Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Near-infrared fluorescent and columnar liquid crystal: synthesis, and photophysical and mesomorphic properties of triphenylene-Bodipy-triphenylene triad†
Xiaoting Fanga,
Hongyu Guo*a,
Fafu Yang *ab and
Jianrong Lina
*ab and
Jianrong Lina
aCollege of Chemistry and Chemical Engineering, Fujian Normal University, Fuzhou 350007, P. R. China. E-mail: yangfafu@fjnu.edu.cn
bFujian Key Laboratory of Polymer Materials, Fuzhou 350007, P. R. China
First published on 2nd May 2017
Abstract
The first near-infrared fluorescent and columnar liquid crystal based on Bodipy was achieved by modifying 3,5-alkenyl substituted Bodipy with two triphenylene units. Triphenylene-Bodipy-triphenylene triad with 3,5-alkenyl groups exhibited hexagonal columnar mesophase and near-infrared fluorescence at 650–800 nm with the quantum yield of 31%.
Over the past decades, columnar liquid crystals have attracted considerable academic and commercial interest based on their unique π–π stacking structures. They exhibited various potential applications for organic field-effect transistors, organic light-emitting diodes, organic photovoltaic cells, gas sensors, etc.1–5 Recently, the columnar liquid crystals with high fluorescence,6–11 such as the perylene liquid crystals,12–16 have received significant attention due to their broad application in novel liquid crystalline materials with photophysical properties. However almost all of the known fluorescent liquid crystals emitted light in the visible spectral region.10 Lately, the near-infrared materials displayed potential application in a number of research fields, such as telecommunications, thermal imaging, and biological imaging.17,18 Thus, the near-infrared-emitting liquid crystals were also prepared, which exhibited interesting near-infrared photophysical properties.19–23 They were usually obtained as organic–inorganic hybrid materials by combining organic liquid crystals with C60, graphene and rare earth metal ions, etc. However, this type of a hybrid material was difficult to construct as a columnar liquid crystal. As for the near-infrared organic columnar liquid crystal, only one example derived from benzo[1,2-c:4,5-c′]bis([1,2,5]thiadiazole) has been presented with good near-infrared fluorescence and hexagonal columnar mesophase to date.10 Studies on the near-infrared columnar liquid crystal have rarely been reported.
On the other hand, 4,4-difluoro-4-borata-3a,4a-diaza-s-indacene (Bodipy) is a class of famous fluorescent dyes with intense fluorescence, good photochemical stability and energy/electron-transfer capabilities.24–28 Some fluorescent Bodipy liquid crystals were reported by introducing long alkyl chains on the Bodipy skeleton.29–34 Recently, triphenylene-Bodipy dyads were also prepared, which exhibited good columnar liquid crystals based on the effective π–π stacking of triphenylene units.13 On the other hand, Bodipy had been applied as a good platform to construct near-infrared materials by extending its aromatic conjugated system.35–40 Therefore, it can be question whether it is possible to design and synthesize the columnar Bodipy liquid crystal with near-infrared fluorescence. This type of near-infrared columnar liquid crystal has not thus far been reported. Based on this consideration, in this study, the first example of near-infrared fluorescent and columnar liquid crystal was designed and synthesized using Bodipy as the building platform. The influences of the triphenylene unit and the alkenyl groups on the mesomorphic and fluorescent properties were investigated. The experimental results showed that the near-infrared fluorescent and columnar liquid crystal were difficult to achieve for the triphenylene-Bodipy dyad, whereas the triphenylene-Bodipy-triphenylene triad with 3,5-alkenyl groups was favourable for good near-infrared fluorescence and hexagonal columnar mesophase. The multiple triphenylene units effectively induced columnar mesophase and the 3,5-alkenyl groups resulted in the near-infrared fluorescence.
