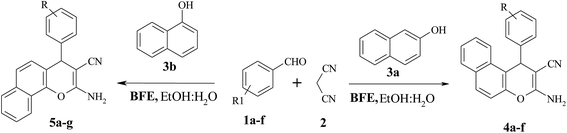
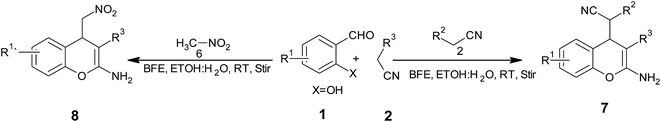
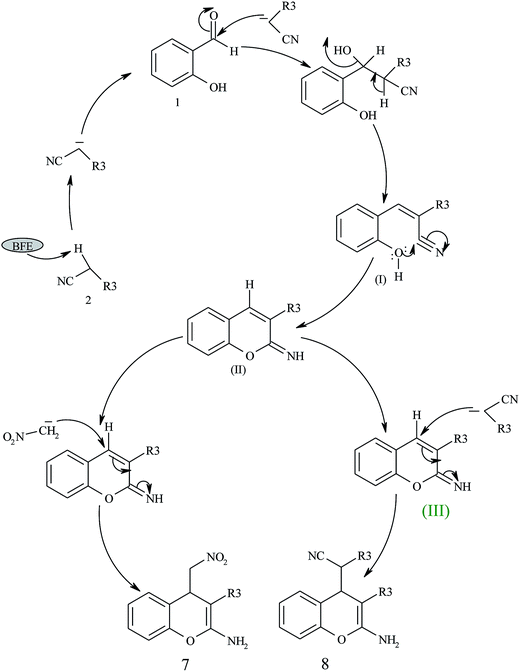
Open Access Article
Open Access ArticleAegle marmelos in heterocyclization: greener, highly efficient, one-pot three-component protocol for the synthesis of highly functionalized 4H-benzochromenes and 4H-chromenes†
Sachin Shinde,
Shashikant Damate,
Smita Morbale,
Megha Patil and
Suresh S. Patil *
*
Synthetic Research Laboratory, PG Department of Chemistry, PDVP College, Tasgaon, Dist. Sangli, - 416312, Affiliated to Shivaji University, Kolhapur, 416004, India. E-mail: sanyujapatil@yahoo.com
First published on 23rd January 2017
Abstract
A facile, one-pot three-component protocol for the synthesis of 2-amino-4H-chromene derivatives has been demonstrated using Bael Fruit Extract (BFE) as a natural catalyst in a green reaction medium. This method offers a mild, efficient and highly economical protocol since the reaction proceeds in natural BFE-catalyst at room temperature under aerobic conditions with a very short reaction time (30 min) under ligand/external catalyst/external promoter-free conditions and, therefore, it is a green and environmentally sound alternative to the existing protocols. The catalyst was obtained by thermal treatment followed by water extraction of the rind of Aegle marmelos (bael) fruit. It was also found to be clean, high-yielding and has the capacity for large scale synthesis.
Introduction
The concept of green chemistry plays an important role in meeting the fundamental scientific challenges of shielding the environment. One of the thrust areas for achieving this target is to investigate alternative reaction media and reaction conditions to carry out the desired chemical transformation with negligible by-products and waste generation as well as elimination of the use of volatile and toxic organic solvents. It is, therefore, of utmost importance to evolve a simple and effective methodology for the different organic transformations that cover the concept of green chemistry.1Multi-component reactions (MCRs) have gained increasing attention for the construction of novel and complex molecular structure because of their environmental-friendly, atom-economy and single-step product formation. This variety can be achieved simply by changing reaction substrate only. For many decades, chemists have been devoting themselves to secure environment by developing new environmental-friendly MCRs for the synthesis of many important biologically active compounds.2
In modern organic chemistry, the improvement of reaction efficiency, the avoidance of toxic reagents, the reduction of waste, and the responsible utilization of our resources have become critical objectives.3,4 By keeping these ideas in mind, a simple and green approach for the synthesis of 4H-benzochromenes and 4H-chromenes has been developed. Bael Fruit Extract (BFE) as a catalyst, ethanol as a solvent and room temperature conditions are enough to afford the 4H-chromene in nearly quantitative yields. Most important of all, the purification procedure is just followed by filtration, washing and drying, and so the waste can be reduced effectively.
