Open Access Article
Open Access ArticleIptycene-functionalized silica gel for the purification of fullerenes using flash chromatography†
Serxho Selmani,
M. Yue Shen and
Derek J. Schipper *
*
Department of Chemistry, Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Ave. W., Waterloo, Ontario N2L 3G1, Canada. E-mail: dschipper@uwaterloo.ca
First published on 30th March 2017
Abstract
Fullerenes have attracted much attention since their discovery but research efforts are plagued by the challenge of fullerene purification. We have developed a functionalized silica gel that allows for the purification of fullerenes using standard flash chromatography. The method relies on a stationary phase consisting of bent aromatic molecules that interact strongly with different types of fullerenes, allowing for their purification on a relatively large scale.
Since their discovery in 1985,1 fullerenes have been at the forefront of materials research due to their electronic and optical properties.2 Fullerenes have been used in biosensors,3,4 photovoltaics,5–7 as molecular containers8–10 and in biological applications.11 The potential for their future advancement is vast but there has been significant lag in their development due to the cost of producing fullerenes12 and, more importantly, the difficulty in purifying fullerene based materials. The difficulty stems from the chemical similarity of fullerenes, consisting entirely of sp2 carbon atoms.
Currently there exist a few main methods for fullerene purification. The most common methods include complexation,13,14 crystallization15 and high performance liquid chromatography (HPLC).16–19 There are several drawbacks to each method. Complexation generally requires specifically designed molecules, which are expensive and may be difficult to recycle. Crystallization is a slow process and has a significant yield to purity trade-off.
Chromatographic methods are currently satisfactory for small-scale purification of not only C60 and higher fullerenes from as-prepared fullerene soot, but also for the purification of modified fullerene derivatives. This is significant because the other methods are not widely adaptable to derivatized fullerenes, which are indispensable in the research and development of fullerene based technologies.20–22 However, the current state of the art relies on HPLC to achieve satisfactory separation of fullerenes. While HPLC is a powerful purification technique, it suffers from high costs and low output quantity. The high cost is not only due to the upfront cost of the HPLC equipment, but also the cost of the high performance silica gel. These HPLC columns can cost tens of thousands of US dollars and have injection volumes on the order of 1 mL, thus are only capable of separating a few milligrams of fullerenes at a time.
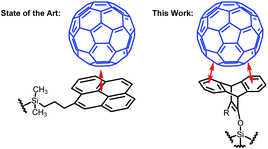
A far more desirable method for fullerene purification would be the use of flash chromatography. Flash chromatography is a routine technique that is used on a daily basis in most organic synthetic labs, making it indispensable to many researchers.23 It allows for the separation of much larger quantities of materials than HPLC and has a far lower cost since it only requires standard glassware. Due to its widespread use, a method for purifying fullerenes with flash chromatography is alluring. However, it has proven difficult to design a stationary phase capable of sufficient interaction with the fullerenes to provide enough retention time for separation to take place. Typical silica gel only interacts with the fullerenes via weak van der Waals interactions, resulting in fast elution even in weak solvents like hexanes. Likewise, reverse phase silica gel results in a similar problem. However, the current state of the art employs a flat, extended aromatic core which is capable of forming π–π interactions with the fullerenes, thus increasing retention time (Fig. 1).24 This introduction of aromatics to the silica gel allowed for a large enough retention time to separate fullerenes on a small scale through the use of HPLC. While this innovation was crucial in establishing a foundation for chromatographic separation of fullerenes, further advancement in the interaction between fullerenes and the stationary phase must be made before standard flash chromatography becomes viable.
Herein we describe a new concept for functionalized stationary phases that enable facile and scalable flash chromatography of fullerenes. Our phase takes advantage of the curvature of fullerenes as well as their aromatic nature to separate the chemically similar mixture of compounds. Current stationary phases rely solely on the π–π interactions that are similar, specifically in C60 and C70, for separation with flash chromatography. Previous reports have shown that triptycene, its derivatives and other bent aromatic molecules are capable of binding selectively to varying sizes of fullerenes and carbon nanotubes.25–27 Based on these reports, we devised a stationary phase consisting of bent aromatic molecules that allows for substantial separation between fullerenes (Fig. 1). The amount of fullerenes loaded in each run was about 25 mg, which is many times larger than what is capable with current HPLC purification techniques. The loading is based on the use of 12 g of silica gel and therefore has potential to be further scaled up.