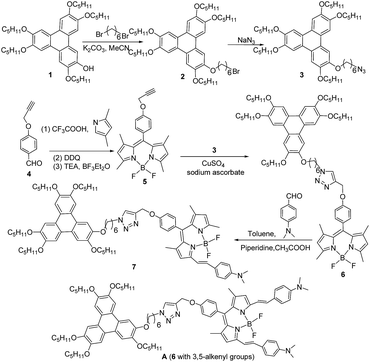
As the triphenylene-Bodipy dyad exhibited columnar liquid crystalline behaviour13 and the near-infrared Bodipy could be prepared by the Knoevenagel condensation reaction on 3,5-methyl of Bodipy,17 we initially tried to design the synthetic route for near-infrared fluorescent and columnar liquid crystal by the Knoevenagel condensation of the triphenylene-Bodipy dyad (Scheme 1). According to the published procedure,41 the triphenylene triazo derivative 3 was prepared by the etherification of triphenylene 1 with 1,6-dibromohexane, with subsequent nucleophilic substitution with NaN3, resulting in a yield of 76%. Moreover, according to the typical procedure for preparing Bodipy,13 the Bodipy derivative containing alkynyl group 5 was obtained by treating 4-(prop-2-ynyloxy)benzaldehyde 4 with 2,4-dimethylpyrrole via sequential condensation, oxidation, and complexation reactions, resulting in the moderate yield of 22% after columnar chromatography. Furthermore, the click reaction of compounds 3 and 5 was carried out smoothly by the catalysis of CuSO4 and sodium ascorbate in DMF solution. Triphenylene-Bodipy dyad 6 was collected in a yield of 66% after columnar chromatography. Finally, by Knoevenagel condensation of compound 6 with p-dimethylaminobenzaldehyde under the catalysis of glacial acetic acid and piperidine, the triphenylene-Bodipy dyad 7 with mono-alkenyl group was prepared in a yield of 20%. As compounds 6 and 7 were prepared, their mesomorphic and photophysical properties were investigated (the detailed experimental data are discussed later). The results implied that compound 6 exhibited a mesophase, but compound 7 showed no liquid crystalline behavior. These phenomena might suggest that one triphenylene unit was not enough to induce the liquid crystalline behavior of compound 7 containing the Bodipy unit with alkenyl group because of its larger rigid structure compared to the normal Bodipy unit. Based on this analysis, it could be deduced that compound A (6 with 3,5-alkenyl groups, as shown in Scheme 1) had no liquid crystalline behavior due to the larger rigid structure of compound A compared to that of compound 7. Moreover, compound A (using MS, this was detected as a by-product formed when preparing compound 7) was difficult to be purified due to similar polarity with compound 7. Thus, the preparation of compound A was abandoned and a means to devise a new strategy to design and synthesize a liquid crystalline molecule containing the Bodipy unit with 3,5-alkenyl groups was sought.
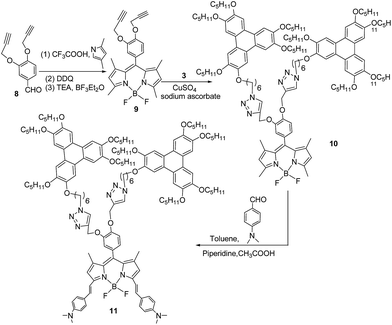
In order to overcome the negative influence of the large rigid structure of the Bodipy unit with alkenyl groups on the mesomorphic properties, increasing the number of triphenylene units may be an effective strategy. More triphenylene units produce stronger induction for the mesophase. The new synthetic route for the triphenylene-Bodipy-triphenylene triad with 3,5-alkenyl groups is presented as Scheme 2. First, the Bodipy derivative, containing two alkynyl groups 9, was synthesized by reacting 4-(prop-2-ynyloxy)benzaldehyde 8 with 2,4-dimethylpyrrole via a similar procedure for the preparation of compound 5. Compound 9 was obtained in the moderate yield of 24% after columnar chromatography. Second, the triphenylene-Bodipy-triphenylene triad 10 was further synthesized in a yield of 64% by reacting compound 9 and 3 via click chemistry. Finally, triphenylene-Bodipy-triphenylene triad 11 with 3,5-alkenyl groups was prepared by the condensation of compound 10 with p-dimethylaminobenzaldehyde under the catalysis of glacial acetic acid and piperidine. The yield was 21% after columnar chromatography. One can see that in the structure of compound 11, the two triphenylene units were favourable for enhancing the inductive effect for mesophase, and the two 3,5-alkenyl groups on the Bodipy unit greatly extended the aromatic conjugated system, resulting in fluorescence in the near-infrared region.