4H-Benzochromene and 4H-chromene derivatives have received significant attention in organic chemistry due to their biological and pharmaceutical properties such as antimicrobial,5 antiviral,6 sex pheromone,7 antitumor,8 anti-inflammatory,9 anti-tubercular,10 and cancer therapy.11 Indeed, vegetables and edible fruits are the food resources that are being characterized by natural products, containing chromene moiety in their structure.12
Synthesis of 4H-benzochromenes has been achieved by condensation of aromatic aldehyde, malononitrile and α/β-naphthols in presence of various acid catalysts such as methanesulphonic acid,13 TiCl4,14 H14[NaP5W30O110],15 p-TSA,16 as well as basic catalysts such as γ-alumina,17 Na2CO3,18 K2CO3,19 piperidine,20 nano sized MgO21 and NaOH.22 This reaction was also reported by employing PTCs such as 1-butyl-3-methylimidazolium hydroxide([bmim]OH),23 hexadecyltrimethylammonium bromide (HTMAB),24 cetyltrimethylammonium bromide (CTAB) coupled with ultrasound,25 triethylbenzylammonium chloride (TEBA),26 cetyltrimethylammonium chloride (CTAC),27 and N,N-dimethyl aminoethyl benzyl dimethyl ammonium chloride.28
Several procedures for the multi-component preparation of 2-amino-4H-chromenes have been reported by employing salicylaldehydes and malononitrile or ethylcyanoacetate over the years,29 some of them catalyzed by using Al2O3 (ref. 30) and molecular sieves.31 They are also obtained in the presence of Zr(KPO4)2,32 and amberlyst-A21.33 On the other hand, nitro methyl derivatives have been prepared from salicylaldehydes, nitromethane and malononitrile or ethylcyanoacetate using NaOAc or KF as base catalysts34 and chiral tertiary amine–thioureas.35 In spite of their merits, most of these methods require the use of transition metals, high catalyst loading, long reaction time, highly corrosive, fuming or expensive catalysts, odorous amines, volatile solvents and consumption of energy for heating of the reaction system.36 Therefore, it is not strange that different MCRs strategies have been developed for the synthesis of 4H-chromenes.
Nowadays, synthetic processes involving bio-based catalysts have received much attention as a viable alternative for the development of green methodologies for organic synthesis.37 In this regard, natural resources as a part of the chemical process offer an excellent alternative to a toxic and harmful catalyst is being more environmental-friendly technologies due to their low toxicity, ease of biodegradability, ability to act as a catalyst, and non-corrosive properties as compared to chemical catalysts.38 Also, due to the high natural abundance their production is potentially less expensive.
Therefore, development of eco-friendly protocol using more efficient and safer catalyst under mild conditions at ambient temperature is of great interest. The urgent need for the development of green and sustainable processes for the use of natural ‘feedstocks’ in chemical synthesis as an alternative to hazardous organic solvents or other metal-based catalysts is widely recognized.39
Keeping this idea in mind and in continuation of our interest in application of natural catalysts for organic transformation,40 herein we wish to report a BFE-catalyst as an efficient and eco-friendly catalyst for the synthesis of 2-amino-4H-benzochromenes based on MCR strategy (Scheme 1). The BFE-catalyst was found to be a non-conventional base, highly active, recyclable with good to excellent yields in short reaction time under a green reaction medium from synthetic point of view. To the best of our knowledge, this is the first report on the use of BFE-catalyst for 2-amino-4H-benzochromenes synthesis even for any organic transformations.
The catalytic medium is sourced from the aqueous extract of ash of rind of bael fruit, which is a tree of Indian origin and known from pre-historic time. It has great historic mythological significance for Indians, and has great nutritional, environmental as well as commercial importance. From literature records it is well known that the Aegle marmelos (Linn.) Correa ex Roxb, locally known as bael in India. Bael is a native of India and is also found in Egypt, Sri Lanka, Pakistan, Nepal, Burma, Bangladesh, Vietnam, Laos, Cambodia, Thailand, Malay Peninsula, Java, Timor Leste, Philippines, Ceylon, Island and Fiji.41 Belongs to the Rutaceae family, having tremendous therapeutic potential and traditionally used as anticancer, remedy for chronic diarrhea,42,43 amoebic dysentery,44 antiviral45,46 antifungal drug,47 antipyretic and analgesic48–50 against peptic ulcer51,52 respiratory infections.53,54 The physico-chemical studies have revealed that bael fruit is rich in mineral and vitamin contents. Calcium is the highest mineral (86.68%) present in bael fruit along with Fe, Cu, Zn and Mn in the range of 1.29–15.82% which are reported very essential in muscle contraction, building strong bones and teeth, blood clotting, nerve impulse, transmission, regulating heartbeat and fluid balance within cells.55
In view of this data and in continuation of our ongoing research in the development of green synthetic routes, we thought this amazing fruit ash extract, having alkaline nature, may serve as a better alternative to harmful corrosive catalysts for organic reactions.
