H. Piao and H. J. Choi, Nanomaterials, 2015, 5, 2249 CrossRef CAS PubMed.
- L. D. Liu and H. Choi, Soft Matter, 2012, 8, 11961 RSC.
- C.-M. Yoon, S. Lee, S. H. Hong and J. Jang, J. Colloid Interface Sci., 2015, 438, 14 CrossRef CAS PubMed.
- S. Kim, Y. Jang, W.-K. Oh, C. Kim and J. Jang, Adv. Healthcare Mater., 2014, 3, 1097 CrossRef CAS PubMed.
- X. Lu, M. Yu, G. Wang, T. Zhai, S. Xie, Y. Ling, Y. Tong and Y. Li, Adv. Mater., 2013, 25, 267 CrossRef CAS PubMed.
- A. N. Murashkevich, A. S. Lavitskaya, T. I. Barannikova and I. M. Zharskii, J. Appl. Spectrosc., 2008, 75, 730 CrossRef CAS.
- B. Ding, H. Kim, C. Kim, M. Khil and S. Park, Nanotechnology, 2003, 14, 532 CrossRef CAS.
- S. Lee, J. Noh, S. Hong, Y. K. Kim and J. Jang, Chem. Mater., 2016, 28, 2624 CrossRef CAS.
- J. P. Huang and K. W. Yu, Phys. Lett. A, 2004, 333, 347 CrossRef CAS.
- H. Block, J. P. Kelly, A. Qin and T. Watson, Langmuir, 1990, 6, 6 CrossRef CAS.
- B. X. Wang and X. P. Zhao, Adv. Funct. Mater., 2005, 15, 1815 CrossRef CAS.
- C. McCullagh, J. M. C. Robertson, D. W. Bahnemann and P. K. J. Robertson, Res. Chem. Intermed., 2007, 33, 359 CrossRef CAS.
- R. Kumar, A. N. Maitra, P. K. Patanjali and P. Sharma, Biomaterials, 2005, 26, 6743 CrossRef CAS PubMed.
- X. Lai, J. Li, B. A. Korgel, Z. Dong, Z. Li, F. Su, J. Du and D. Wang, Angew. Chem., 2011, 123, 2790 CrossRef.
- J.-Y. Hong, M. Cho, C. Kim and J. Jang, J. Colloid Interface Sci., 2010, 347, 177 CrossRef CAS PubMed.
- S. Lee, C.-M. Yoon, J.-Y. Hong and J. Jang, J. Mater. Chem. C, 2014, 2, 6010 RSC.
- Z.-G. Feng and E. E. Michaelides, J. Fluids Eng., 2001, 123, 841 CrossRef.
- J. Noh, C.-M. Yoon and J. Jang, J. Colloid Interface Sci., 2016, 470, 237 CrossRef CAS PubMed.
- R. C. Kanu and M. T. Shaw, J. Rheol., 1998, 42, 657 CrossRef CAS.
- J. A. Stratton, Electromagnetic Theory, McGraws-Hill, New York and London, 1941 Search PubMed.
- S. S. Dukhin, Dielectric Properties of Disperse Systems, Wiley-Interscience, New York, 1971 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (1) SEM micrographs of SiO2 rod, ST/CS rod, and HST rod (L/D = 3), (2) TEM micrographs HCl untreated and calcined SiO2 rod and resulting HST rod, (3) TEM micrographs of shell thickness controlled HST rod, (4) TEM micrographs of SiO2 rod, ST/CS rod, and HST materials with varying aspect ratio (L/D = 1, 2, 6), (5) low-magnified TEM micrographs of various HST materials (L/D = 1, 2, 6), (6) physical parameters of various HST materials, (7) porosity, pore volume, and BET surface area of HST rod (L/D = 3), (8) EDS analysis of SiO2 rod, ST/CS rod, and HST rod (L/D = 3), (9) STEM elemental mapping of SiO2 rod (L/D = 3), (10) FT-IR spectra and XRD pattern of SiO2 rod, ST/CS rod, and HST rod (L/D = 3), (11) FT-IR spectra of HCl treated and untreated SiO2 rod, (12) atomic compositions of various HST materials (L/D = 1, 2, 6), (13) shear stress of HST material-based ER fluids with varying aspect ratio (L/D = 1, 2, 6), (14) dielectric properties of HST sphere. See DOI: 10.1039/c7ra01786c |
| This journal is © The Royal Society of Chemistry 2017 |