Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface charge switchable and core cross-linked polyurethane micelles as a reduction-triggered drug delivery system for cancer therapy†
Chang Liu,
Yayuan Guan,
Yuling Su,
Lili Zhao,
Fancui Meng,
Yongchao Yao and
Jianbin Luo*
College of Chemistry and Environmental Protection Engineering, Southwest University for Nationalities, 610041 Chengdu, China. E-mail: luojb1971@163.com
First published on 13th February 2017
Abstract
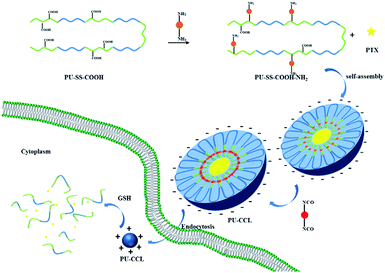
The extracellular stability versus intracellular drug release and the long blood stream circulation versus enhanced cell uptake are two dilemmas for micellar drug delivery systems. To resolve the above two problems, a novel core cross-linked polyurethane micelles with redox sensitive and pH-responsive surface charge switchable properties were prepared and investigated as anti-cancer drug carriers. Firstly, amphiphilic multi-blocked polyurethane with pendant carboxyl groups and disulfides in the hard segment (PU-SS-COOH) was synthesized. Afterwards, 1,6-diaminohexane was connected onto some of the pendant carboxyl groups via dicyclohexylcarbodiimide (DCC) condensation, which resulted in polyurethane with pendant amine and carboxyl groups (PU-SS-COOH-NH2). Then the PU-SS-COOH-NH2 was assembled into micelles and the hexamethylene-1,6-diisocyanate (HDI) was added into the micelle solution. The hydrophobic diisocyanate penetrated into the hydrophobic core of PU micelles and reacted with amine groups, which resulted in core crosslinked PU micelles (PU-CCL). PTX and doxorubicin hydrochloride (DOX) were chosen as model hydrophobic drugs to evaluate the loading and redox-triggered release of the PU micelles. The drug release from the cross-linked polyurethane micelles (PU-CCL) demonstrated that the PU-CCL has superior particle stability compared with the uncross-linked polyurethane micelles in a phosphate buffer saline (PBS) solution without reducing agents, whereas the drug release rate is markedly accelerated with the addition of a reducing agent, glutathione (GSH). Notably, PU micelles with both amine and carboxyl groups, i.e. PU-SS-COOH-NH2 and PU-CCL micelles, showed pH-responsive charge switchable properties. The in vitro cytotoxicity and cell uptake of the PTX (or DOX used to illustrate the cell uptake) loaded micelles was also assessed in HepG2 cells. It was found that the redox-sensitive polyurethane micelles can rapidly enter tumor cells. In vitro cytotoxicity studies demonstrated that the DOX-loaded PU-CCL display lower cytotoxicity in vitro compared to either free DOX or DOX-loaded uncrosslinked PU micelles. The cross-linked reduction responsive biodegradable micelles, having superior extracellular stability and providing rapid intracellular drug release, may hold great potential as a bio-triggered drug delivery system for cancer therapy.
Introduction
Recently, self-assembled polymer micelles based on multi-blocked polyurethanes have been widely studied as anti-cancer drug carriers due to their highly tunable composition and good compatibility.1–5 After circulation in blood vessels, the nanoscale drug carriers accumulated passively at tumor sites owing to the enhanced permeation and retention (EPR) effect.6–10 To achieve a better therapeutic effect, three more steps should be completed after the circulation and passive accumulation. The drug loaded carriers should penetrate into deep tumor tissues, be internalized by tumor cells and release drugs spontaneously in response to a specific stimulus specific to the intracellular micro-environment of the tumors, i.e. pH and redox.11–15 Redox-sensitive nanoscopic vehicles containing disulfide bonds which can be cleaved at millimolar concentrations of glutathione (GSH) are particularly interesting due to the great difference in the redox potential between the intracellular and extracellular compartments of tumor cells.16 As a consequence, polyurethanes with disulfide linkages in the backbone or side chains were extensively studied as efficient drug release systems.17,18The rapid dilution of polymeric micelles after intravenous administration could cause the polymer concentration to fall below the critical micelle concentration (CMC), leading to premature drug release following injection at undesired sites.19–21 The early drug release not only lowers the local therapeutic efficacy, but also causes undesirable toxicity to normal organs.22 Crosslinked polymer micelles have been developed to improve the blood circulation stability of drug carriers and to decrease premature drug release.23–28 A PEG stealth strategy was widely used to prolong the circulation time of the nanoparticles in the blood by escaping the clearance of the nanoparticles by a reticuloendothelial system (RES).29–32 Neutral or negatively charged polymer micelles which avoid nonspecific binding during circulation have a longer circulation time than positively charged ones.33 However, the positively charged micelles have shown an enhanced cell uptake compared with negatively charged ones.34 To resolve the charge dilemma of drug carriers, surface charge switchable polymer micelles are highly anticipated.35,36 Polymer micelles that bear a negative charge in a neutral environment result in less protein adhesion and a prolonged circulation time in blood. When reaching tumor tissues there is an uptake by tumor cells when an acidic microenvironment is present; the negative surface charge of the polymer nanocarriers transforms to a positive one, leading to an enhanced cell uptake and lysosome escape.11
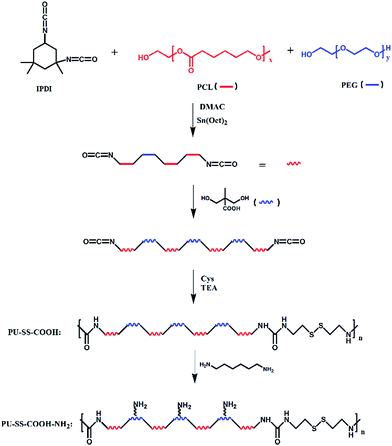
Herein, novel core cross-linked polyurethane micelles with redox sensitive and pH-responsive surface charge switchable properties were prepared and investigated as anti-cancer drug carriers. The process for the preparation of this kind of polyurethane micelles is shown in Scheme 1. Firstly, amphiphilic multi-blocked polyurethane with pendant carboxyl groups and disulfides in the hard segment (PU-SS-COOH) was synthesized by using poly(ethylene glycol) (PEG) and poly(ε-caprolactone)diol (PCL) as the soft-segment, and 2,2-bis(hydroxymethyl)propionic acid (DMPA) and cystamine dihydrochloride as the chain extenders. Afterwards, 1,6-diaminohexane was connected onto the partially pendant carboxyl groups via dicyclohexylcarbodiimide (DCC) condensation which resulted in polyurethane with pendant amine and carboxyl groups (PU-SS-COOH-NH2). Then the PU-SS-COOH-NH2 was assembled into micelles and the hexamethylene-1,6-diisocyanate (HDI) was added into the micelle solution. The hydrophobic hexamethylene-1,6-diisocyanate penetrated into the hydrophobic core of PU micelles and reacted with amine groups, which resulted in core crosslinked PU micelles (PU-CCL). PTX and doxorubicin hydrochloride (DOX) were chosen as model hydrophobic drugs to evaluate the loading and redox-triggered release of the PU micelles. All the three kinds of PU micelles released their payloads in the presence of GSH, while PU micelles with both amine and carboxyl groups, i.e. PU-SS-COOH-NH2 and PU-CCL micelles, showed pH-responsive charge switchable properties. Furthermore, the stability of the core crosslinked PU micelles enhanced dramatically while the premature release decreased and the drug release of the PU-CCL slowed down compared with non-crosslinked micelles. The in vitro cytotoxicity and cell uptake of the PTX (or DOX, used to illustrate the cell uptake) loaded micelles were also assessed in HUVECs and HepG2 cells.