During this study, bael fruits were obtained from the local area and species were authenticated by the Department of Botany and the catalyst was obtained by thermal treatment. For this, initially rind of dry fruits (Fig. 1a) was broken into small pieces manually with a knife (Fig. 1b), and then thermally treated at heating rate 2 °C min−1 in muffle furnace to 900 °C and this temperature was maintained for 3 h. The thermal treatment had two parts: in the first part, most of the organic materials were burnt out for 30 minutes, whereas in the second part it was transformed into soft ash (Fig. 1c). Further, the BFE was suspended in distilled water and then carefully stirred for 1 h at room temperature. The mixture was then filtered to get clear extract (Fig. 1d) and denominated as BFE, which was found to be alkaline (pH = 12.6).
 | ||
| Fig. 1 (a) Rind of dry bael fruit, (b) pieces of bael fruit, (c) ash of bael fruit, (d) water extract of BFE. | ||
Results and discussion
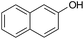
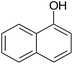
The efficiency of the catalytic medium was evaluated by the reacting naphthols, aromatic aldehyde and malononitrile in BFE in the absence of any other ligands or promoters. Initially we focused on identifying the optimal reaction conditions for our proposed synthetic conversion and these are shown in Table 1. To carry out this synthetic protocol, a model reaction of 4-chlorobenzaldehyde (1.0 mmol), malononitrile (1.0 mmol) and β-naphthol (1.0 mmol) was reacted by applying truly non-toxic conditions using the natural feedstock BFE at room temperature.| Entry | BFE-catalyst (mL) | Solvent (5 mL) | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: β-naphthol (1.0 mmol), 4-chloro benzaldehyde (1.0 mmol), malononitrile (1.0 mmol) in presence of various amount of catalyst in different solvent (5 mL) at RT.b The yields refers to isolated product 4d.c Amount of catalyst 1, 3, 7, 10 mL.d 10 wt% BFE catalyst. | |||||
| 1 | — | — | RT | 120 | — |
| 2 | — | — | Reflux | 120 | — |
| 3 | 5 | — | RT | 120 | 62 (59, 61, 62, 62)c |
| 4 | 5 | — | Reflux | 120 | 62 |
| 5 | 3 | MeOH | RT | 30 | 86 |
| 6 | 3 | EtOH | RT | 30 | 94 |
| 7 | 3 | Toluene | RT | 120 | 31 |
| 8 | 3 | DCM | RT | 120 | 30 |
| 9 | 3 | THF | RT | 120 | 29 |
| 10 | 3 | Acetonitrile | RT | 120 | 43 |
| 11 | 3 | Water | RT | 60 | 54 |
| 12 | 10d | EtOH | RT | 30 | 94 |
We first studied the impact of the amount of catalyst equivalents with respect to the substrate on this reaction. An initial reaction of equimolar quantity of reactants (1 mmol each) was conducted under solvent-free condition at room temperature using 5 mL BFE. We were quite satisfied to see that our reaction proceeded at room temperature and up to 62% product was isolated after a reaction time of 2 h and no further improvement in the yield was observed after increasing or decreasing the catalytic amount (entry 3) even after prolonged reaction time and even after at reflux condition (entry 4).
The result reveals that, examination of solvent system needs improvement in the yield of product. At first, to optimize a suitable reaction medium, the model reaction was performed in presence of different organic solvents such as methanol, ethanol, toluene, DCM, THF and acetonitrile as well as water using catalytic amount of BFE (3 mL) at ambient temperature and results are incorporated in Table 1. In aqueous medium, after 2 h, result of model reaction was poor with 54% product yield (entry 11). However, when ethanol was used as a solvent, considerable enhancement in the conversion of reactants into product was observed after 30 minutes with 94% product yield (entry 6), and pure product was obtained simply by recrystallization without using any chromatographic separation technique. Moreover, when toluene, THF, DCM or acetonitrile were used as solvent, product formation started but the reaction could not proceed satisfactorily and it resulted into lower yield even after 2 h (entries 7–10). Furthermore, we also performed the model reaction directly in presence of bael fruit ash (10 wt%) in ethanol, the result was equally good (entry 12), but separation of catalytic residue from the product became a tedious job.
