Open Access Article
Open Access ArticleLoess surface grafted functional copolymer for removing basic fuchsin†
Tingjun Lu,
Li Wang,
Yufeng He*,
Jing Chen and
Rong-Min Wang *
*
Key Lab. Eco-Environment-Related Polymer Materials of Ministry of Education, College of Chemistry & Chemical Engineering, Northwest Normal University, Lanzhou 730070, China. E-mail: heyufeng@nwnu.edu.cn; wangrm@nwnu.edu.cn
First published on 27th March 2017
Abstract
Loess clay (LC), a very abundant clay with granules and high hydrophilicity, was modified by surface grafting copolymerization of functional monomers, such as acrylamide (AM) and sodium p-styrene sulfonate (StS), and a cross-linking agent (N,N-methylenebisacrylamide: MBA), which afforded a LC surface grafting copolymer (LC-PAmS). Its structure and composition were characterized by Fourier transform infrared spectroscopy (FT-IR), thermal gravimetric analysis (TGA) and scanning electron microscopy (SEM). Acting as a novel and low-cost Loess-based polymer adsorbent, its adsorption behaviors were investigated with removing basic fuchsin (BF) in aqueous solution. After optimizing conditions, the removal rate got to 98.4% in 40 min at room temperature. Its adsorption mechanism was also investigated. It was found that the adsorption isotherm model could meet the Freundlich isotherm requirements and the dynamics were consistent with a pseudo-second-order kinetic model. In summary, LC-PAmS is a kind of polymer adsorbent for practically applied in wastewater treatment.
1 Introduction
As we know, dyes have made a great contribution to the progress of this beautiful and bright world as many commodities, such as dyestuffs, paper, textiles and plastics, have been industrially dyed. However, many kinds of dyes are classified as hazardous pollutants as they are very toxic even at very low concentrations.1 Basic fuchsin (BF), a water-soluble cationic dye, is widely used in textile industries for dyeing cotton, wool and silk. It is a great threat to aquatic organisms and also potentially harmful to humans as it can burn the eyes and irritate the skin.2 Therefore, it is necessary to remove BF from industrial wastewater before being discharged. Many techniques have been developed for the removal of dye, which include ion exchange, photocatalysis, chemical precipitation sonochemical degradation and adsorption.3–5 Adsorption is a common and simple separation process for treatment of waste among them.6 The exploitation of novel, low costs and more effective adsorbents is always an important focus in this fields.7 Loess clay (LC), a typical silicate minerals, is a kind of rich soil on the earth. It shows a certain adsorptive property because of small granule and high hydrophilicity. In order to enhance adsorption capacity, the loess had been modified by surfactants.8–11 We found that LC could be modified by copolymer using in situ polymerization, which afforded a new kind of polymer/inorganic mineral composites. This Loess-based copolymer exhibited excellent adsorption activity for removing Pb(II) ions.12 However, in this polymer/inorganic mineral composites, the dosage of copolymer is relatively high, which increase the cost of polymer adsorbent. Here, in order to decrease the ratio of polymer, the surface grafting polymerization was employed to modify LC. Using acrylamide (AM) and sodium p-styrene sulfonate (StS) as functional monomers, N,N-methylenebisacrylamide (MBA) as cross-linking agent, the loess surface grafted copolymer (LC-PAmS) was successfully synthesized by grafting polymerization. Then, the obtained LC-PAmS was applied to remove BF from aqueous solutions, and optimal conditions, such as initial BF concentration and contact time on the LC-PAmS, were measured. Its adsorption mechanism was also investigated.2 Experimental section
2.1. Materials and reagents
Loess clay (LC) was collected from the local hill near Lanzhou of China. It was ground and sifted through a 100-mesh sieve, and then stored in a desiccator for further use. 3-Methacryloxypropyl trimethoxysilane (KH-570), StS, AM and MBA were obtained commercially from Shanghai Zhongqin Chem. Reagent Co. Ltd, Shanghai Jingchun Reagent Co. Ltd, and Tianjin Baishi Chem. Ind. Co. Ltd, respectively. Potassium persulfate (KPS) was obtained from Yantai Shuangshang Chem. Ind. Co., Ltd BF, acetic acid (HAc), ammonia, hydrochloric acid (HCl) and ethanol are were commercially obtained with analytical grade.2.2. Preparation of LC-PAmS
2.3. Characterization of LC-PAmS
FT-IR spectra of LC-PAmS were recorded between 400 and 4000 cm−1 through the KBr method with a FTS3000 spectrophotometer. Thermal gravimetric analysis (TGA) were carried out using TG analyzer (PerkinElmer, model Pyris Diamond, USA). A morphology analysis of certain features was visualized by using a SEM microscope (ULTRA Plus, at 5 kV, Germany).2.4. Adsorption behaviours
Batch tests were conducted to investigate adsorption of LC-PAmS for removing dye, and BF was selected as dye model. The content of residual BF was measured using a visible spectrum. The removal rate and adsorption capacity were calculated by eqn (1) and eqn (2).