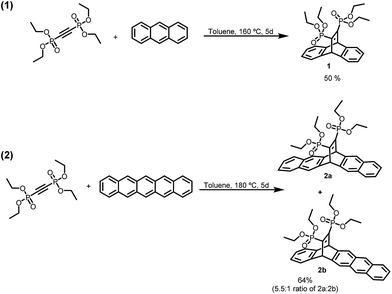
Bent aromatic molecule 1 was chosen due to its facile synthesis consisting of only a single step from commercially available materials. Additionally, the diethyl phosphonate groups, such as those of 1, have been shown to be excellent groups for the functionalization of various metal oxides including silica gel.28 The Diels–Alder reaction between anthracene and bis(diethoxyphosphoryl)acetylene proceeded smoothly to afford 1 in 50% yield (Scheme 1, reaction (1)).
1 was then used to functionalize silica gel (see ESI 1.3†) by refluxing in acetonitrile followed by solvent removal and heating in a vacuum oven. Because 1 contains four possible sites for functionalization, it was important to determine the optimal ratio of silica gel to 1 in order to maximize the functionalization of the silica while limiting the amount of unused 1. To this end, we tried a number of small-scale reactions and determined the extent of the functionalization by the use of 29Si SSNMR (solid-state nuclear magnetic resonance). It was determined that 28 w/w% of 1 to silica gel was required to sufficiently functionalize the silica gel as evidenced by the 29Si SSNMR (Fig. 2). The peak at −109 ppm (designated Q4) is attributed to the internal silicon atoms which have no free –OH groups and thus are not available for functionalization and the size of the peak remains constant throughout the trials. At −99 ppm is the peak associated with silicon atoms with a single –OH group (designated Q3).29 This peak is used to determine the extent of functionalization because as the Si–OH bond is replaced by an Si–O–R bond, this peak decreases.
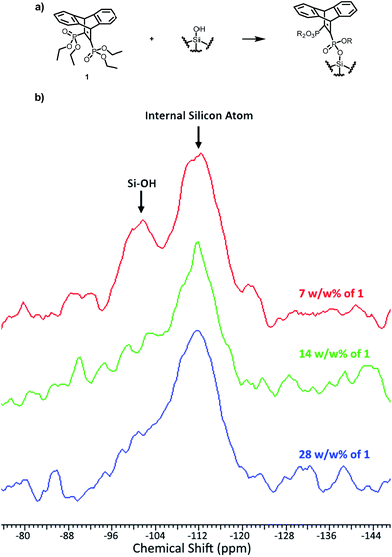 | ||
| Fig. 2 (a) The functionalization of silica gel with 1. (b) 29Si SSNMR of functionalized silica at varying w/w% of 1 with silica gel (red: 7% 1, green: 14% 1, blue: 28% 1). | ||
The effectiveness of the 1-functionalized silica gel was evaluated by attempting to separate a C60/C70 mixture using flash chromatography with an in-line UV-Vis spectrophotometer. The resolution was also compared to that of unfunctionalized silica gel. The results showed no improvement between using silica gel functionalized with 1 and unfunctionalized silica gel, resulting in insignificant separation and a retention time of only 8 minutes (Fig. 3). Based on the retention time, it is reasonable to conclude that the interactions between 1 and the fullerenes are too weak to afford meaningful retention and, therefore, resolution. We believed that by incorporating more aromatic rings into the stationary phase, there would be a larger surface area for increased π–π interactions with the fullerenes, resulting in longer retention times and better resolution. Therefore, we proceeded to synthesize the bent, extended aromatic compound 2a, via a Diels–Alder reaction between pentacene and bis(diethoxyphosphoryl)acetylene (Scheme 1, reaction (2)). However, we discovered that the reaction led to two inseparable isomers, 2a and 2b, in a 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio respectively. We hypothesized that 2b should behave in a similar manner due to its similar shape and aromatic units, so we continued on, using this mixture of isomers.
1 ratio respectively. We hypothesized that 2b should behave in a similar manner due to its similar shape and aromatic units, so we continued on, using this mixture of isomers.
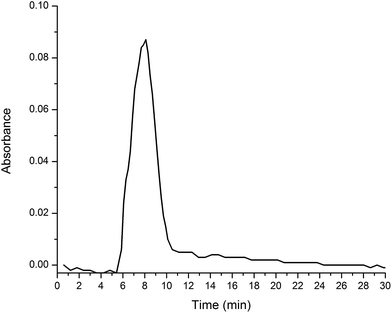 | ||
| Fig. 3 UV-Vis chromatogram of C60/C70 mixture being eluted on a flash column with 1-functionalized silica gel as the stationary phase and hexanes as the mobile phase. | ||
2(2a/b mixture) was then used to functionalize silica gel under the same functionalization conditions as 1. The 2-functionalized silica gel was then used to separate a C60/C70 fullerene mixture using flash chromatography which resulted in significant separation between C60 and C70. Moreover, not only is there increased resolution (Fig. 4) but also, drastically increased retention times (45 minutes for C60 and 90 minutes for C70). These results verify the hypothesis that increased conjugated area of 2 leads to stronger π–π interactions with fullerenes. Due to the unique shape of 2, it is possible that the subsequent interactions with fullerenes may lead to a different elution order from that the flat aromatic stationary phase. However, upon identification of the fractions it was confirmed that that C60 elutes first, followed by C70, which indicates that C70 forms stronger interactions with the stationary phase than C60. Consistent with previous reports on the interaction of fullerenes with extended conjugated triptycene motifs,30 the stronger interaction of C70 may in part be due to the boat-like cavity of the 2-functionalized stationary phase being better matched to interact with oval-shaped C70 as opposed the spherical shape of C60.