 | ||
| Scheme 2 The synthetic route of the triphenylene-Bodipy-triphenylene triad with 3,5-alkenyl groups 11. | ||
The target compounds were fully characterized by 1H NMR, 13C NMR, HR-MS spectra and elemental analysis. The MS spectra showed corresponding molecular ion peaks (M+, MH+ or MNa+, see ESI†), indicating condensation. In their 1H NMR spectra, all peaks were well assigned to the corresponding structures (see ESI†). For example, one and two singlets for the Bodipy skeleton of compounds 11 and 7 certainly indicated the bis-substituted and mono-substituted alkenyl groups, respectively. The 13C NMR spectra also supported the corresponding structures of the target compounds.
The liquid crystalline properties of new compounds 6, 7, 10 and 11 were investigated preliminarily by differential scanning calorimeter (DSC). Their DSC curves for second heating and cooling are illustrated in Fig. 1. The corresponding phase transition temperatures and enthalpy changes are summarized in Table 1. One can see that triphenylene-Bodipy dyad 6 showed two phase transition peaks on cooling at 100.4 °C and 37.8 °C and upon second heating at 38.2 °C and 104.9 °C. These data might indicate the mesophase of compound 6, which was confirmed further by POM and XRD analysis. However, compound 7 with alkenyl group derived from compound 6 exhibited only one peak upon cooling and second upon heating, suggesting the crystalline phase-isotropic phase transition without mesophase. These differences in mesomorphic properties of compounds 6 and 7 could be explained by the fact that the alkenyl group on the Bodipy skeleton of compound 7 enlarged the rigid aromatic structure and destroyed the mesomorphic property. These results may suggest that one triphenylene unit was not enough to induce liquid crystalline behavior in the Bodipy skeleton with an alkenyl group. On the contrary, both triphenylene-Bodipy-triphenylene triad 10 and its condensation derivative 11 presented two phase transition peaks on cooling (114.8 °C and 23.7 °C for 10, and 109.2 °C and 10.2 °C for 11) and second heating (24.2 °C and 118.0 °C for 10, and 19.6 °C and 112.5 °C for 11). These data implied that both compounds 10 and 11 had good reversible phase transition behaviors with mesophase. Moreover, the results suggested that although two alkenyl groups were introduced onto the Bodipy skeleton of compound 11, the latter still possessed liquid crystalline behaviour due to the strong mesomorphic-induced effect of two triphenylene units. Thus, it could be concluded that compounds 6, 10, and 11 were liquid crystalline molecules, but compound 7 presented no mesophase. More triphenylene units on Bodipy resulted in a strong mesomorphic-induced effect.
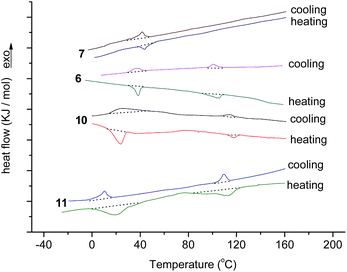 | ||
| Fig. 1 The DSC traces of compounds 6, 7, 10 and 11 on second heating and cooling (scan rate 10 °C min−1). | ||
| Compd | Phase transitiona | Heating scan T(ΔH) | Cooling scan (ΔH) |
|---|---|---|---|
| a Cr = crystalline, Col = columnar mesophase, Iso = isotropic. | |||
| 6 | Cr–Col | 38.2(12.4) | 37.8(10.4) |
| Col–Iso | 104.9(8.8) | 100.4(6.7) | |
| 7 | Cr–Iso | 43.8(21.3) | 41.5(23.6) |
| 10 | Cr–Col | 24.2(16.6) | 23.7(14.3) |
| LC–Iso | 118.0(2.3) | 114.8(2.8) | |
| 11 | Cr–Col | 19.6(18.8) | 10.2(9.8) |
| LC–Iso | 112.5(10.9) | 109.2(6.2) | |
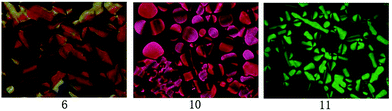
Moreover, the mesophase of compounds 6, 7, 10 and 11 were studied by polarizing optical microscopy (POM). Although only Cr–Iso phase transition was observed for compound 7, the Cr–Col and Col–Iso phase transition of compounds 6, 10 and 11 were observed at the approximate temperatures of DSC curves. Their liquid crystalline textures clearly appeared after gradual cooling from the isotropic phase. Fig. 2 illustrates the mesomorphic textures at 60 °C. All textures were pseudo-confocal conic types, suggesting that columnar liquid crystal structures exist for compounds 6, 10 and 11.