Experimental
Materials
Polyethylene glycol (PEG) (Mn = 1000, Sunshine Biotechnology (Nanjing) Co., Ltd. China) and poly(ε-caprolactone)diol (PCL Mn = 2000, Dow Chemical, USA) were dehydrated under reduced pressure at 100 °C for 2–3 h before use. N,N-Dimethylacetamide (DMAc) was dried over CaH2, vacuum distilled before use. Isophorone diisocyanate (IPDI), hexamethylene-1,6-diisocyanate (HDI) and stannous octoate (Sn(Oct)2) were purchased from Aladdin Industrial Corporation, China, and used without further purification. Triethylamine (TEA, Aladdin Industrial Corporation, China) was distilled under vacuum. Cystamine dihydrochloride (Cys, Aladdin Industrial Corporation, China), 2,2-bis(hydroxymethyl)propionic acid (DMPA) and dicyclohexylcarbodiimide (DCC) were used as received. PTX (99.5%) and doxorubicin hydrochloride (DOX, –NH3+Cl− salt form, >98%) were obtained from AstaTech (Chengdu) Pharmaceutical Co. Ltd., China. Glutathione and sodium dodecyl sulfate (SDS) were purchased from Kelong Chemical Reagent Factory, China. The HUVEC cell lines were donated from the Shanghai Cell Bank of the Chinese Academy of Sciences. The HepG2 cell lines were obtained from the Wuhan Procell Life Science and Technology Co., Ltd. South American fetal bovine serum was obtained from CLARK Bioscience Co., Ltd. 0.25% of trypsin (+EDTA), RPMI/medium, double resistance and PBS buffer were produced by Hyclone Co., Ltd. CCK8 was obtained from Biosharp Co., Ltd. DAPI was obtained from Roche Co., Ltd.Synthesis of PU-SS-COOH
Polyurethane with pendant carboxyl groups and disulfide linkages on the main chain was synthesized from PEG, PCL, IPDI, Cys and DMPA by the traditional two step solution polymerization process. The feed ratios are listed in Table 1. In brief, anhydrous PEG and PCL-diol were dissolved in DMAc under a dry nitrogen atmosphere. IPDI was added into the DMAc solution of PCL and PEG and pre-polymerized in the presence of 0.1% stannous octoate (Sn(Oct)2) at 90 °C for 4 h under stirring. Afterwards, the prepolymer was chain extended by DMPA at 90 °C for another 4 h. Then, the reaction solution was cooled to 0 °C by ice water and the chain extender Cys with the same amount of triethylamine (TEA, used to neutralize the hydrogen chloride of Cys) was added, and the mixture was kept at 0 °C for 1 h and at room temperature for 2 h. Finally, the mixture was reacted at 90 °C for 4 h to complete the chain extending reaction. After that, the reaction mixture was cooled to room temperature. To remove the organic solvents and impurities, the crude product was subsequently poured into a water and methanol mixture with a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The precipitant was redissolved in DMAc and precipitated by a water and methanol mixture. The polymer was harvested and dried at 40 °C in the atmosphere for 5 h and at 60 °C in a reduced pressure for 2 days to get the final product.
1. The precipitant was redissolved in DMAc and precipitated by a water and methanol mixture. The polymer was harvested and dried at 40 °C in the atmosphere for 5 h and at 60 °C in a reduced pressure for 2 days to get the final product.
