Open Access Article
Open Access ArticleMicrowave-enhanced catalytic wet peroxide oxidation of quinoline: the influence of pH and H2O2 dosage and identification of reactive oxygen species†
Bo Zhang *a,
Hong Youab and
Fei Wanga
*a,
Hong Youab and
Fei Wanga
aState Key Laboratory of Urban Water Resource and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, 73, Huanghe Rd., Nangang Dist., Harbin, 150090, PR China. E-mail: zhangbovivi@163.com; Fax: +86 451 8628 3118; Tel: +86 451 8628 3118
bSchool of Marine Science and Technology, HIT at Weihai, Weihai 264209, PR China
First published on 7th March 2017
Abstract
This article presents a comprehensive study about the influence of initial pH and H2O2 dosage on quinoline mineralization efficiency (denoted by TOC abatement) by microwave-catalytic wet peroxide oxidation (MW-CWPO) with supported Cu–Ni bimetallic oxides as a catalyst and reactive oxygen identification on the basis of inhibition of quinoline mineralization using the specific radical scavengers. In an initial pH range from 2.0 to 8.0, this MW-CWPO was powerful, with the reaction at pH 7.0 obtaining the highest 81.12% TOC abatement under MW 500 W, H2O2 22.75 mmol L−1, Cu/Ni-catalyst 4 g L−1, and T 333 K. Five carboxylic acids, namely acetic acid, formic acid, succinic acid, oxalic acid, and glycolic acid, were quantified to elucidate the pH fall during mineralization under alkaline and neutral conditions. The experiments of reactive oxygen identification on the basis of inhibition of quinoline mineralization under the corresponding scavenger for ˙OH and O2˙− exhibited ˙OH and O2˙− immersed in supported bimetallic Cu/Ni oxides catalyzing MW-CWPO, and that ˙OH was a direct product and O2˙− was a secondary oxidant produced from ˙OH-involved reactions. Based on the results of stability and metal leaching experiments, it could be concluded that supported Cu–Ni bimetallic oxides could be a proper catalyst.
1. Introduction
Heterogeneous catalytic wet peroxide oxidation (CWPO), a Fenton-like technology, has appeared as a potential technique for advanced oxidation processes (AOPs) and has aroused growing interest in wastewater purification owing to its natural advantages, such as wide-ranging pH, no metal sludge, and recyclable catalyst. Many solid catalysts containing monometallic transition metals like Fe1,2 and Cu3,4 have been confirmed to be effective in heterogeneous CWPO treatment of refractory wastewater. For example, Yan et al.,2 who examined phenol degradation using Fe2O3/MCM-41 as a heterogeneous CWPO catalyst in a fixed bed reactor, found that the conversion of TOC reached 72.5% under optimum conditions. Additionally, Huang et al.5 reported the degradation of phenol using copper hydroxyl sulfates as a catalyst, and the phenol removal efficiency and COD removal were 99% and 97%, respectively, when the phenol concentration was 100 mg L−1 under the best conditions. However, iron species as a traditional catalyst show excellent catalytic activities,6 but the redox reaction between H2O2 and iron-containing catalysts is somewhat unstable.7 Such drawbacks have induced great efforts to improve them. Copper species have received a lot of attention in the last 10 years because the redox reaction between H2O2 and Cu species is more stable than that with iron.7 This characteristic promotes the application of copper-containing catalysts in water remediation.Now, bimetallic catalytic materials have come to be a research hotspot in the field of heterogeneous CWPO over the last 10 years. In heterogeneous CWPO mineralization of phenol, Xu et al.,8 who tested heterogeneous CWPO to treat phenol over Cu/Fe-flokite used as a catalyst in a glass batch reactor, found that the removal of phenol using Cu/Fe-flokite was 1.37 times under the same reaction conditions than pure Cu-flokite, indicating that bimetallic catalytic materials may present better catalytic performance. Considering that Ni presents great catalytic performance in treatment of phenol and its derivatives,9–11 our previous work confirmed that supported bimetallic Cu/Ni oxides could be successfully applied in the heterogeneous MW-CWPO process to obtain high catalytic performance.12 The two-metal materials had enhanced catalytic activity owing to the synergistic effects of the two metals.