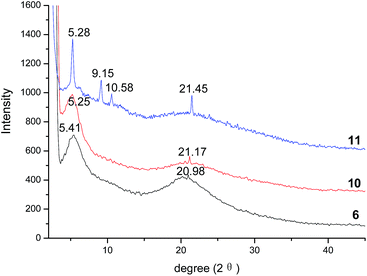
As the liquid crystalline behaviors of compounds 6, 10 and 11 were confirmed by DSC and POM, the X-ray diffraction (XRD) was further employed to investigate the columnar stacking behaviors of mesophase. The XRD traces at 60 °C are illustrated in Fig. 3. In the small-angle region, the strong peaks are observed at 5.41°, 5.25°, 5.28° for compounds 6, 10 and 11, respectively. The corresponding d-spacings calculated using the formula d = λ/(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) were 16.32 Å, 16.81 Å and 16.72 Å for compounds 6, 10 and 11, respectively. These data were in agreement with the [100] reflections of the column phase. Thus, the lattice parameter α for compounds 6, 10 and 11 could be calculated as 18.85 Å, 19.41 Å and 19.30 Å, respectively. In the wide-angle region, the broad halos between 15° and 25° suggest that the mean distances of 4.5 Å approximately could be assigned to the reflection of the very short correlation length of the molten alkyl chains. The small reflections at 20.98°, 21.17°, and 21.45°, indicating the spacings of 4.23 Å, 4.19 Å, and 4.14 Å, respectively, could be distinguished for the typical characteristic of π–π interactions for the intracolumnar distance of columnar liquid crystals. On the other hand, it is clear that by comparing the XRD traces of compounds 6 and 10, sample 11 showed not only a strong and narrow peak at 5.28°, but also two new peaks at 9.15° and 10.58°. These results suggested that compounds 6 and 10 might possess a mixed columnar phase or a disordered columnar phase, but compound 11 had an ordered columnar phase. Based on the reflections at 5.28°, 9.15° and 10.58° of compound 11, the d-spacings following the calculations were found to be 16.72 Å, 9.65 Å and 8.36 Å, which were in accordance with the ratio of 1
θ) were 16.32 Å, 16.81 Å and 16.72 Å for compounds 6, 10 and 11, respectively. These data were in agreement with the [100] reflections of the column phase. Thus, the lattice parameter α for compounds 6, 10 and 11 could be calculated as 18.85 Å, 19.41 Å and 19.30 Å, respectively. In the wide-angle region, the broad halos between 15° and 25° suggest that the mean distances of 4.5 Å approximately could be assigned to the reflection of the very short correlation length of the molten alkyl chains. The small reflections at 20.98°, 21.17°, and 21.45°, indicating the spacings of 4.23 Å, 4.19 Å, and 4.14 Å, respectively, could be distinguished for the typical characteristic of π–π interactions for the intracolumnar distance of columnar liquid crystals. On the other hand, it is clear that by comparing the XRD traces of compounds 6 and 10, sample 11 showed not only a strong and narrow peak at 5.28°, but also two new peaks at 9.15° and 10.58°. These results suggested that compounds 6 and 10 might possess a mixed columnar phase or a disordered columnar phase, but compound 11 had an ordered columnar phase. Based on the reflections at 5.28°, 9.15° and 10.58° of compound 11, the d-spacings following the calculations were found to be 16.72 Å, 9.65 Å and 8.36 Å, which were in accordance with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
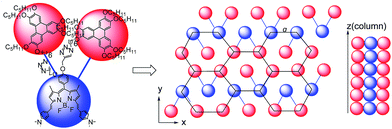
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√4 for (100), (110) and (200) reflections. These results supported the assumption that compound 11 had the typical reflection mode for the hexagonal columnar liquid crystal. The possible molecular stacking mode of hexagonal columns of compounds 11 was proposed, as shown in Fig. 4. The XRD analyses implied that compounds 6, 10 and 11 were columnar liquid crystals. In particular, compound 11 exhibited ordered hexagonal columnar mesophase.