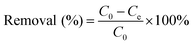 | (1) |
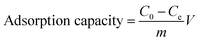 | (2) |
3 Results and discussion
3.1. Preparation of LC surface grafting copolymer (LC-PAmS)
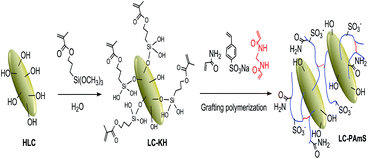
As a kind of typical silicate minerals, loess is a sort of natural inorganic polymer adsorbent. It was found that the acid activation with HCl solution could dredge channel of loess,13 and improve its adsorption capacity. The acid activation could dissolve carbonate and other impurities in pores of loess, which increase the content of hydroxyl groups on clay surface. Therefore, we have also acidized LC before surface grafting modification. The reaction in preparation of LC-PAmS is showed in Scheme 1. Firstly, KH-570, a typical silane coupling agents with vinyl group,14 was grafted onto the LC surface, which allowed the double bond to be introduced. Secondly, using AM and StS as functional monomers, and MBA as cross-linking agent, the copolymer (PAmS) was grafted onto the LC surface by graft copolymerization, which afforded loess surface grafting copolymer (LC-PAmS). In copolymer chain of LC-PAmS, the highly hydrophilic group (–SO3−) of StS could improve its dispersity in aqueous environment and the salt resistance, and units of AM could improve its affinity to organic reagents. Using cross-linking agent, a few network structure could be formed between multiple linear copolymer, which is conducive to the adsorption.In order to evaluate influence of different side groups in polymer chains for adsorption capacity, LC surface grafting polyacrylamide (LC-PAM) and LC surface grafting polystyrene sulfonate (PStS) were prepared by similar procedure (ESI†).
3.2. Characterization of LC-PAmS
The FT-IR spectra of LC-PAmS and its materials (LC, HLC, LC-KH) were measured and showed in Fig. S1.† For HLC, the absorption peaks at 3645 cm−1, 3452 cm−1 and 1631 cm−1 are correspond to the –OH stretching vibrations and bending vibrations. A sharp peak at 797 cm−1 can be assigned to quartz. The bands observed at 1093 cm−1, 538 cm−1 and 479 cm−1 can be assigned to the Si–O–Si stretching vibrations and bending vibrations. It was found that main characteristic peaks in LC-KH and LC-PAmS are similar to HLC as silicate structure was retained basically. In LC-KH, there are five peaks from 3000 cm−1 to 2800 cm−1, which are attributed to![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H(νs), –CH3(νs), –CH3(νas), –CH2(νs), and –CH3(νas), of KH-570, respectively. The characteristic peaks near, 1720, 1600 cm−1 are attributed to ester carbonyl and C
C–H(νs), –CH3(νs), –CH3(νas), –CH2(νs), and –CH3(νas), of KH-570, respectively. The characteristic peaks near, 1720, 1600 cm−1 are attributed to ester carbonyl and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, respectively. In LC-PAmS, the peak at 1183 cm−1 represents sulfonate group (–SO3−) of StS in polymer chain. The bands near 1670 cm−1 and 1450 cm−1 are attributed to amide (C
C stretching vibrations, respectively. In LC-PAmS, the peak at 1183 cm−1 represents sulfonate group (–SO3−) of StS in polymer chain. The bands near 1670 cm−1 and 1450 cm−1 are attributed to amide (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and C–N stretching vibrations conferred by AM. The characteristic peak of
O stretching) and C–N stretching vibrations conferred by AM. The characteristic peak of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching vibrations near 3000 cm−1 disappears in LC-PAmS. It indicates that C
C–H stretching vibrations near 3000 cm−1 disappears in LC-PAmS. It indicates that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of LC-KH had copolymerized with Am and StS. And it means copolymers of AM and StS have been successfully grafted onto the loess surface.