On the other hand, microwave-enhanced catalytic oxidation has gained wide interest from numerous scientific communities in the field of wastewater treatment13,14 because it has higher energy efficiency, shorter reaction time, and the transmission of charge supports the surface.15 Additionally, earlier research confirmed that the non-thermal effects of MW can promote the decomposition of H2O2 into hydroxyl radical (˙OH).16,17 In heterogeneous CWPO mineralization of p-chlorophenol, Zhao et al.18 found that the TOC abatement was above 90% at 343 K within 15 minutes with MW irradiation, whereas it was merely 62.8% in an identical system without MW irradiations, demonstrating that under MW irradiation, the H2O2 utilization efficiency was enhanced. Analogous conclusions were attained during p-nitrophenol mineralization with CuO/Al2O3 catalyst.19 The utilization efficiency of H2O2 is an important parameter to assess H2O2-based systems20,21 because H2O2 utilization efficiency not only considerably impacts the operation cost in real-world applications, but also significantly affects the mineralization efficiency.
Furthermore, the effect of pH on mineralization performance is relatively different. Fajerwerg et al.22 found that phenol mineralization in heterogeneous CWPO decreased with increasing pH and the TOC abatement was 81.4% in 360 min with an initial pH of 2, and the mineralization efficiency was obviously impacted by the initial solution pH in a wide range of 2.0–7.0. Whether heterogeneous MW-CWPO could still retain high-pitched catalytic performance under neutral conditions is a key issue to be explored in detail. Additionally, most of the current studies on the mechanism involved in heterogeneous Fenton-like systems are based on the Haber–Weiss mechanism,23 mainly concentrating on yield of ˙OH.20 Nevertheless, few reports are available on the involvement of other radicals in heterogeneous Fenton-like systems.
The environmental persistence contaminant of quinoline, which has an N atom in the cyclic system, is very easy to be assembled in water environments.24,25 Besides, it is very harmful toward the health of humans and animals.26 It was chosen as the target pollutant to study the mineralization efficiency of supported bimetallic Cu/Ni oxides catalyzing MW-CWPO.
As part of our ongoing work in this field, this work presents the influence of initial pH and H2O2 dosage on quinoline mineralization to assess whether supported bimetallic Cu/Ni oxides catalyzing MW-CWPO could carry out the mineralization efficiently under neutral conditions, in addition to H2O2 utilization efficiency. Moreover, five carboxylic acids, namely acetic acid, formic acid, succinic acid, oxalic acid, and glycolic acid, were quantified to elucidate the pH fall during mineralization under alkaline and neutral conditions. Finally, specific radical scavengers were used to identify the oxygen radicals involved in supported bimetallic Cu/Ni oxides catalyzing MW-CWPO.
2. Experimental
2.1. Chemicals and reagents
All reagents utilized in this research were of analytical grade and were utilized without further purification. Quinoline was obtained from Sigma-Aldrich. The main characteristics of quinoline are presented in Table S1 of the ESI.† Hydrogen peroxide (H2O2) (35%, w/w, stabilized), nitrobenzene (NB) and carbon tetrachloride (CT) were obtained from J&K Scientific. All solutions were prepared with Milli-Q water.2.2. Preparation and characterization of catalyst
The supported Cu/Ni catalyst was prepared by the wet impregnation method with 50 mL of mixture (1.5 mol L−1; Cu(NO3)2 and Ni(NO3)2; CCu(NO3)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNi(NO3)2 = 5
CNi(NO3)2 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The detailed synthetic methods are illustrated in Text S1.† All the samples were kept in a desiccator for the subsequent experiments. The SEM images, EDX analysis, and XRD patterns of the as-synthesized catalyst is provided in Fig. S1–S3.† Furthermore, the elemental content of the as-prepared catalyst is provided in Table S2.† The BET surface area, pore size, and pore volume of the as-synthesized catalyst were 136.64 m2 g−1, 0.34 cm3 g−1, and 10.11 nm, respectively.
1). The detailed synthetic methods are illustrated in Text S1.† All the samples were kept in a desiccator for the subsequent experiments. The SEM images, EDX analysis, and XRD patterns of the as-synthesized catalyst is provided in Fig. S1–S3.† Furthermore, the elemental content of the as-prepared catalyst is provided in Table S2.† The BET surface area, pore size, and pore volume of the as-synthesized catalyst were 136.64 m2 g−1, 0.34 cm3 g−1, and 10.11 nm, respectively.