During the solvent-optimization study, as mentioned above, when water was used as a solvent lower yield of product was obtained, it may be due to immiscibility of reactants in aqueous phase containing active catalyst, while in case of less polar organic solvents like toluene, THF and DCM the catalyst was not homogeneous in the solvent phase containing substrates resulting in poor yield of the product and taking comparatively more time for conversion.
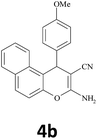
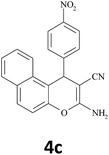
To evaluate the generality of this model reaction, we prepared a range of 2-amino 4H-benzochromene derivatives by reacting α and β-naphthols with a variety of differently substituted aromatic aldehydes and malononitrile under optimized reaction conditions in presence of the BFE-catalyst. The results are shown in Table 2. As shown, aromatic aldehydes with substituent's carrying either electron-donating or electron-withdrawing groups reacted successfully and gave the expected products in high yields. It was found that the aromatic aldehydes with electron-withdrawing groups reacted faster than those with electron-donating groups as was expected.
| Entry | Aldehydes | α/β-Naphthols | Product (4a–f/5a–q) | Time (min) | Yieldb (%) | M. P. (°C) |
|---|---|---|---|---|---|---|
| a Reaction conditions: α/β-naphthols (1 mmol), aldehydes (1 mmol), malononitrile (1 mmol) and BFE-catalyst (3 mL) in EtOH (5 mL) solvent at RT.b Isolated yield based on aldehydes.c Scale up reaction: β-naphthol (100 mmol), 4-chlorobenzaldehyde (100 mmol), and malononitrile (100 mmol), BFE catalyst (10 mL), EtOH (15 mL) at RT. | ||||||
| 1 |  |
 |
 |
20 | 90 | 286–287 |
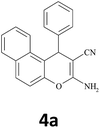
| 2 |  |
 |
 |
30 | 90 | 190–192 |
| 3 | 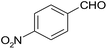 |
 |
 |
20 | 95 | 185–186 |
| 4c | 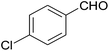 |
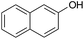 |
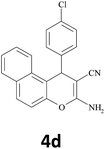 |
30 | 94 | 206–208 |
| 5 | 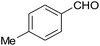 |
 |
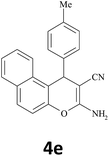 |
35 | 89 | 269–270 |
| 6 |  |
 |
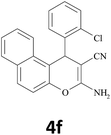 |
15 | 92 | 268–269 |
| 7 | 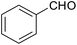 |
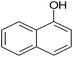 |
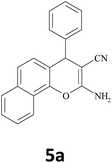 |
15 | 92 | 205–207 |
| 8 | 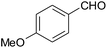 |
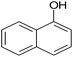 |
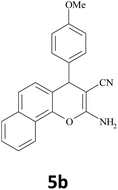 |
30 | 89 | 193–194 |
| 9 | 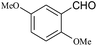 |
 |
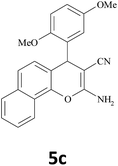 |
30 | 89 | 245–247 |
| 10 |  |
 |
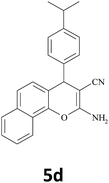 |
25 | 90 | 204–205 |
| 11 | 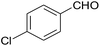 |
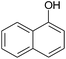 |
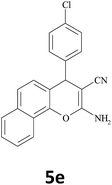 |
15 | 93 | 229–230 |
| 12 | 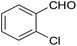 |
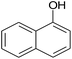 |
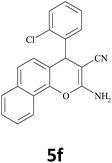 |
15 | 92 | 253–254 |
| 13 | 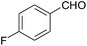 |
 |
 |
45 | 90 | <300 |
| 14 |  |
 |
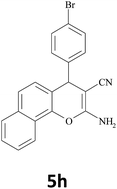 |
35 | 90 | 240–241 |
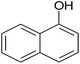
| 15 | 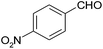 |
 |
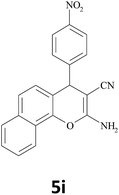 |
10 | 93 | 238–239 |
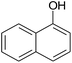
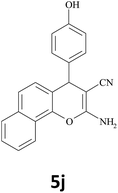
| 16 | 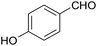 |