1/√4 for (100), (110) and (200) reflections. These results supported the assumption that compound 11 had the typical reflection mode for the hexagonal columnar liquid crystal. The possible molecular stacking mode of hexagonal columns of compounds 11 was proposed, as shown in Fig. 4. The XRD analyses implied that compounds 6, 10 and 11 were columnar liquid crystals. In particular, compound 11 exhibited ordered hexagonal columnar mesophase.
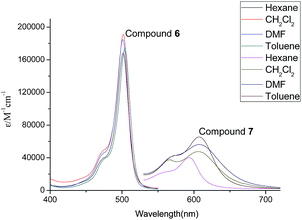
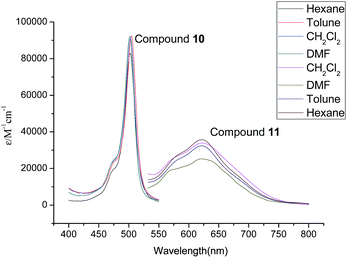
The photophysical properties of compounds 6, 7, 10 and 11 were studied by absorption spectra and fluorescent spectra. Their corresponding spectra in different organic solvents (hexane, toluene, CH2Cl2 and DMF) are illustrated in Fig. 5–8. It can be seen that compounds 6 and 10 without alkenyl group showed similar absorption spectra with strong peaks observed at 501 nm. These results could be attributed to the same structure of their Bodipy units. The different solvents had little influence on the absorption spectra. However, compound 7 with the mono-alkenyl group exhibited a clear red shift from 501 nm to 607 nm, and compound 11 with the 3,5-alkenyl groups presented a very large red shift from 501 nm to 622 nm. These phenomena could be explained by the larger aromatic conjugated structures of Bodipy units with alkenyl groups. In relation to the fluorescent spectra, compounds 6 and 10 without alkenyl groups possessed narrow fluorescent emission. The strongest fluorescence intensity appeared at 515 nm in the DMF solution. Compounds 7 and 11 with alkenyl groups had broad emissions with large red shifts, compared with compounds 6 and 10. The emission peaks of compound 7 with a mono-alkenyl group were between 600–700 nm in different solvents. It is noteworthy that compound 11 with 3,5-alkenyl groups displayed the near-infrared emission of 650–800 nm, as was expected. The solvents also greatly influenced the emission of compounds 7 and 11. Compounds 7 and 11 exhibited the strongest emissions in toluene, but the emissions became significantly weaker and were seen to red shift in the DMF solution. The low fluorescences and further red shifts in DMF solution could be attributed to polar-solvent effect and potential aggregation. These results implied that by comparing with compounds 6 and 10, compounds 7 and 11 with the larger aromatic conjugated structures of Bodipy units exhibited red shifts of fluorescence and stronger π–π stacking for aggregation.