C groups of LC-KH had copolymerized with Am and StS. And it means copolymers of AM and StS have been successfully grafted onto the loess surface.
TG and DTG curves of LC-PAmS and its materials (LC, HLC, LC-KH) are showed in Fig. S2.† It is obviously that LC-PAmS is not stable than its materials. There are only a few weight loss in LC and HLC.
The weight loss of LC-KH increase to 3.2%, which indicates KH-570 was grafted onto LC particles. The total weight loss of LC-PAmS till 800 °C is only 13.1%, and most of them losing from 300 °C to 500 °C is degradation of polymer chains. Compared with polymer/loess composites,12 the content of polymer in loess surface grafting copolymer (LC-PAmS) decreased greatly.
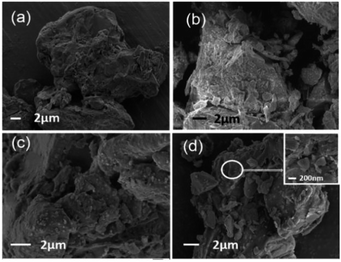
SEM images of LC, HLC, LC-KH and LC-PAmS (d) are showed in Fig. 1. Obviously, the surface of LC (a) is rough. Particles of HLC (b) show clear boundary, and there are a lot of tiny crack. The morphology of LC-KH (c) is not much different to HLC because LC was modified by small molecular KH-570 on surface. However, the loess surface of LC-PAmS is wrapped by a layer of polymer film. This suggests that the copolymer was successfully grafted onto the surface of loess.
3.3. Adsorption behaviours of LC-PAmS
LC-PAmS was applied to remove BF, a typical dye, in aqueous solution. In order to investigate the influence of functional groups in polymer chains, the adsorption behaviours of LC-PStS, LC-PAM, and its materials were also measured. The results are showed in Table 1.| Adsorbents | Removal (%) | Adsorption capacity (mg g−1) |
|---|---|---|
| a Adsorbents: 0.05 g/50 mL; time: 30 min; temp: 25 °C. | ||
| LC | 27.60 | 13.77 |
| HLC | 44.36 | 22.18 |
| LC-PAM | 46.70 | 23.35 |
| LC-PStS | 41.80 | 20.90 |
| LC-PAmS | 94.98 | 47.49 |
The adsorption capacity of LC-PAmS is higher than that of materials (LC, HLC). It is also higher than that LC surface grafting polyacrylamide (LC-PAM) and LC surface grafting polystyrene sulfonate (LC-PStS). We consider there are synergistic effects among two kinds of functional groups when LC-PAmS removes dyes.
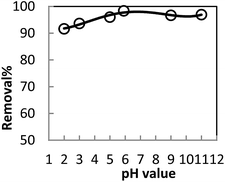
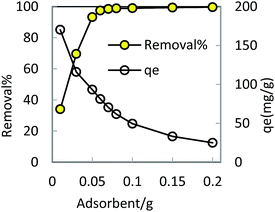
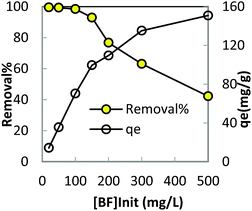
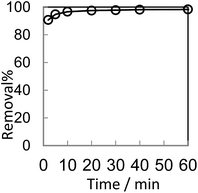
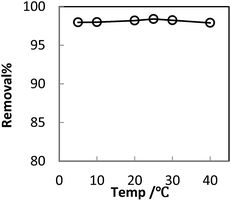
In order to optimize conditions for removing BF, some parameters for affecting the adsorption process, such as initial concentration dyes, adsorbent dosage, contact time, temperatures, pH value were investigated.