2.3. Degradation experiments
Batch degradation experiments were carried out in a 500 mL glass flask equipped with a mechanical agitator, a reflux condenser and a thermocouple under MW irradiation. More information is offered in Text S2.†Samples were taken at set time intervals: some sample was promptly quantified for H2O2 concentration; Na2SO3 was introduced to the remainder to impede the ongoing redox reaction before TOC (Total Organic Carbon) measurement.27 In probing the oxygen radicals included in the heterogeneous MW-CWPO catalyzed by supported Cu/Ni bimetallic oxides, NB and carbon CT were utilized as scavengers for ˙OH and O2˙−, respectively, assessing the variation of the removal rate of 100 mg L−1 quinoline with 22.75 mmol L−1 H2O2, 4 g L−1 Cu/Ni-catalyst, temperature of 333 K, and initial pH of 7.0 under MW (500 W) irradiation to identify relevant radicals.
2.4. Sample analysis
The H2O2 concentration in the solution was measured by titration with potassium permanganate.28 TOC in solution was quantified by a TOC-V analyzer (Shimadzu Co., Tokyo, Japan). Agilent ion exclusion HPLC with an Aminex HPX-87H column was employed to quantify the amount of generated carboxylic acids.27 The calculated equation of H2O2 utilization efficiency (η) is presented in Text S3.†29 The pH value was quantified and adjusted with a pH meter (FiveEasyPlus™ pH meter FEP 20, Mettler). The mineralization reaction of quinoline can be expressed as follows (eqn. (1)):| C9H7N + 23.5H2O2 → 9CO2 + 27H2O + NO2 | (1) |
The theoretical H2O2 dosage for complete oxidation of 100 mg L−1 quinoline was determined from eqn (1) to be 18.20 mmol L−1 (denote it by ▲H2O2).
3. Results and discussion
3.1. Influence of initial pH on quinoline mineralization in supported Cu/Ni bimetallic oxides catalyzing MW-CWPO
The solution pH value can substantially impact the MW-CWPO reaction. Thus, the influence of initial pH on quinoline mineralization by supported Cu/Ni bimetallic oxides catalyzing MW-CWPO was determined, as presented in Fig. 1.Fig. S4† presents the TOC abatement with different initial pH values, and it was witnessed that the influence of initial pH on quinoline mineralization was coupled with reaction time. During the first 9 min, the TOC removal efficiency diminished with increasing pH from 3 to 7, while during the remaining 9 min, the TOC abatement increased with increasing pH from 3 to 7. This may be ascribed to the variation of solution pH (Table 1) resulting from the production of some acidic intermediates. The variation of oxidation efficiency for the initial pH of 2.0 and 8.0 during 18 min was analogous to that at pH 6.0 and pH 5.0, respectively. After 18 min, the quinoline mineralization efficiency followed the order: pH 7.0 > pH 6.0 > pH 5.0 > pH 4.0 > pH 3.0 > pH 2.0 > pH 8.0; the reaction at the initial pH of 7.0 attained the best catalytic performance with 81.12% TOC abatement. The tendency of pH influence on TOC abatement in this study was not the same as that reported earlier regarding the decrease of mineralization efficiency with increasing pH.30 In most studies, the optimal pH was close to 3.0.31,32 Actually, the solution pH reduced with reaction time, as presented in Table 1.
| Time (min) | pH | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 | 7.00 | 8.00 |
| 9 | 1.95 | 2.81 | 3.34 | 3.87 | 3.99 | 3.97 | 3.85 |
| 12 | 2.01 | 2.76 | 3.27 | 3.45 | 3.47 | 3.54 | 3.45 |
| 15 | 2.01 | 2.76 | 3.31 | 3.25 | 3.30 | 3.75 | 3.27 |
| 18 | 1.98 | 2.79 | 3.54 | 3.64 | 3.85 | 4.01 | 3.49 |
Even at an initial pH of 7.0 and 8.0, the solution pH reached acidic conditions of about pH 3.5 before 15 min owing to the generation of some acidic intermediates. In the CWPO degradation of quinoline, some organic acids, including oxalic acid, succinic acid, glycolic acid, acetic acid, and formic acid, were discovered in the study by Thomsen et al.33 The decrease of pH based on the Fenton reaction was also stated in the elimination of acidic red,34 bisphenol A,35 tannery wastewater,36 and landfill leachate.37 However, the pH began to decrease from 0 min, which might be attributable to incomplete decomposition of the produced organic acids in this study, which will be revealed in-depth in the variation of generated carboxylic acids in Section 3.4.