| Samples | Feed ratio | Molecular weights (g mol) | Size (nm) | Zeta (mV) | Loading Content (LC) (%) | Encapsulation Efficiency (EE) (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCL | PEG | IPDI | DMPA | Cys | Mw | Mn | Mw/Mn | PTX | DOX | PTX | DOX | |||
| PU-SS-COOH | 3 | 1 | 8.1 | 3 | 1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.440 | 104.93 ± 0.9 | −17.8 ± 0.6 | ||||
| PU-SS-COOH-NH2 | 3 | 1 | 8.1 | 3 | 1 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.271 | 87.02 ± 1.4 | −18.27 ± 0.7 | ||||
| PU-CCL | 3 | 1 | 8.1 | 3 | 1 | 77.27 ± 1.8 | −18.9 ± 0.7 | |||||||
| PU-SS-COOH(PTX)/PU-SS-COOH(DOX) | 3 | 1 | 8.1 | 3 | 1 | 106.5 ± 1.2 | −18.27 ± 0.8 | 3.8 | 4.8 | 8.02 | 25.6 | |||
| PU-SS-COOH-NH2(PTX)/PU-SS-COOH-NH2(DOX) | 3 | 1 | 8.1 | 3 | 1 | 98.61 ± 1.4 | −18.17 ± 0.4 | 4.2 | 5.0 | 8.84 | 26.9 | |||
| PU-CCL(PTX)/PU-CCL(DOX) | 3 | 1 | 8.1 | 3 | 1 | 90.58 ± 1.6 | −18.53 ± 0.5 | 1.4 | 4.1 | 2.90 | 22.8 | |||
Synthesis of PU-SS-COOH-NH2
PU-SS-COOH (5 g) was dissolved in a mixture of DMF (15 ml) and THF (20 ml) at room temperature with stirring. 1,6-Diaminohexane (0.8 mmol) and 1-hydroxybenzotriazole (HOBT, 0.3 mmol) was added and the reaction solution was placed into ice-salt baths for 15 min. Afterwards, dicyclohexylcarbodiimide (DCC, 2 mmol) was added under stirring and reacted for 18 h to complete the DCC condensation reaction. Polyurethane was purified using the same procedure as that to purify PU-SS-COOH. The final product was denoted as PU-SS-COOH-NH2.Characterization of polyurethanes
1H NMR was recorded on an Agilent-NMR-vnmrs 400 (400 MHz) spectrometer using deuterated chloroform (CDCl3-d6) as the solvent. The molecular weights and molecular weight distributions of PU-SS-COOH and PU-SS-COOH-NH2 were determined by a Waters-1515 Gel Permeation Chromatogram. Tetrahydrofuran (THF) was used as the mobile phase at a flow rate of 1 ml min−1 at 40 °C and the molecular weights are reported relative to polystyrene (PS) standards.Preparation of polyurethane micelles
A dialysis method was used to prepare the polyurethane micelles. Typically, polyurethane (PU-SS-COOH or PU-SS-COOH-NH2, 20 mg) was completely dissolved in 4 ml DMF. Afterwards, the solution was added dropwise into 10 ml distilled water under vigorous stirring. To prepare the PU-CCL micelles, HDI (5 mg ml−1 in DMF) was added slowly into the PU-SS-COOH-NH2 micelles in the presence of catalytic amounts of stannous octoate (Sn(Oct)2) under intense stirring at room temperature. The reaction mixture was reacted under stirring at room temperature for 24 hours. Subsequently, the micelle solution was transferred to a dialysis bag (MWCO, 3.5 kDa) and dialyzed against distilled water for 72 h to eliminate the organic solvent at room temperature. The micelle solution was passed through a 0.45 μm pore-sized syringe filter (Millipore, Carrigtwohill, Co. Cork, Ireland), and stored at 4 °C.Characterization of polyurethane micelles
The critical micelle concentration (CMC) of the obtained polyurethanes in distilled water was determined by fluorescence spectroscopy using pyrene as a probe. The polyurethane concentration varied from 1.0 × 10−5 to 0.2 mg ml−1 and the final pyrene concentration was fixed at 5.2 × 10−6 M. The combined solution of pyrene and micelles was sonicated for 4 h in the dark before the fluorescence measurement. Fluorescence excitation spectra were recorded by a fluorometer (Varian Cary Eclipse Fluorescence Spectrophotometer, USA) at a wavelength range from 285 to 355 nm, with the emission wavelength at 372 nm and slits at 5 nm for both excitation and emission. Size and zeta potentials of the nanoparticles in aqueous solution were measured with a Zetasizer analyzer (Malvern ZetasizerNano, Zen 3690+MPT2, Malvern, UK). The kinetic stability of the PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL in DI water was studied in the presence of SDS acting as a destabilizing agent. The micelles (1 mg ml−1) were mixed with SDS aqueous solution (20 mg ml−1) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio and the scattered light intensity was monitored by DLS over a period of 48 h.28 The morphologies of PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles were observed by transmission electron microscopy (TEM) (Tecnai G2 F20 transmission electron microscope, Royal Dutch Philips Electronics Ltd., Holland) at an accelerating voltage of 200 kV with phosphotungstic acid 2% negative staining.
1 v/v ratio and the scattered light intensity was monitored by DLS over a period of 48 h.28 The morphologies of PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles were observed by transmission electron microscopy (TEM) (Tecnai G2 F20 transmission electron microscope, Royal Dutch Philips Electronics Ltd., Holland) at an accelerating voltage of 200 kV with phosphotungstic acid 2% negative staining.
Preparation of PTX-loaded micelles
To prepare the PTX-loaded PU-SS-COOH and PU-SS-COOH-NH2 micelles, a clear solution consisting of the purified, dried PU (10 mg) and PTX (5 mg) in DMF (1 ml) was added drop-wise to water (10 ml). To prepare the PTX-loaded PU-CCL micelles, HDI (5 mg ml−1 in DMF) was added slowly into the PTX-loaded PU-SS-COOH-NH2 micelles in the presence of a catalytic amount of stannous octoate (Sn(Oct)2) under intense stirring at room temperature. The reaction mixture was reacted under stirring at room temperature for 24 hours. Subsequently, the micelle solution was transferred to the dialysis bag (MWCO, 3.5 kDa) and dialyzed against distilled water for 72 h to eliminate the organic solvent at room temperature. The excess of PTX was removed by a 0.45 μm filter. The concentration of the drugs in the loaded micelles was evaluated using a high-performance liquid chromatography (HPLC) system (Waters Isocratic HPLC Pump, US) equipped with a reverse-phase C18 column (4.6 × 250 mm, 5 μm). An acetonitrile–water (60/40 v/v) mixture was used as the mobile phase and the flow rate was 1.0 ml min−1. The UV adsorption of PTX that flowed out from the chromatographic column was recorded by a Waters 2489 UV/Visible Detector at a wavelength of 227 nm. The loading content (%) and encapsulation efficiency (%) were calculated based on the equations below:| The loading content (LC) (%) = mass of drugs in micelles/total mass of loaded micelles × 100% |