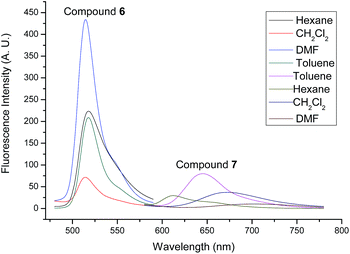 | ||
| Fig. 7 Fluorescence emission spectra of compounds 6 and 7 in different solvents (10−5 M). The excitation wavelength was 500 nm for compound 6 and 570 nm for compound 7. | ||
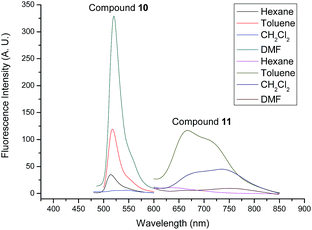 | ||
| Fig. 8 Fluorescence emission spectra of compounds 10 and 11 in different solvents (10−5 M). The excitation wavelength was 500 nm for compound 10 and 600 nm for compound 11. | ||
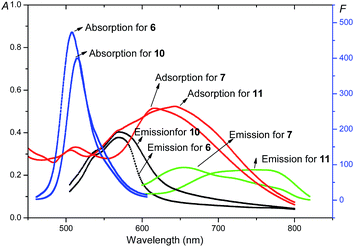
Furthermore, the stokes shifts and the fluorescent quantum yields (ΦF) of compounds 6, 7, 10 and 11 were calculated, and the results are listed in Table 2. It can be seen that compounds 7 and 11 with alkenyl groups show a larger stokes shift compared with compounds 6 and 10. The stokes shifts of compound 11 were as large as 114 nm and 129 nm in CH2Cl2 and DMF, respectively. On the other hand, the ΦF of compounds 6, 7, 10 and 11 fluctuated between 0.02 and 0.79 in different solvents. The ΦF of compounds 7 and 11 with alkenyl groups were lower than that of compounds 6 and 10. These phenomena also could be ascribed to the larger aromatic conjugated structures of Bodipy units in compounds 7 and 11, which showed a stronger π–π stacking for potential aggregation resulting in fluorescent quenching. Nevertheless, compound 11, possessing the near-infrared fluorescence, had a moderate fluorescence quantum yield of 19% in CH2Cl2 and 31% in toluene. On the other hand, the absorption and fluorescence emission spectra of compounds 6, 7, 10 and 11 were investigated in solid films. The results are shown in Fig. 9. The fluorescence quantum yields were 0.10, 0.12, 0.02 and 0.03 for the solid films of compounds 6, 7, 10 and 11, respectively, which were lower than those in solutions. On comparing with corresponding spectra in solutions, clear red shifts were observed in solid films. These results suggested that strong aggregations existed in solid films, resulting in the red shifts of spectra and a decrease in the fluorescence quantum yields. The emission spectra of compounds 6, 7, 10, and 11 at different temperatures (25 °C and 50 °C) were also studied. The results are exhibited in Fig. S23 and S24.† One can see that the fluorescence maintained stable as a whole, with some decrease in the fluorescent intensities, which may be attributed to the influence of the lower solvent viscosity at higher temperatures. Based on the above analysis, it could be concluded that all of these Bodipy derivatives 6, 7, 10 and 11 showed good photophysical properties with moderate fluorescence quantum yields. Particularly, triphenylene-Bodipy-triphenylene triad 11 with 3,5-alkenyl groups was found to emit fluorescence in the near-infrared region with a reasonable fluorescence quantum yield of 31% in toluene.
| Comp. | Solvent | λabs (nm) | λem (nm) | Stokes shift (nm) | ΦF |
|---|---|---|---|---|---|
| 6 | Hexane | 501 | 518 | 17 | 0.48 |
| CH2Cl2 | 501 | 514 | 13 | 0.16 | |
| Toluene | 503 | 517 | 14 | 0.37 | |
| DMF | 500 | 514 | 14 | 0.76 | |
| 7 | Hexane | 593 | 612 | 17 | 0.12 |
| CH2Cl2 | 607 | 674 | 67 | 0.20 | |
| Toluene | 608 | 645 | 37 | 0.30 | |
| DMF | 609 | 701 | 92 | 0.02 | |
| 10 | Hexane | 501 | 514 | 13 | 0.14 |
| CH2Cl2 | 502 | 515 | 13 | 0.03 | |
| Toluene | 503 | 518 | 15 | 0.32 | |
| DMF | 501 | 520 | 19 | 0.79 | |