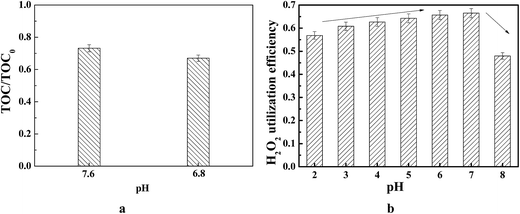
In order to explore the mineralization performance of Cu/Ni bimetallic oxides catalyzing MW-CWPO under neutral conditions without the influence of alteration of solution pH by produced organic acids, experiments were executed in buffer solutions of pH 6.8 and pH 7.6 including Na2HPO4·7H2O and NaH2PO4·H2O. From Fig. 1a, it was apparent that TOC removal substantially decreased with a stable pH of 6.8 and 7.6. In Fenton reactions the optimum pH is usually 2.0–4.0.
The benefit of supported Cu/Ni bimetallic oxides over soluble metal salts as a catalyst in CWPO lies in avoiding the shortcoming owing to metal ion precipitation. The reduced mineralization efficiency with buffers at pH 6.8 and 7.6 could be based on the fact that ˙OH has an oxidation-reduction potential of +1.5 V in basic media and +2.8 V in acidic media.38 In addition, it was noticed that the utilization of H2O2 with buffers of pH 6.8 and 7.6 was considerably inhibited, consequently diminishing the amount of reactive species for quinoline mineralization.
Fig. 1b displays the variation of H2O2 utilization efficiency with different initial pHs. As the pH rises from 2.0 to 7.0 in solution, the H2O2 utilization efficiency increased from 0.56 at pH 2.0 to 0.67 at pH 7.0 at 18 min. Higher H2O2 utilization efficiency indicates a lower required amount of H2O2 for the same mineralization efficiency. The values above 0.56 indicate that less TOC was removed than the theoretical value calculated from the amount of H2O2 in the reaction.29
The higher H2O2 utilization efficiency along with the highest TOC removal at initial pH of 7.0 in supported Cu/Ni bimetallic oxides catalyzing MW-CWPO would avoid the inherent disadvantages of homogeneous wet peroxide oxidation, including high H2O2 dosage and regulation of initial pH to be strongly acidic.39,40
3.2. Influence of H2O2 dosage on quinoline mineralization by supported Cu/Ni bimetallic oxides catalyzing MW-CWPO
The influence of H2O2 dosage was explored by altering the initial H2O2 concentration to 0.75 × ▲H2O2, 1 × ▲H2O2, 1.25 × ▲H2O2, 1.5 × ▲H2O2, and 1.75 × ▲H2O2 mmol L−1 theoretical H2O2 dosage for mineralization of 100 mg L−1 quinoline.Fig. S5† shows that the TOC removal efficiency increased with H2O2 dosage over the range from 0.75 × ▲H2O2 to 1.75 × ▲H2O2 at 18 min. During the 18 min reaction time, the TOC abatement with 1.25 × ▲H2O2 was significantly higher than that at other H2O2 dosages; 1.5 × ▲H2O2, gave 81.12%, indicating that an excessive dosage of H2O2 was not essential to attain satisfactory performance. Actually, with the exception of the reaction with 0.75 × ▲H2O2, there was some H2O2 left in the solution after 18 min, which were 0.72 mM, 0.99 mM, 2.01 mM, and 2.73 mM for the reactions with 1 × ▲H2O2, 1.25 × ▲H2O2, 1.5 × ▲H2O2, and 1.75 × ▲H2O2, respectively.
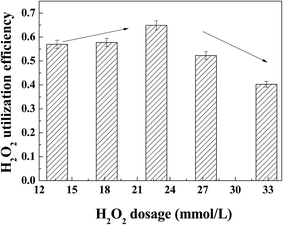
The H2O2 utilization efficiency in Fig. 2 demonstrates that the reaction with 1.25 × ▲H2O2 had the highest H2O2 utilization efficiency of 0.65. With increasing the dosage of H2O2 from 1.25 × ▲H2O2 to 1.75 × ▲H2O2, the utilization efficiency of H2O2 diminished from 0.65 to 0.40, which might be attributed to the scavenging of ˙OH by excess H2O2.19,41
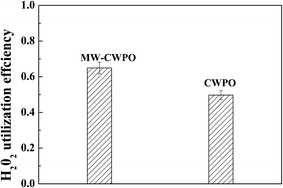
To acquire high efficiency of both TOC abatement and H2O2 utilization, the H2O2 dosage could be limited to the range of 1 × ▲H2O2 to 1.5 × ▲H2O2 for mineralization of 100 mg L−1 quinoline. The high H2O2 utilization efficiency in this study poses a vital highlight of supported Cu/Ni bimetallic oxides catalyzing MW-CWPO, which is to reduce the H2O2 dosage and operation cost. Comparison of the H2O2 utilization efficiency for CWPO and MW-CWPO is displayed in Fig. 3.