| Encapsulation efficiency (EE) (%) = mass of drugs in micelles/initial amount of feeding drugs × 100%. |
Preparation of DOX-loaded micelles using dialysis method
Water (5 ml) was added drop-wise to a clear solution consisting of the purified, dried PU (10 mg), DOX (2 mg), and Et3N (3 molar equivalents to DOX) in DMF (2 ml). The resulting dispersion was dialyzed over water (500 ml) for 2 days, yielding DOX-loaded micelles of PU at 1.0 mg ml−1. The excess DOX was removed by a 0.45 μm filter. The concentration of the drugs in the loaded micelles was evaluated using a UV-vis spectrophotometer (UnicamUA500, Thermo Electron Corporation). The UV adsorption of DOX was recorded at a wavelength of 482 nm. The loading content (%) and encapsulation efficiency (%) were calculated based on the equations below:| The loading content (LC) (%) = mass of drugs in micelles/total mass of loaded micelles × 100% |
| Encapsulation efficiency (EE) (%) = mass of drugs in micelles/initial amount of feeding drugs × 100%. |
In vitro release of PTX
In vitro drug release from the drug-loaded micelles was performed using a dialysis method. In brief, 2.5 ml of the drug loaded micelle solution (PU-SS-COOH, PU-SS-COOH-NH2, PU-CCL) was added to a dialysis bag (MWCO: 8000) and immersed in 25 ml of PBS (0.01 M, pH 7.4) containing 0.1 M sodium salicylate with or without 10 mM GSH. The dialysis system was kept at 37 °C in a thermostatic incubator with a shaking speed of 110 rpm. Samples were taken out and replaced with the same volume of fresh medium at the desired time intervals. The concentration of PTX released from the drug loaded micelles was analyzed using a HPLC.Cell culture
Human liver cell line HepG2 cells were cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 U ml−1 penicillin and 10% fetal bovine serum (FBS) (FBS, CLARK, UT) at 37 °C in a humidified atmosphere containing 5% CO2 (Sanyo Incubator, MCO-18AIC, Japan).HUVECs were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco Life, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, CLARK, USA), 2 mM L-glutamine and 1% (v/v) antibiotics mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U of penicillin and 10 mg of streptomycin) (Hyclone). The cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C (Sanyo Incubator, MCO-18AIC, Japan).
000 U of penicillin and 10 mg of streptomycin) (Hyclone). The cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C (Sanyo Incubator, MCO-18AIC, Japan).
Cellular uptake and intracellular release of payloads
Confocal laser scanning microscopy (CLSM) was employed to examine the cellular uptake and intracellular release behaviors of polyurethane micelles. The DOX-loaded micelles were incubated with HepG2 cells for 1 h at 37 °C. After removal of the medium, the cells were washed three times with cold PBS, fixed with 1 ml of 4% paraformaldehyde for 30 min at 4 °C, and stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, Roche) for 10 min. Finally, the slides were mounted with 10% glycerol solution and observed by a LeicaTCS SP8 (Leica Microscopy Systems Ltd., Germany).Cell viability assay
To evaluate the antitumor activity of PTX-loaded polyurethane micelles and the cytocompatibility of the drug-free micelles, HepG2 cells and HUVECs were seeded in 96-well plates at 4 × 103 cells per well and incubated for 24 h.37 The culture medium was removed and replaced with 100 ml medium containing various concentrations of micelle solutions for another 24 h of incubation. Then, 10 μm of Cell Counting Kit-8 (CCK-8) solution (100T ml−1, Biosharp Co., Ltd.) was added to each well. After incubating the cells for 4 h, the absorbance was measured at a wavelength of 450 nm. The cell viability was normalized to that of cells cultured in the full culture media. The dose–effect curves were plotted and the median inhibitory concentration (IC50) was determined using the software IBM SPSS Statistics (SPPS, Inc., USA).Results and discussion
Synthesis and characterization of polyurethanes
The synthesis processes of the reduction-sensitive biodegradable multi-block polyurethanes are listed in Scheme 2. For PU-SS-COOH, the feed ratio of PCL to PEG was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as listed in Table 1. As we have reported previously,18 the disulfide bonds and carboxyl bonds were mainly located between the hydrophobic PCL segments, which resulted in the core location of carboxyl groups in the self-assembled micelles of PU-SS-COOH and PU-SS-COOH-NH2. Polyurethane with pendant primary amine groups and carboxyl groups (PU-SS-COOH-NH2) were prepared by grafting 1,6-diaminohexane onto carboxyl groups using the DCC condensation reaction, as shown in Scheme 2.
1 as listed in Table 1. As we have reported previously,18 the disulfide bonds and carboxyl bonds were mainly located between the hydrophobic PCL segments, which resulted in the core location of carboxyl groups in the self-assembled micelles of PU-SS-COOH and PU-SS-COOH-NH2. Polyurethane with pendant primary amine groups and carboxyl groups (PU-SS-COOH-NH2) were prepared by grafting 1,6-diaminohexane onto carboxyl groups using the DCC condensation reaction, as shown in Scheme 2.
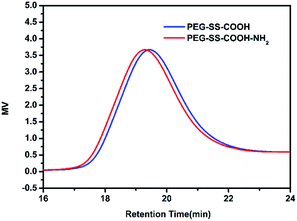
The unimodal GPC curves of purified PU-SS-COOH and PU-SS-COOH-NH2 (Fig. 1) confirmed a successful polymerization. Notably, as is shown in Fig. 1, the retention time of PU-SS-COOH-NH2 is a little shorter than that of PU-SS-COOH, suggesting the successful grafting of 1,6-diaminohexane. The average molecular weight and polydispersity of PU-SS-COOH and PU-SS-COOH-NH2 are listed in Table 1. As shown in Table 1, PU-SS-COOH had Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 g mol−1 with Mw/Mn = 1.44, while the Mn of PU-SS-COOH-NH2 was 16
749 g mol−1 with Mw/Mn = 1.44, while the Mn of PU-SS-COOH-NH2 was 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 g mol−1 with Mw/Mn = 1.27.
615 g mol−1 with Mw/Mn = 1.27.