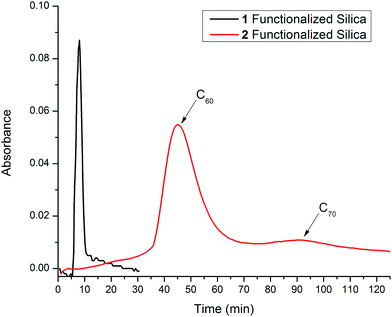 | ||
| Fig. 4 UV-Vis chromatogram of a C60/C70 mixture being eluted on a flash column with 1-(black) or 2-(red) functionalized silica gel as the stationary phase and hexanes as the mobile phase. | ||
After confirming that the newly functionalized silica gel performed satisfactorily using hexanes as the mobile phase, additional eluent systems were evaluated in hopes of decreasing the retention time, thus speeding up the separation time. As can be seen in Fig. 5, the elution time can be decreased significantly by increasing the amount of toluene in the mobile phase. In pure toluene, the retention time can be reduced to just 6.5 and 7.5 minutes for C60 and C70, respectively. The two peaks are visible but not completely resolved. As a result, we tested a number of other mobile phases and found that 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexanes/toluene allowed for easy separation of C60 from C70 while decreasing elution times to 33 and 59 minutes, respectively. Experimental results show slight decreases in performance over a number of trials with loss of separation after 10 trials. However, utilizing more traditional and robust anchoring groups such as chlorosilanes will likely avoid this issue.
1 hexanes/toluene allowed for easy separation of C60 from C70 while decreasing elution times to 33 and 59 minutes, respectively. Experimental results show slight decreases in performance over a number of trials with loss of separation after 10 trials. However, utilizing more traditional and robust anchoring groups such as chlorosilanes will likely avoid this issue.
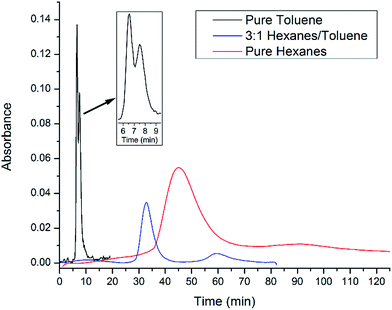 | ||
| Fig. 5 UV-Vis chromatograms of C60/C70 mixture being eluted on a flash column with 2-functionalized silica gel with various eluent systems. | ||
Conclusions
In conclusion, we have demonstrated the effectiveness of a stationary phase functionalized with bent aromatic molecules for scalable purification of fullerenes by flash chromatography. Both the size of the conjugated aromatic system and the shape of the active component of the stationary phase proved to be essential to the successful resolution of fullerene derivatives by increasing the extent of π–π interactions. This concept should be widely applicable given that flash chromatography is a scalable technique extensively utilized in many synthetic laboratories and does not require any expensive specialty equipment.Acknowledgements
We thank NSERC, the University of Waterloo and the Canada Research Chairs Program (CRC-Tier II, D. J. S.) for financial support. We thank SiliCycle Inc. for providing all silica gel for this work. We also thank Dr François Béland for valuable discussion.Notes and references
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS
.
- S. Rondeau and F. Morin, in Functional Materials, ed. M. Leclerc and R. Gauvin, De Gruyter, Berlin/Boston, 1st edn., 2004, pp. 37–60 Search PubMed
.
- V. G. Gavalas and N. A. Chaniotakis, Anal. Chim. Acta, 2000, 409, 131–135 CrossRef CAS
.
- J. Han, Y. Zhuo, Y. Q. Chai, Y. Xiang and R. Yuan, Anal. Chem., 2015, 87, 1669–1675 CrossRef CAS PubMed
.
- B. C. Thompson and J. M. J. Frechet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed
.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297–303 CrossRef CAS
.