The MW-CWPO systems had a significantly higher efficiency of H2O2 utilization than the CWPO systems, increased by more than 15%. Furthermore, the MW-CWPO systems had better and quicker TOC removal efficiency. Further work was conducted to clarify the reason for the quite high utilization efficiency of H2O2 in the MW-CWPO systems.
3.3. Time course of generated carboxylic acids
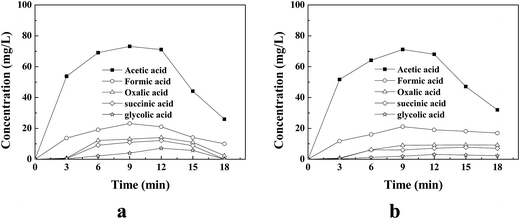
In order to reveal the explanation for the pH fall during the supported Cu/Ni bimetallic oxides catalysis of the wet peroxide oxidation under microwave irradiation of quinoline, several short linear aliphatic carboxylic acids, namely acetic acid, formic acid, succinic acid, oxalic acid, and glycolic acid, were detected by HPLC after degradation with an initial pH of 7.0.Fig. 4a displays the time-profile of carboxylic acids with 22.75 mmol L−1 H2O2 (1.25 × ▲H2O2); acetic acid was the major constituent of the carboxylic acids, achieving the highest concentration of 73.15 mg L−1 at 9 min in mineralization of 100 mg L−1 quinoline. However, formic acid, oxalic acid, succinic acid, and glycolic acid were gathered to the highest values of 23.14 mg L−1, 14.1 mg L−1, 12.11 mg L−1, and 7.11 mg L−1 at 9 min, 12 min, 12 min, and 12 min, respectively. At the end of mineralization at 18 min, succinic acid and glycolic acid disappeared and only small amounts of acetic acid and oxalic acid existed, because most of them were degraded between 12 min and 18 min; acetic acid was the chief constituent of the residual carboxylic acids. The sum of TOC contributed by residual acetic acid, formic acid, and oxalic acid, was 16.35 mg L−1, similar to the measured TOC value at 18 min of 17.16 ± 2 mg L−1. As for the pH increase starting from 12 min presented in Table 1, it might be linked to the degradation of carboxylic acids for the reason that the carboxylic acids were substantially degraded during this period. When the H2O2 dosage was reduced to 18.20 mmol L−1 H2O2 (1.00 × ▲H2O2), the time-profile of produced carboxylic acids was quite dissimilar to that with 22.75 mmol L−1 H2O2 (1.25 × ▲H2O2). Fig. 4b shows the carboxylic acids produced with 18.20 mmol L−1 H2O2 (1.00 × ▲H2O2). The succinic acid, formic acid, glycolic acid, and oxalic acid were unable to be decomposed while acetic acid was slowly but surely decomposed with 18.20 mmol L−1 H2O2 (1.00 × ▲H2O2), which was probably caused by the inadequate production of reactive species like ˙OH owing to the lower H2O2 dosage. When decreasing the dosage of H2O2 in practical applications, an approach should be approved in which the effluent from supported Cu/Ni bimetallic oxides catalyzing MW-CWPO with high biodegradability is further clarified by successive biological treatment.