| 11 | Hexane | 622 | 632 | 10 | 0.02 |
| CH2Cl2 | 622 | 736 | 114 | 0.19 | |
| Toluene | 623 | 667 | 44 | 0.31 | |
| DMF | 623 | 752 | 129 | 0.03 |
Conclusions
In summary, triphenylene-Bodipy dyad 6 and its derivative 7 with mono-alkenyl group, and triphenylene-Bodipy-triphenylene triad 10 and its derivative 11 with 3,5-alkenyl groups were designed and synthesized via the click chemistry reaction and Knoevenagel condensation. Compound 6 exhibited mesophase but compound 7 showed no liquid crystalline behavior. Both compound 10 and its derivative 11 exhibited mesomorphic properties based on the strong mesomorphic-induced effect of two triphenylene units. The investigation of photophysical properties suggested that all compounds emitted fluorescence with moderate fluorescent quantum yields. Compound 11 displayed the near-infrared emission of 650–800 nm. These results implied that the triphenylene-Bodipy-triphenylene triad 11 with 3,5-alkenyl groups was the first example of a columnar Bodipy liquid crystal with near-infrared fluorescence. The multiple triphenylene units were favourable for columnar mesophase and the 3,5-alkenyl groups on Bodipy led to near-infrared fluorescence. This study presents a model on how to design and synthesis of novel columnar liquid crystal with near-infrared fluorescence.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21406036), Fujian Natural Science Foundation of China (No. 2017J01571) and the Program for Innovative Research Team in Science and Technology in Fujian Province University are greatly acknowledged.Notes and references
- S. Kumar, in Chemistry of discotic liquid crystals: from monomers to polymers, CRC Press, Boca Raton, FL, 2011 Search PubMed.
- S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38–68 CrossRef CAS PubMed.
- R. J. Bushby and K. Kawata, Liq. Cryst., 2011, 38, 1415–1426 CrossRef CAS.
- T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed.
- A. A. Vieira, H. Gallardo, J. Barberá, P. Romero, J. L. Serrano and T. Sierra, J. Mater. Chem., 2011, 21, 5916–5922 RSC.
- M. H. Ryu, J. W. Choi, H. J. Kim, N. Park and B. K. Cho, Angew. Chem., Int. Ed., 2011, 50, 5737–5740 CrossRef CAS PubMed.
- J. F. Xiong, S. H. Luo, J. P. Huo, J. Y. Liu, S. X. Chen and Z. Y. Wang, J. Org. Chem., 2014, 79, 8366–8373 CrossRef CAS PubMed.
- L. Vega, S. Cleuvenbergen, G. Depotter, E. M. García-Frutos, B. Gómez-Lor, A. Omenat, R. M. Tejidor, J. L. Serrano, G. Hennrich and K. Clays, J. Org. Chem., 2012, 77, 10891–10896 CrossRef PubMed.
- X. Li, A. Liu, S. Xun, W. Qiao, X. Wan and Z. Y. Wang, Org. Lett., 2008, 10, 3785–3787 CrossRef CAS PubMed.
- R. Chaudhuri, M. Y. Hsu, C. W. Li, C. Wang, C. J. Chen, C. K. Lai, L. Y. Chen, S. H. Liu, C. C. Wu and R. S. Liu, Org. Lett., 2008, 10, 3053–3756 CrossRef CAS PubMed.
- M. G. Zhu, H. Y. Guo, F. F. Yang and Z. S. Wang, Liq. Cryst., 2016, 43, 1875–1883 CrossRef CAS.
- X. T. Fang, H. Y. Guo, J. R. Lin and F. F. Yang, Tetrahedron Lett., 2016, 57, 4939–4943 CrossRef CAS.
- H. Y. Guo, M. G. Zhu, Z. S. Wang and F. F. Yang, Tetrahedron Lett., 2016, 57, 4191–4195 CrossRef CAS.
- M. G. Zhu, Z. S. Wang, F. F. Yang and H. Y. Guo, Dyes Pigm., 2016, 133, 387–394 CrossRef CAS.
- M. G. Zhu, H. Y. Guo, F. F. Yang and Z. S. Wang, RSC Adv., 2017, 7, 4320–4328 RSC.
- Y. Ni and J. S. Wu, Org. Biomol. Chem., 2014, 12, 3774–3793 CAS.