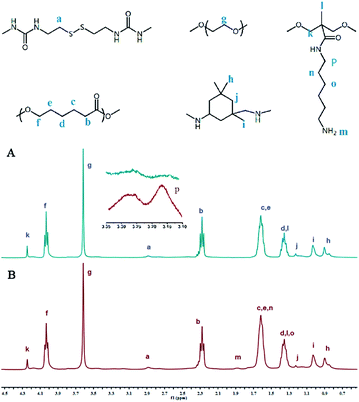
The representative 1H NMR spectra of PU-SS-COOH and PU-SS-COOH-NH2 and the assignment of the peaks are presented in Fig. 2. The resonance peak centered at 3.62 ppm (peak g) was assigned to the protons on the PEG units while the peaks at 4.03 ppm (peak f), 2.28 ppm (peak b), 1.62 ppm (peaks c and e) and 1.36 ppm (peak d) are attributed to the protons of the PCL units. The resonances at 1.22 ppm (peak j), 1.02 ppm (peak i) and 0.9 ppm (peak h) are ascribed to the methylene and methyl protons of the IPDI units in the products. The peaks at 2.89 ppm (peak a) are attributed to the methylene protons of the Cys units, indicating the presence of the Cys units in the resulting polyurethanes. The resonances at 4.24 ppm (peak k) and 1.35 ppm (peak l) are ascribed to the methylene and methyl protons of the DMPA units in the products, indicating the presence of the carboxyl groups in the PU-SS-COOH.
The resonances at 3.16 ppm (peak p), 1.88 ppm (peak m), 1.62 ppm (peak n) and 1.35 ppm (peak o) are ascribed to the amine and methyl protons of the 1,6-diaminohexane units in PU-SS-COOH-NH2, suggesting the successful attachment of 1,6-diaminohexane onto the carboxyl groups of PU-SS-COOH and the presence of the primary amine groups in PU-SS-COOH-NH2. Based on the peak area ratio of peak k to peak p, which correspond to the methane protons of DMPA and 1,6-diaminohexane, respectively, about 63% (molar ratio) of the carboxyl groups were grafted by 1,6-diaminohexane. Therefore, the molar ratio of primary amine to the carboxyl groups is 63/37 in PU-SS-COOH-NH2. The presence of both primary amine and carboxyl groups in the PU-SS-COOH-NH2 results in the charge switchable properties of PU-SS-COOH-NH2 based micelles.
Characterization of PU micelles
The amphiphilic multi-blocked polyurethanes with redox responsive properties self-assembled in aqueous solution into micelles having a hydrophobic PCL core and a hydrophilic PEG shell. The core–shell structure of the polyurethane micelles was confirmed from fluorescence measurements using pyrene as a probe (ESI: Fig. S1A and B†).The critical micellar concentration (CMC) was determined by fluorescence spectroscopy with a pyrene probe. The ratio of the intensity of the peak at 337 nm to that at 333.5 nm is plotted against the log of polymer concentration and the concentration corresponding to the intersection of the two tangential lines is the CMC value (ESI: Fig. S1C†). The CMCs of PU-SS-COOH and PU-SS-COOH-NH2 were determined to be 1.66 × 10−3 mg ml−1 and 7.7 × 10−4 mg ml−1, respectively. The CMC of PU-SS-COOH-NH2 is much lower than that of PU-SS-COOH, suggesting PU-SS-COOH-NH2 micelles are more stable than PU-SS-COOH micelles.38 Kim et al.39 lowered the CMC of PEG–PTMC block polymers by incorporating strong H-bonding urea-functional groups on the side chain of the block polymers, which resulted in more kinetically stable block polymer micelles. The lower CMC of PU-SS-COOH-NH2 is, therefore, attributed to an enhanced hydrogen bonding interaction between the pendant amine groups of PU-SS-COOH-NH2.
Size and zeta potentials of the PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles were determined by dynamic light scattering (DLS) at 25 °C using a Zetasizer analyzer (Malvern ZetasizerNano, Zen 3690+MPT2, Malvern, UK). As presented in Table 1, the hydrodynamic diameters of the PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles determined by DLS are 104.93 nm, 87.02 nm and 77.27 nm, respectively, with a unimodal size distribution (Fig. 3A). Notably, the particle sizes of PU-SS-COOH-NH2 and PU-CCL micelles are reduced by ca. 18 and 28 nm, respectively, compared with that of PU-SS-COOH micelles, indicating the successful amidation of PU-SS-COOH and core-crosslinking of PU-SS-COOH-NH2 micelles. As reported in the literature,40 the particle size of crosslinked polymer micelles is usually smaller that of than their uncrosslinked micelles due to the more compact chain package of crosslinked micelles. In addition, the size reduction of PU-SS-COOH-NH2 micelles compared with PU-SS-COOH micelles may be ascribed to the enhanced interchain H-bonding among the urethane groups and the amide groups of PU-SS-COOH-NH2.40 After loading the drugs, the three kinds of micelles grew larger due to the encapsulation of the hydrophobic drugs into the core of the micelles (Fig. 3B). Notably, a negative surface charge at pH 7.4 was found for all three kinds of PU micelles, as shown in Table 1, suggesting a long circulation time of these micelles in the blood.
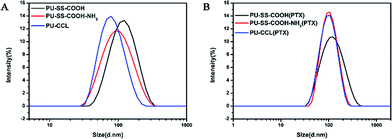 | ||
| Fig. 3 Size distribution of the PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles (A) and their PTX loaded micelles (B). | ||
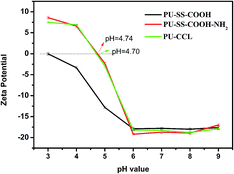
The change of surface charge with solution pH was monitored by the relationship of zeta potential and pH value (Fig. 4). The existence of carboxyl groups and amine groups on PU-SS-COOH-NH2 and PU-CCL rendered the surface charge of these two kinds of PU micelles pH-responsive characteristics. The zeta potential of all three kinds of PU micelles remained constant at ca. −18 mV when the solution's pH value stayed at above 6 due to the deprotonation of the carboxyl groups in these PU materials. The negative-charged PU micelles will have good protein resistance and long blood stream circulation times.35 However, zeta potential values of all the polyurethane micelles increased with a decrease of the solution's pH value when the solution was in an acidic environment (pH < 6) as a result of the protonation of the primary amine groups and the carboxyl groups. Interestingly, as shown in Fig. 4, the surface charge of the PU-SS-COOH-NH2 and PU-CCL micelles reversed to positive at pH 4.74 and 4.70, respectively, due to the protonation of the amine, which resulted in an enhanced cell uptake and the lysosome escape of these two PU micelles.