3.4. Identification of free radicals utilizing the chemical probe method
Although ˙OH has been widely acknowledged as the most active species in modified systems based on the Fenton system, the production of other free radicals, for example, O2˙−, had also been witnessed in some Fenton-like reactions.42Smith et al.43 confirmed that O2˙− is the main reductive species in the degradation of CT. Hence, it is quite probable that under specific situations, both redox reactions may promote the elimination of pollutants in the MW-CWPO systems at the same time. Hereafter, further research was carried out in this study to detect the generation of ˙OH and O2˙− utilizing free radical probe tests. Based on the reaction with each of the free radicals potentially present in the MW-CWPO system, NB and CT were utilized as scavengers for ˙OH and O2˙−, respectively, because NB reacts with ˙OH rapidly (k˙OH = 3.9 × 109 M−1 s−1)44 and CT has high reactivity with O2˙− (k˙OH < 2 × 106 M−1 s−1, ke = 1.6 × 1010 M−1 s−1).45
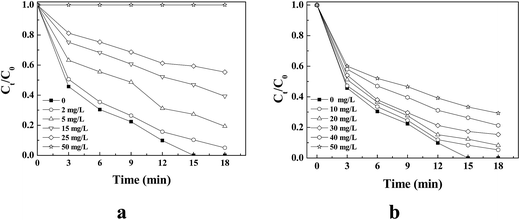
Fig. 5a shows the impact of NB on quinoline degradation over the range of 0–50 mg L−1. The degradation rate of quinoline substantially declined with NB compared with that without NB. The results imply that ˙OH was present in MW-CWPO, which caused quick quinoline removal. Furthermore, the quinoline removal in 18 min was 100% in the absence of NB. However, quinoline degradation was substantially impeded after NB addition and the impact of quinoline degradation was obviously dependent on NB dosage (Fig. 5a). The production of O2˙− in the MW-CWPO system, quantified by CT, is shown in Fig. 5b. Quinoline degradation was diminished to different degrees over the range of 0–50 mg L−1, but the impact degrees were less than that for NB. These results show that O2˙− was present in the MW-CWPO systems, participated in the quinoline degradation, and played a slight role in the quinoline degradation.
Based on the influence of NB and CT on quinoline degradation in supported bimetallic Cu/Ni oxides catalyzing catalytic wet peroxide oxidation under microwave irradiation, it could be concluded that ˙OH and O2˙− were present in the MW-CWPO systems and the relative contribution of these two radicals followed the order: ˙OH > O2˙−. Furthermore, quinoline degradation was completely impeded by scavenging ˙OH, implying that O2˙− was produced from reactions with the involvement of ˙OH.46 So, a simple mechanism of supported Cu/Ni bimetallic oxides catalyzing MW-assisted CWPO is proposed in Fig. 6.
3.5. Catalytic activity of Cu/Ni-catalyst in repeated runs
In exploring the stability and reusability of the Cu/Ni-catalyst in heterogeneous MW-CWPO, the catalytic reaction was conducted repeatedly six times with the Cu/Ni-catalyst under the standard reaction conditions. As seen in Fig. S6,† the activity of the Cu/Ni-catalyst decreased gradually on the basis of TOC abatement and H2O2 utilization efficiency, probably owing to the leaching of iron from the catalyst surface as determined in the former section (Table 2). However, during the later third runs, TOC removal and H2O2 utilization efficiency were similar (Fig. S6†), implying that the catalyst may be stable.| Cycle number | Leached metal/total metal (%) | |
|---|---|---|
| Cu | Ni | |
| 1st run | 0.007 | 0.002 |
| 2nd run | 0.004 | 0.0007 |
| 3rd run | 0.001 | 0.0003 |
| 4th run | — | — |
| 5th run | — | — |
| 6th run | — | — |
Additionally, atomic absorption spectroscopy was utilized to measure whether there were any free Cu and Ni ions in the reaction solution after each reaction. The amount of metal leaching was analyzed after each experimental run (Table 2).
As with the metal Cu and Ni leaching, quinoline mineralization efficiency and H2O2 utilization efficiency are reduced in the third experimental run (Fig. S6†). This agrees well with previous reports that showed that catalysts were deactivated as a result of the active ingredient leaching.47,48 However, dissolved Cu and Ni ions could not be detected from the fourth to sixth runs, and TOC abatement and H2O2 utilization efficiency were almost stable (Fig. S6†), implying that the Cu/Ni-catalyst was relatively constant in the MW-CWPO. Further work is required to reduce the leaching of the active ingredient from the surface of the Cu/Ni-catalyst and to develop an effective regeneration process.
4. Conclusions
Supported bimetallic Cu/Ni oxides catalyzing heterogeneous MW-CWPO are a future technology for mineralization of organic pollutants with intrinsic advantages over homogeneous wet peroxide oxidation, such as wider initial pH range and higher H2O2 utilization efficiency. In the mineralization of aqueous quinoline solution, this heterogeneous MW-CWPO could perform proficiently over an initial pH range from 2.0 to 8.0 accompanied with higher H2O2 utilization efficiency than other CWPO processes.During quinoline mineralization with an initial of pH 7.0, formic acid, acetic acid, oxalic acid, succinic acid, and glycolic acid were produced and instigated the fall of the solution pH to acidic conditions, favorable for high mineralization performance. In order to attain satisfactory mineralization efficiency and H2O2 utilization efficiency, the H2O2 dosage should be 1.25 times the theoretical value of H2O2 usage in mineralization of 100 mg L−1 quinoline.