- T. Fromherz, F. Padinger, D. Gebeyehu, C. Brabec, J. C. Hummelen and N. S. Sariciftci, Sol. Energy Mater. Sol. Cells, 2000, 63, 61–68 CrossRef CAS
.
- G. C. Vougioukalakis, M. M. Roubelakis and M. Orfanopoulos, Chem. Soc. Rev., 2010, 39, 817–844 RSC
.
- M. Murata, Y. Murata and K. Komatsu, Chem. Commun., 2008, 6083–6094 RSC
.
- H. Shinohara, Rep. Prog. Phys., 2000, 63, 843–892 CrossRef CAS
.
- T. Da Ros and M. Prato, Chem. Commun., 1999, 663–669 RSC
.
- A. Anctil, C. W. Babbitt, R. P. Raffaelle and B. J. Landi, Environ. Sci. Technol., 2011, 45, 2353–2359 CrossRef CAS PubMed
.
- J. L. Atwood, G. A. Koutsantonis and C. L. Raston, Nature, 1994, 368, 229–231 CrossRef CAS
.
- K. Nagata, E. Dejima, Y. Kikuchi and M. Hashiguchi, Org. Process Res. Dev., 2005, 9, 660–662 CrossRef CAS
.
- N. Coustel, P. Bernier, R. Aznar, A. Zahab, J.-M. Lambert and P. Lyard, Chem. Commun., 1992, 1402–1403 RSC
.
- J. Xiao, M. R. Savina, G. B. Martin, A. H. Francis and M. E. Meyerhoff, J. Am. Chem. Soc., 1994, 116, 9341–9342 CrossRef CAS
.
- J. M. Hawkins, T. A. Lewis, S. D. Loren, A. Meyer, J. R. Heath, Y. Shibato and R. J. Saykally, J. Org. Chem., 1990, 55, 6250–6252 CrossRef CAS PubMed
.
- H. Ajie, M. M. Alvarez, S. J. Anz, R. D. Beck, F. Diederich, K. Fostiropoulos, D. R. Huffman, W. Kratschmer, Y. Rubin, K. E. Schriver, D. Sensharma and R. L. Whetten, J. Phys. Chem., 1990, 94, 8630–8633 CrossRef CAS
.
- P. D. W. Boyd and C. A. Reed, Acc. Chem. Res., 2005, 38, 235–242 CrossRef CAS PubMed
.
- M. Prato, J. Mater. Chem., 1997, 7, 1097–1109 RSC
.
- C. Dragonetti, A. Colombo, M. Fontani, D. Marinotto, F. Nisic, S. Righetto, D. Roberto, F. Tintori and S. Fantacci, Organometallics, 2016, 35, 1015–1021 CrossRef CAS
.
- F. Gibbons, H. M. Zaid, M. Manickam, J. A. Preece, R. E. Palmer and A. P. G. Robinson, Small, 2007, 3, 2076–2080 CrossRef CAS PubMed
.
- W. Clark, W. C. Still, M. Kahn and A. Mitra, J. Org. Chem., 1978, 43, 2923–2925 CrossRef
.
- K. Kimata, K. Hosoya, T. Araki and N. Tanaka, J. Org. Chem., 1993, 58, 282–283 CrossRef CAS
.
- D. V. Konarev, N. V. Drichko, R. N. Lyubovskaya, Y. M. Shul’ga, A. L. Litvinov, V. N. Semkin, Y. A. Dubitsky and A. Zaopo, J. Mol. Struct., 2000, 526, 25–29 CrossRef CAS
.
- J. Zhou, H. Li, J. Lu, G. Luo, L. Lai, R. Qin, L. Wang, S. Nagase, Z. Gao, W. Mei, G. Li, D. Yu and S. Sanvito, Nano Res., 2010, 3, 1–11 CrossRef
.
- P. Hammershøj, P. H. H. Bomans, R. Lakshminarayanan, J. Fock, S. H. Jensen, T. S. Jespersen, T. Brock-Nannestad, T. Hassenkam, J. Nygård, N. A. J. M. Sommerdijk, K. Kilså, T. Bjørnholm and J. B. Christensen, Chem.–Eur. J., 2012, 18, 8716–8723 CrossRef PubMed
.
- M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777–3807 CrossRef PubMed
.
- I. Lukd, M. Borbaruah and L. D. Quin, J. Am. Chem. Soc., 1994, 116, 1737–1741 CrossRef
.
- S. Z. Hu and C. F. Chen, Chem. Commun., 2010, 46, 4199–4201 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction procedures, chromatographic methods, spectroscopic data. See DOI: 10.1039/c7ra01575e |
| This journal is © The Royal Society of Chemistry 2017 |