TEM micrographs of the PU micelles demonstrated that the assembled PU micelles had a spherical morphology with an average diameter of ∼63, ∼50 and ∼44 nanometers for PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL, respectively (Fig. 5A–C). The difference in micelle sizes between DLS and TEM can be attributed to the dehydrated state of the micelles.
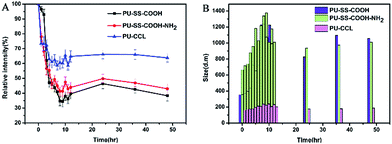
The micelle kinetic stability was studied by dynamic light scattering (DLS) in the presence of sodium dodecyl sulfate (SDS) known as a destabilizing agent.39 As is shown in Fig. 6A, a significant decrease in the scattered light intensity was observed in the first 10 h for SDS-treated PU-SS-COOH and PU-SS-COOH-NH2 micelles, and the relative intensity was then maintained at 40–45% of the original value. This suggests the dissociation of a large percentage of the micelles. In contrast, only a 35% drop in scattered light intensity was observed for PU-CCL micelles in 48 h, indicating an enhanced stability of PU-CCL micelles. Fig. 6B shows the size change of the PU micelles after treatment with SDS as a function of time. Notably, the size of the PU-CCL micelles after treatment with SDS remained stable with an average diameter less than 200 nm. However, the sizes of the PU-SS-COOH and PU-SS-COOH-NH2 micelles grew instantly to about 1000 nm or more after treatment with SDS, suggesting a low stability of these two kinds of PU micelles.
Drug loading and release
PTX was used as a model hydrophobic drug to investigate the loading capacity of the redox active polyurethane micelles. A micelle extraction technique was used to load the PTX into polyurethane micelles. The excess PTX was removed by filtration through a 0.45 μm filter. The loading level of PTX for PTX-loaded micelles was determined using HPLC. The drug loading content (LC) and encapsulation efficiency for PTX loaded micelles are shown in Table 1.The drug release behavior experiments of PTX-loaded polyurethane nanoparticles were conducted in PBS buffer solutions (pH 7.4, 10 mM) with and without 10 mM of GSH at 37 °C. The accumulative drug release profiles as a function of time are plotted in Fig. 7. Without the presence of GSH, an obvious initial burst release was observed for PTX-loaded PU-SS-COOH and PU-SS-COOH-NH2 micelles, while this was markedly suppressed for drug loaded PU-CCL micelles. For instance, only 19% of PTX was released from drug encapsulated PU-CCL micelles at 48 h without the presence of GSH (Fig. 7), and 43% and 33% were released from PTX encapsulated uncross-linked PU-SS-COOH and PU-SS-COOH-NH2 at 48 h. This indicated a better stability of PTX loaded PU-CCL micelles in PBS than that of the other two drug loaded uncross-linked PU micelles. In addition, drug loaded PU-SS-COOH-NH2 micelles show an enhanced stability over PTX loaded PU-SS-COOH micelles, which is consistent with the CMC results. However, PTX release from all the three PU micelles was enhanced dramatically when 10 mM GSH was present in the PBS solution, causing 78%, 62% and 60% to be released from PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles, respectively, in 48 h. The enhanced drug release in the presence of GSH arose from the disassembly of polyurethane micelles due to the GSH-induced disulfide cleavage.
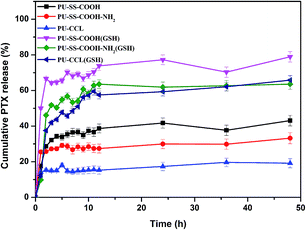 | ||
| Fig. 7 Time dependent cumulative release of PTX from PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles in PBS buffer solutions (pH 7.4, 10 mM) with and without 10 mM of GSH at 37 °C. | ||
Internalization and intracellular release of the polyurethane micelle payload
The cellular uptake and intracellular drug release behavior of DOX-loaded nanoparticles in HepG2 cells were analyzed by confocal laser scanning microscopy (CLSM). Since PTX molecules are not fluorescent, doxorubicin (DOX, red fluorescence) was encapsulated in hydrophobic micellar cores using the dialysis method to label the nanocarriers and to track the internalization and intracellular localization of DOX (red fluorescence) in HepG2 cells.40 The nuclei of HepG2 cells were stained by DAPI, which presented blue fluorescence to distinguish it from the red fluorescence. Fig. 8 shows the CLSM images of HepG2 cells incubated with DOX-loaded polyurethane micelles for 2 h. As shown in Fig. 8, after 2 h of cell incubation, a strong red fluorescence of free DOX was observed in nuclei, which even turned pink in the merged fluorescence images as a result of the overlapping fluorescence of DAPI and DOX. DOX fluorescence was observed in the cytoplasm and nuclei of DOX loaded PU-SS-COOH-NH2, PU-SS-COOH and PU-CCL micelles, which suggests that DOX-loaded polyurethane micelles were internalized and DOX was released to reach the cell nuclei.41,42 In addition, DOX loaded PU-CCL showed a weaker fluorescence intensity as compared to that of drug loaded non-crosslinked micelles due to the enhanced stabilities of PU-CCL.26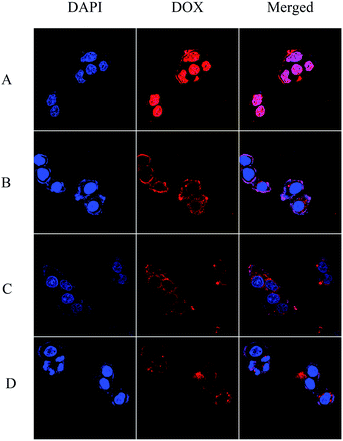 | ||
| Fig. 8 CLSM images of HepG2 cells incubated with free DOX (A), and DOX loaded PU-SS-COOH (B), PU-SS-COOH-NH2 (C) and PU-CCL (D) micelles for 2 h. Scale bar = 10 μm. | ||
In vitro cytotoxicities of polyurethane micelles and PTX-loaded polyurethane micelles
The in vitro cytotoxicity of drug-free and drug-loaded polyurethane micelles was evaluated in the CCK8 assay. Fig. 9 shows the effect of PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL concentration on the viability of HUVECs and HepG2 cells. The results demonstrate that empty PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles show very low cytotoxicity (greater than 90% cell viability) in two different cell lines even at micelle concentrations up to 1 mg ml−1; suggesting the nontoxic nature of polyurethane micelles to HUVECs and HepG2 cells.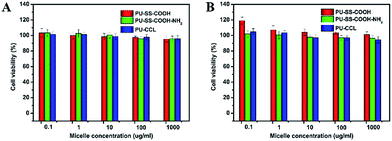 | ||