To identify the free radical species, CT and NB were introduced to the MW-CWPO system. Differences between the probes' reactivity and the potential free radical species were perceived, indicating that ˙OH was a direct product and O2˙− was a secondary oxidant produced from ˙OH-based reactions. The results of the stability and metal leaching experiments indicated that supported Cu–Ni bimetallic oxides could be applied as a proper catalyst.
5. Conflict of interest statement
The authors declare that there are no conflicts of interest.Acknowledgements
The present paper was supported by SKLUWRE of HIT (No. 2016DX12 and No. 2013DX09).References
- Y. Yan, S. Jiang, H. Zhang and X. Zhang, Chem. Eng. J., 2015, 259, 243–251 CrossRef CAS.
- Y. Yan, X. Wu and H. Zhang, Sep. Purif. Technol., 2016, 171, 52–61 CrossRef CAS.
- K. M. Valkaj, A. Katovic and S. Zrncevic, Ind. Eng. Chem. Res., 2011, 50, 4390–4397 CrossRef CAS.
- K. Huang, Y. Xu, L. Wang and D. Wu, RSC Adv., 2015, 5, 32795–32803 RSC.
- K. Huang, J. Wang, D. Wu and S. Lin, RSC Adv., 2015, 5, 8455–8462 RSC.
- A. Rey, A. B. Hungria, C. J. Duran-Valle, M. Faraldos, A. Bahamonde, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2016, 181, 249–259 CrossRef CAS.
- A. S. Albuquerque, M. V. C. Tolentino, J. D. Ardisson, F. C. C. Moura, R. de Mendonça and W. A. A. Macedo, Ceram. Int., 2012, 38, 2225–2231 CrossRef CAS.
- J. Q. Xu, F. Guo, J. Li, X. Z. Ran and Y. Tang, in Material Sciences and Technology, Pts 1 & 2, ed. Y. Li, Trans Tech Publications Ltd, Stafa-Zurich, 2012, vol. 560–561, pp. 869–872 Search PubMed.
- T.-L. Lai, J.-Y. Liu, K.-F. Yong, Y.-Y. Shu and C.-B. Wang, J. Hazard. Mater., 2008, 157, 496–502 CrossRef CAS PubMed.
- T.-L. Lai, W.-F. Wang, Y.-Y. Shu, Y.-T. Liu and C.-B. Wang, J. Mol. Catal. A: Chem., 2007, 273, 303–309 CrossRef CAS.
- A. B. Ahmed, B. Jibril, S. Danwittayakul and J. Dutta, Appl. Catal., B, 2014, 156–157, 456–465 CrossRef CAS.
- B. Zhang, H. You, Z. Yang and F. Wang, RSC Adv., 2016, 6, 66027–66036 RSC.
- V. Homem, A. Alves and L. Santos, Chem. Eng. J., 2013, 220, 35–44 CrossRef CAS.
- N. Serpone, S. Horikoshi and A. V. Emeline, J. Photochem. Photobiol., C, 2010, 11, 114–131 CrossRef CAS.
- Q.-S. Liu, T. Zheng, P. Wang and Y.-J. Li, Sep. Sci. Technol., 2013, 49, 68–73 CrossRef CAS.
- Y. J. Wang, H. Y. Zhao, J. X. Gao, G. H. Zhao, Y. G. Zhang and Y. L. Zhang, J. Phys. Chem. C, 2012, 116, 7457–7463 CAS.
- H. Iboukhoulef, A. Amrane and H. Kadi, Environ. Technol., 2013, 34, 853–860 CrossRef CAS PubMed.
- G. H. Zhao, B. Y. Lv, Y. Jin and D. M. Li, Water Environ. Res., 2010, 82, 120–127 CrossRef CAS PubMed.
- W. Pan, G. Zhang, T. Zheng and P. Wang, RSC Adv., 2015, 5, 27043–27051 RSC.
- L. Xu and J. Wang, Environ. Sci. Technol., 2012, 46, 10145–10153 CrossRef CAS PubMed.
- N. Masomboon, C. Ratanatamskul and M.-C. Lu, Environ. Sci. Technol., 2009, 43, 8629–8634 CrossRef CAS PubMed.
- K. Fajerwerg, J. N. Foussard, A. Perrard and H. Debellefontaine, Water Sci. Technol., 1997, 35, 103–110 CrossRef CAS.