- G. Qian and Z. Y. Wang, Chem.–Asian J., 2010, 5, 1006–1010 CrossRef CAS PubMed.
- K. G. Gutierrez-Cuevas, L. Wang, C. M. Xue, G. Singh, S. Kumar, A. Urbas and Q. Li, Chem. Commun., 2015, 51, 9845–9849 RSC.
- R. V. Deun, D. Moors, B. D. Fré and K. Binnemans, J. Mater. Chem., 2003, 13, 1520–1522 RSC.
- R. C. Liu, V. Marinova, S. H. Lin, M. S. Chen, Y. H. Lin and K. Y. Hsu, Opt. Lett., 2014, 39, 3320–3323 CrossRef CAS PubMed.
- M. D. J. Quinn, J. Du, B. J. Boyd, A. Hawley and S. M. Notley, Langmuir, 2015, 31, 6605–6609 CrossRef CAS PubMed.
- A. M. Tan, I. S. Sidorov, S. É. Putilin, Y. Sapurina and N. V. Kamanina, Tech. Phys., 2007, 52, 1515–1518 CrossRef CAS.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4973 RSC.
- W. Dehaen, V. Leen and N. Boens, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- M. Castillo, S. L. Raut, S. Price, I. Bora, L. P. Jameson, C. Qiu, K. A. Schug, Z. Grycznski and S. Dzyuba, RSC Adv., 2016, 6, 68705–68708 RSC.
- S. Sengupta, U. K. Pandey and E. U. Athresh, RSC Adv., 2016, 6, 73645–73649 RSC.
- F. Camerel, L. Bonardi, M. Schmutz and R. Ziessel, J. Am. Chem. Soc., 2006, 128, 4548–4549 CrossRef CAS PubMed.
- F. Camerel, L. Bonardi, G. Ulrich, L. Charbonniare, B. Donnio, C. Bourgogne, D. Guillon, P. Retailleau and R. Ziessel, Chem. Mater., 2006, 18, 5009–5021 CrossRef CAS.
- F. Camerel, G. Ulrich, J. Barbera and R. Ziessel, Chem.–Eur. J., 2007, 13, 2189–2200 CrossRef CAS PubMed.
- J. H. Olivier, F. Camerel, G. Ulrich, J. Barberá and R. Ziessel, Chem.–Eur. J., 2010, 16, 7134–7142 CrossRef CAS PubMed.
- J. H. Olivier, J. Barberá, E. Bahaidarah, A. Harriman and R. Ziessel, J. Am. Chem. Soc., 2012, 134, 6100–6103 CrossRef CAS PubMed.
- M. Benstead, G. A. Rosser, A. Beeby, G. H. Mehl and R. W. Boyle, New J. Chem., 2011, 35, 1410–1417 RSC.
- X. D. Jiang, S. Li, J. Guan, T. Fang, X. Liu and L. J. Xiao, Curr. Org. Chem., 2017, 21, 1736–1744 Search PubMed.
- Y. Ni, R. K. Kannadorai, J. J. Peng, S. W. K. Yu, Y. T. Chang and J. S. Wu, Chem. Commun., 2016, 52, 11504–11507 RSC.
- X. F. Zhang, Y. D. Zhang, L. C. Chen and Y. Xiao, RSC Adv., 2015, 5, 32283–32289 RSC.
- J. T. Zhang, M. Yang, C. Li, N. Dorh, F. Xie, F. T. Luo, A. Tiwari and H. Y. Liu, J. Mater. Chem. B, 2015, 3, 2173–2184 RSC.
- X. D. Jiang, D. M. Xi, J. L. Zhao, H. F. Yu, G. T. Sun and L. J. Xiao, RSC Adv., 2014, 4, 60970–60973 RSC.
- T. Sarma, P. K. Panda and J. I. Setsune, Chem. Commun., 2013, 49, 9806–9808 RSC.
- B. Q. Hong, F. F. Yang, H. Y. Guo and Z. Y. Jiao, Tetrahedron Lett., 2014, 55, 252–255 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, synthesis and characterisation of new compounds. See DOI: 10.1039/c7ra00982h |
| This journal is © The Royal Society of Chemistry 2017 |