| Fig. 9 Viability of HUVECs (A) and HepG2 cells (B) after 48 h of incubation with various concentrations of empty reduction-sensitive polyurethane micelles determined by the CCK8 assay. | ||
Further, the in vitro cytotoxicity of PTX-loaded PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL micelles is shown in Fig. 10. Fig. 10A shows the cytotoxicity results given as a function of PTX concentration from 1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng ml−1. As the test concentration was increased to 100 ng ml−1, PU-CCL encapsulated PTX exhibited a higher cell viability (65.61%) than PU-SS-COOH and PU-SS-COOH-NH2 encapsulated PTX. The IC50 (i.e., inhibitory concentration to produce 50% cell death) values of PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL encapsulated PTX were determined to be ∼357, ∼2239 and ∼2655 ng ml−1, respectively, for HepG2 cells (P < 0.05). The results reveal that the PU-CCL micelles provide a less efficient intracellular delivery of PTX as compared to PU-SS-COOH micelles. The reason is that the PU-CCL micelles were more stable than PU-SS-COOH, which leads to a less efficient intracellular delivery. HepG2 cells were treated for 24 h or 72 h with PTX-loaded micelles (PTX dosage: 100 ng ml−1) as shown in Fig. 10B. Interestingly, cell viabilities of 65.6% and 59.4% (P < 0.05) were observed for HepG2 cells following incubation with PTX-loaded crosslinked micelles for 24 and 72 h, respectively, which are analogous to those obtained for PTX-loaded non-crosslinked controls (cell viabilities of 65% and 55.5% (P < 0.05) for 24 and 72 h incubation, respectively). The cell viability declined with an extending time of cultivation. This phenomenon shows a drug sustained release.
000 ng ml−1. As the test concentration was increased to 100 ng ml−1, PU-CCL encapsulated PTX exhibited a higher cell viability (65.61%) than PU-SS-COOH and PU-SS-COOH-NH2 encapsulated PTX. The IC50 (i.e., inhibitory concentration to produce 50% cell death) values of PU-SS-COOH, PU-SS-COOH-NH2 and PU-CCL encapsulated PTX were determined to be ∼357, ∼2239 and ∼2655 ng ml−1, respectively, for HepG2 cells (P < 0.05). The results reveal that the PU-CCL micelles provide a less efficient intracellular delivery of PTX as compared to PU-SS-COOH micelles. The reason is that the PU-CCL micelles were more stable than PU-SS-COOH, which leads to a less efficient intracellular delivery. HepG2 cells were treated for 24 h or 72 h with PTX-loaded micelles (PTX dosage: 100 ng ml−1) as shown in Fig. 10B. Interestingly, cell viabilities of 65.6% and 59.4% (P < 0.05) were observed for HepG2 cells following incubation with PTX-loaded crosslinked micelles for 24 and 72 h, respectively, which are analogous to those obtained for PTX-loaded non-crosslinked controls (cell viabilities of 65% and 55.5% (P < 0.05) for 24 and 72 h incubation, respectively). The cell viability declined with an extending time of cultivation. This phenomenon shows a drug sustained release.
Conclusions
In summary, novel core cross-linked polyurethane micelles with redox sensitive and pH-responsive surface charge switchable properties were prepared and investigated as anti-cancer drug carriers. As a starting material, amphiphilic multi-blocked polyurethane with pendant carboxyl groups and disulfides in the hard segment (PU-SS-COOH) was synthesized firstly. The PU-SS-COOH was further functionalized by 1,6-diaminohexane, which resulted in polyurethane with pendant amine and carboxyl groups (PU-SS-COOH-NH2). Then the PU-SS-COOH-NH2 was assembled into micelles and hexamethylene-1,6-diisocyanate (HDI) was reacted with the amine groups which resulted in core crosslinked PU micelles (PU-CCL). All the three kinds of PU micelles released their payloads in the presence of GSH, while PU micelles with both amine and carboxyl groups, i.e. PU-SS-COOH-NH2 and PU-CCL micelles, showed pH-responsive charge switchable properties. Furthermore, the stability of the core crosslinked PU micelles (PU-CCL) enhanced dramatically while the premature release decreased compared with non-crosslinked micelles. The in vitro cytotoxicity and cell uptake of the PTX (or DOX, used to illustrate the cell uptake) loaded micelles was also assessed in HepG2 cells. The cross-linked reduction responsive biodegradable micelles, having superior extracellular stability and providing rapid intracellular drug release, may hold great potential as a bio-triggered drug delivery system for cancer therapy.Acknowledgements
This work was financially supported by Applied Basic Research Programs Foundation of Sichuan Province (No. 2015JY0126) and the Innovation Scientific Research Program for Graduates in Southwest University for Nationalities (CX2015SZ056).Notes and references
- R. Savic, L. Luo, A. Eisenberg and D. Maysinger, Science, 2003, 300, 615 CrossRef CAS PubMed.
- N. Ma, Y. Li, H. Xu, Z. Wang and X. Zhang, J. Am. Chem. Soc., 2010, 132, 442 CrossRef CAS PubMed.
- M. Ding, J. Li, X. He, N. Song, H. Tan, Y. Zhang, L. Zhou, Q. Gu, H. Deng and Q. Fu, Adv. Mater., 2012, 24, 3639 CrossRef CAS PubMed.
- S. Yu, C. He, J. Ding, Y. Cheng, W. Song, X. Zhuang and X. Chen, Soft Matter, 2013, 9, 2637 RSC.
- D. Xu, Y. Su, L. Zhao, F. Meng, C. Liu, Y. Guan, J. Zhang and J. Luo, J. Biomed. Mater. Res., Part A, 2017, 105(2), 531 CrossRef CAS PubMed.