- L. Xu and J. Wang, Appl. Catal., B, 2012, 123–124, 117–126 CrossRef CAS.
- B.-h. Tuo, J.-b. Yan, B.-a. Fan, Z.-h. Yang and J.-z. Liu, Bioresour. Technol., 2012, 107, 55–60 CrossRef CAS PubMed.
- J. Jing, J. Feng, W. Li and W. W. Yu, J. Colloid Interface Sci., 2013, 396, 90–94 CrossRef CAS PubMed.
- D. Rameshraja, V. C. Srivastava, J. P. Kushwaha and I. D. Mall, Chem. Eng. J., 2012, 181–182, 343–351 CrossRef CAS.
- W. Li, Y. Wang and A. Irini, Chem. Eng. J., 2014, 244, 1–8 CrossRef CAS.
- B. Cornish, L. A. Lawton and P. K. J. Robertson, Appl. Catal., B, 2000, 25, 59–67 CrossRef CAS.
- W. Luo, L. Zhu, N. Wang, H. Tang, M. Cao and Y. She, Environ. Sci. Technol., 2010, 44, 1786–1791 CrossRef CAS PubMed.
- Y. Kojima, T. Fukuta, T. Yamada, M. S. Onyango, E. C. Bernardo, H. Matsuda and K. Yagishita, Water Res., 2005, 39, 29–36 CrossRef CAS PubMed.
- H. C. Li, X. Yu, H. W. Zheng, Y. M. Li, X. H. Wang and M. X. Huo, RSC Adv., 2014, 4, 7266–7274 RSC.
- S. Zhang, Y. Han, L. Wang, Y. Chen and P. Zhang, Chem. Eng. J., 2014, 252, 141–149 CrossRef CAS.
- A. B. Thomsen and H. H. Kilen, Water Res., 1998, 32, 3353–3361 CrossRef CAS.
- L. C. Almeida, S. Garcia-Segura, C. Arias, N. Bocchi and E. Brillas, Chemosphere, 2012, 89, 751–758 CrossRef CAS PubMed.
- L. Lyu, L. Zhang, Q. Wang, Y. Nie and C. Hu, Environ. Sci. Technol., 2015, 49, 8639–8647 CrossRef PubMed.
- S. G. Schrank, H. J. José, R. F. P. M. Moreira and H. F. Schröder, Chemosphere, 2005, 60, 644–655 CrossRef CAS PubMed.
- H. Zhang, X. Ran and X. Wu, J. Hazard. Mater., 2012, 241–242, 259–266 CrossRef CAS PubMed.
- J. L. Wang and L. J. Xu, Crit. Rev. Environ. Sci. Technol., 2012, 42, 251–325 CrossRef CAS.
- S.-T. Liu, J. Huang, Y. Ye, A.-B. Zhang, L. Pan and X.-G. Chen, Chem. Eng. J., 2013, 215–216, 586–590 CrossRef CAS.
- N. Li, P. Wang, C. Zuo, H. L. Cao and Q. S. Liu, Environ. Eng. Sci., 2010, 27, 271–280 CrossRef CAS.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1–84 CrossRef CAS.
- B. A. Smith, A. L. Teel and R. J. Watts, Environ. Sci. Technol., 2004, 38, 5465–5469 CrossRef CAS PubMed.
- B. A. Smith, A. L. Teel and R. J. Watts, J. Contam. Hydrol., 2006, 85, 229–246 CrossRef CAS PubMed.
- G. M. S. ElShafei, F. Z. Yehia, O. I. H. Dimitry, A. M. Badawi and G. Eshaq, Appl. Catal., B, 2010, 99, 242–247 CrossRef CAS.
- Z. Miao, X. Gu, S. Lu, M. L. Brusseau, N. Yan, Z. Qiu and Q. Sui, J. Hazard. Mater., 2015, 300, 530–537 CrossRef CAS PubMed.
- L. Zhou, W. Song, Z. Q. Chen and G. C. Yin, Environ. Sci. Technol., 2013, 47, 3833–3839 CrossRef CAS PubMed.
- F. Arena, R. Giovenco, T. Torre, A. Venuto and A. Parmaliana, Appl. Catal., B, 2003, 45, 51–62 CrossRef CAS.
- A. Santos, P. Yustos, A. Quintanilla and F. Garcia-Ochoa, Top. Catal., 2005, 33, 181–192 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01350g |
| This journal is © The Royal Society of Chemistry 2017 |