3.4. Adsorption isotherm study
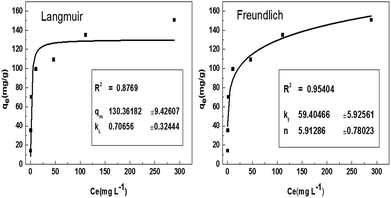
Adsorption isotherms are important for describing adsorption process of adsorbate on adsorbent surface. Hence, the correlation of equilibrium data using either a theoretical or empirical equation is essential for the adsorption interpretation and prediction of the extent of adsorption.21 The process for removing BF by LC-PAmS was evaluated with nonlinear form of Langmuir (eqn (3)) and Freundlich (eqn (4)) isotherm models:Langmuir:
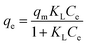 | (3) |
Freundlich:
| qe = KfC1/ne | (4) |
The plots of Langmuir and Freundlich for adsorption of BF are showed in Fig. 7. The correlation coefficients of Langmuir and Freundlich are 0.8769 and 0.9540, respectively, which indicate the best fitting of the data to the Freundlich equation. This phenomenon suggests that heterogeneous adsorption take place on the surface of LC-PAmS.
3.5. Adsorption kinetics
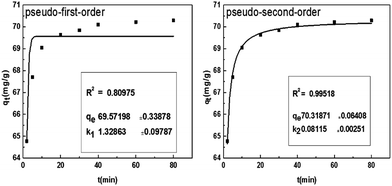
Adsorption kinetics is one of characteristics for defining adsorption efficiency and explaining adsorption mechanism. Here, the nonlinear forms of pseudo-first-order and pseudo-second-order kinetics were used to test the experimental data. The pseudo-first-order model can be explained as follows:| qt = qe − expk1t | (5) |
The pseudo-second-order model can be expressed as follows:
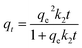 | (6) |
The analysis of the data are showed in Fig. 8 by plotting qt as a function of the contact time t. The value of correlation coefficient (R2) obtained from pseudo-second-order kinetics is higher than that of pseudo-first-order kinetics and the calculated qe,cal (70.32 mg g−1) is closer to experimental qe,exp(70.30 mg g−1). It indicates that the pseudo-second-order model is appropriate for the adsorption phenomena.
4 Conclusions
The copolymer of AM and StS was grafted onto surface of loess particles by graft copolymerization. It was found that the obtained loess surface grafted functional copolymer (LC-PAmS), was an effective adsorbent for the removal of basic fuchsin from aqueous solutions. The removal rate reached to 98.4%. Kinetic and isotherm studies were carried out. The adsorption isotherms of LC-PAmS for basic fuchsin could be described well by the Freundlich model. The experimental data fitted the pseudo-second-order kinetic model very well. In summary, LC-PAmS is a kind of polymer adsorbent for removing BF from solution and will be practically applied in wastewater treatment.Acknowledgements
The project was supported by the National Natural Science Foundation (21364012; 21263024) of China.References
- (a) G. P. Hao, W. C. Li, S. Wang, S. Zhang and A. H. Lu, Carbon, 2010, 48, 3330 CrossRef CAS; (b) Y. F. Hao, L. G. Yan, H. Q. Yu, K. Yang, S. J. Yu, R. R. Shan and B. Du, J. Mol. Liq., 2014, 199, 202 CrossRef CAS; (c) V. Chandane and V. K. Singh, Desalin. Water Treat., 2016, 57, 4122 CrossRef CAS.
- (a) T. A. Khan and E. A. Khan, Appl. Clay Sci., 2015, 107, 70 CrossRef CAS; (b) Y. He, H. Li, Z. Zhang, T. Lu and R. M. Wang, Key Eng. Mater., 2017, 726, 345 CrossRef.
- (a) S. Raghu and C. A. Basha, J. Hazard. Mater., 2007, 149, 324 CrossRef CAS PubMed; (b) Y. Dong, Z. Han, C. Liu and F. Du, Sci. Total Environ., 2010, 408, 2245 CrossRef CAS PubMed; (c) M. X. Zhu, L. Lee, H. H. Wang and Z. Wang, J. Hazard. Mater., 2007, 149, 735 CrossRef CAS PubMed.