- Y. Matsumura and H. Maeda, Cancer Res., 1986, 46, 6387 CAS.
- M. Talelli, M. Barz, C. J. F. Rijcken, F. Kiessling, W. E. Hennink and T. Lammers, Nano Today, 2015, 10, 93 CrossRef CAS PubMed.
- H. Maeda, H. Nakamura and J. Fang, Adv. Drug Delivery Rev., 2013, 65, 71 CrossRef CAS PubMed.
- H. Maeda, J. Controlled Release, 2012, 164, 138 CrossRef CAS PubMed.
- R. K. Jain and T. Stylianopoulos, Nat. Rev. Clin. Oncol, 2010, 7, 653 CrossRef CAS PubMed.
- Q. Sun, X. Sun, X. Ma, Z. Zhou, E. Jin, B. Zhang, Y. Shen, E. A. Van Kirk, W. J. Murdoch, J. R. Lott, T. P. Lodge, M. Radosz and Y. Zhao, Adv. Mater., 2014, 01, 554 Search PubMed.
- Q. Zhang, Na R. Ko and J. K. Oh, Chem. Commun., 2012, 48, 7542 RSC.
- E. Fleige, M. A. Quadir and R. Haag, Adv. Drug Delivery Rev., 2012, 64, 866 CrossRef CAS PubMed.
- F. Meng, R. Cheng, C. Deng and Z. Zhong, Mater. Today, 2012, 15, 436 CrossRef CAS.
- W. Chen, P. Zhong, F. Meng, R. Cheng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2013, 169, 171 CrossRef CAS PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991 CrossRef CAS PubMed.
- X. He, M. Ding, J. Li, H. Tan, Q. Fu and L. Li, RSC Adv., 2014, 4, 24736 RSC.
- Y. Yao, H. Xu, C. Liu, Y. Guan, D. Xu, J. Zhang, Y. Su, L. Zhao and J. Luo, RSC Adv., 2016, 6, 9082 RSC.
- S. Yu, J. Ding, C. He, Y. Cao, W. Xu and X. Chen, Adv. Healthcare Mater, 2014, 3, 752 CrossRef CAS PubMed.
- Y. Li, K. Xiao, W. Zhu, W. Deng and K. S. Lam, Adv. Drug Delivery Rev., 2014, 66, 58 CrossRef CAS PubMed.
- W. Chen, F. Meng, R. Cheng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2015, 210, 125 CrossRef CAS PubMed.
- H. S. Han, Ki Y. Choi, H. Ko, J. Jeon, G. Saravanakumar, Y. D. Suh, D. S. Lee and J. H. Park, J. Controlled Release, 2015, 200, 158 CrossRef CAS PubMed.
- R. K. O'Reilly, C. J. Hawker and K. L. Wooley, Chem. Soc. Rev., 2006, 35, 1068 RSC.
- E. S. Read and S. P. Armes, Chem. Commun., 2007, 3021 RSC.
- Y.-L. Li, L. Zhu, Z. Liu, R. Cheng, F. Meng, J.-H. Cui, S.-J. Ji and Z. Zhong, Angew. Chem., Int. Ed., 2009, 48, 9914 CrossRef CAS PubMed.
- J. Dai, S. Lin, D. U. Cheng, S. Zou and X. Shuai, Angew. Chem., Int. Ed., 2011, 50, 9404 CrossRef CAS PubMed.
- A. Na Koo, H. Jae Lee, S. Eun Kim, J. H. Chang, C. Park, C. Kim, J. H. Park and S. C. Lee, Chem. Commun., 2008, 6570 RSC.
- R. Sun, Q. Luo, C. Gao, Y. Wang, L. Gao, H. Du, Y. Huang, X. Li, Z. Shen and W. Zhu, Polym. Chem., 2014, 5, 4879 RSC.
- G. Kwon, S. Suwa, M. Yokoyama, T. Okano, Y. Sakurai and K. Kataoka, J. Controlled Release, 1994, 29, 17 CrossRef CAS.
- H. Liu, S. Farrell and K. Uhrich, J. Controlled Release, 2000, 68, 167 CrossRef CAS PubMed.
- R. Savic, A. Eisenberg and D. Maysinger, J. Drug Targeting, 2006, 14, 343 CrossRef CAS PubMed.
- Y. Yao, D. Xu, C. Liu, Y. Guan, J. Zhang, Y. Su, L. Zhao, F. Meng and J. Luo, RSC Adv., 2016, 6, 97684 RSC.
- J.-W. Yoo, N. Doshi and S. Mitragotri, Adv. Drug Delivery Rev., 2011, 63, 1247 CrossRef CAS PubMed.
- V. Mailander and K. Landfester, Biomacromolecules, 2009, 10(9), 2379 CrossRef PubMed.
- Y. Huang, Z. Tang, X. Zhang, H. Yu, H. Sun, X. Pang and X. Chen, Biomacromolecules, 2013, 14, 2023 CrossRef CAS PubMed.
- L. Wu, L. Zhang, G. Shi and C. Ni, Mater. Sci. Eng., C, 2016, 61, 278 CrossRef CAS PubMed.
- Y. Tao, R. Liu, M. Chen, C. Yang and X. Liu, J. Mater. Chem., 2012, 22, 373 RSC.
- J. Liu, F. Zeng and C. Allen, Eur. J. Pharm. Biopharm., 2007, 65, 309 CrossRef CAS PubMed.
- S. H. Kim, J. P. K. Tan, F. Nederberg, K. Fukushima, J. Colson, C. Yang, A. Nelson, Y.-Y. Yang and J. L. Hedrick, Biomaterials, 2010, 31, 8063 CrossRef CAS PubMed.
- Y. Wu, W. Chen, F. Meng, Z. Wang, R. Cheng, C. Deng, H. Liu and Z. Zhong, J. Controlled Release, 2012, 164, 338 CrossRef CAS PubMed.
- Z. Ge and S. Liu, Chem. Soc. Rev., 2013, 42, 7289 RSC.
- P. S. Pramod, R. Shah and M. Jayakannan, Nanoscale, 2015, 7, 6636 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00346c |
| This journal is © The Royal Society of Chemistry 2017 |