- A. Taamallah, S. Merouani and O. Hamdaoui, Desalin. Water Treat., 2016, 57, 27314 CrossRef CAS.
- L. H. Huang, J. Kong, W. L. Wang, C. L. Zhang, S. Niu and B. Y. Gao, Desalination, 2012, 286, 268 CrossRef CAS.
- (a) A. S. K. Kumar, R. Ramachandran, S. Kalidhasan, V. Rajesh and N. Rajesh, Chem. Eng. J., 2012, 211, 396 CrossRef; (b) Y. F. He, F. Li, R. M. Wang, F. Li, Y. Wang and Z. Zhang, Water Sci. Technol., 2010, 61, 1235 CrossRef CAS PubMed.
- X. Yang, Y. H. Li, Q. J. Du, J. K. Sun, L. Chen, S. Hu, Z. H. Wang and L. H. Xia, J. Colloid Interface Sci., 2015, 453, 107 CrossRef CAS PubMed.
- H. Chen, R. Yang, K. Zhu, W. Zhou and M. Jiang, J. Hazard. Mater., 2002, 94, 91 CrossRef.
- Q. Yang, J. Zhang, Q. Yang, Y. Yu and G. Yang, Desalin. Water Treat., 2012, 39, 10 CrossRef CAS.
- J. H. Park and D. I. Jung, Desalination, 2011, 269, 104 CrossRef CAS.
- T. C. An, H. Chen, H. Y. Zhan and R. Berndtsson, Environ. Geol., 2005, 47, 467 CrossRef CAS.
- Y. F. He, L. Zhang, R. M. Wang, H. R. Li and Y. Wang, J. Hazard. Mater., 2012, 227, 334 CrossRef PubMed.
- H. M. Yang, A. D. Tang, J. O. Ouyang, M. Li and S. Mann, J. Phys. Chem. B, 2010, 114, 2390 CrossRef CAS PubMed.
- (a) S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi and R. Kumar, Prog. Polym. Sci., 2013, 38, 1232 CrossRef CAS; (b) X. Jin, C. Yu, Y. Li, Y. Qi and L. Yang, J. Hazard. Mater., 2011, 186, 1672 CrossRef CAS PubMed; (c) Y. Zhao, Y. Chen, M. Li, S. Zhou, A. Xue and W. Xing, J. Hazard. Mater., 2009, 171, 640 CrossRef CAS PubMed.
- Ş. Tokalıoğlu, E. Yavuz, A. Aslantaş, H. Şahan, F. Taşkin and Ş. Patat, Spectrochim. Acta, Part A, 2015, 149, 378 CrossRef PubMed.
- (a) Y. B. Song, X. D. Song, C. J. Cheng and Z. G. Zhao, RSC Adv., 2015, 5, 87030 RSC; (b) H. Qiao, Y. M. Zhou, F. Yu, E. A. Wang, Y. H. Min, Q. Huang, L. F. Pang and T. S. Ma, Chemosphere, 2015, 141, 297 CrossRef CAS PubMed.
- (a) J. Xiao, H. Zhang, Y. Xia, Z. Li and W. Huang, RSC Adv., 2016, 6, 39861 RSC; (b) M. El Haddad, J Taibah Univ. Sci., 2016, 10, 664 CrossRef.
- L. H. Huang, J. J. Kong, W. L. Wang, C. L. Zhang, S. F. Niu and B. Y. Gao, Desalination, 2012, 286, 268 CrossRef CAS.
- Y. Liu, Y. Kang, B. Mu and A. Q. Wang, Chem. Eng. J., 2014, 237, 403 CrossRef CAS.
- W. J. He, Y. F. He, D. Z. Yan, Y. Wang and R. M. Wang, J. Dispersion Sci. Technol., 2014, 35, 1378 CrossRef CAS.
- (a) L. Wang, W. J. He, Y. F. He, H. Li and R. M. Wang, Key Eng. Mater., 2015, 633, 165 CrossRef CAS; (b) Y. Bulut and H. Karaer, J. Dispersion Sci. Technol., 2015, 36, 61 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00610a |
| This journal is © The Royal Society of Chemistry 2017 |