 |
 |
35 | 89 | 244–245 |
| 17 | 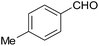 |
 |
 |
30 | 91 | 205–207 |
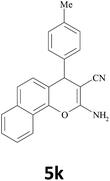
| 18 |  |
 |
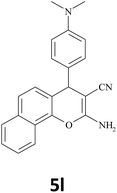 |
25 | 88 | 176–178 |
| 19 | 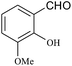 |
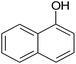 |
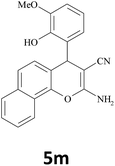 |
30 | 89 | 205–207 |
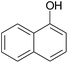
| 20 | 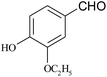 |
 |
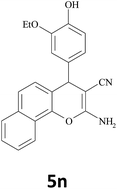 |
35 | 88 | 223–225 |
| 21 | 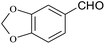 |
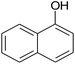 |
 |
30 | 89 | 179–180 |
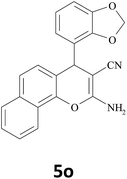
| 22 | 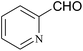 |
 |
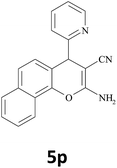 |
45 | 86 | 200–202 |
| 23 | 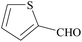 |
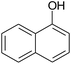 |
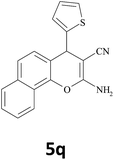 |
30 | 89 | 188–190 |
In addition, the synthesis of 4d derivative was also performed with 100 mmol of 4-chlorobenzaldehyde as mentioned in Table 2. 93% of the desired product (entry 4) was isolated indicating that this method could also be used for large scale synthesis without any undesired products. In all the cases, the products were isolated in pure form and were characterized by IR, 1H NMR, and 13C NMR spectral data (ESI†).
Inspired by these tempting results obtained for cyclocondensation of 4H-benzochromenes, we extended the same protocol for synthesis of 2-amino-4H-chromenes reacting various salicylaldehydes with malononitrile (or ethylcyanoacetate) and nitromethane (Scheme 2). However, the three-component reaction of salicylaldehyde, malononitrile (or ethylcyanoacetate) and nitromethane required longer reaction times compared to the reaction of salicylaldehyde with malononitrile (or ethylcyanoacetate) under similar reaction conditions (Table 3).
| Entry | Aldehydes | R3 | Products | Time (min) | Yieldb (%) | M. P. (°C) |
|---|---|---|---|---|---|---|
| a Reaction conditions: different salicylaldehydes (1 mmol), malononitrile/ethylcyanoacetate (2 mmol) or nitromethane (2.5 mmol), BFE (3 mL) and EtOH (5 mL) at RT.b Isolated yield based on salicylaldehydes. | ||||||
| 1 | 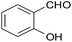 |
–CN | 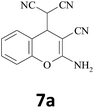 |
20 | 89 | 165–167 |
| 2 | 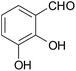 |
–CN | 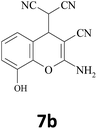 |
25 | 90 | 166–168 |
| 3 | 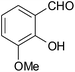 |
–CN | 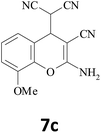 |
20 | 90 | 313–315 |
| 4 | 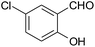 |
–CN | 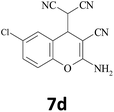 |
20 | 91 | 153–154 |
| 5 | 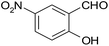 |
–CN | 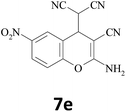 |
10 | 92 | 181–183 |
| 6 | 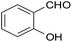 |
–COOEt | 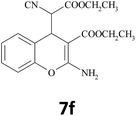 |
15 | 90 | 131–132 |
| 7 | 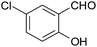 |
–COOEt | 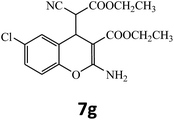 |
15 | 91 | 124–126 |
| 8 | 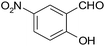 |
–COOEt | 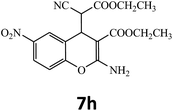 |
10 | 92 | 174–176 |
| 9 | 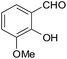 |
–COOEt | 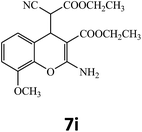 |
25 | 90 | 128–129 |
| 10 | 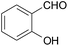 |
–CN | 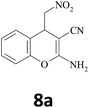 |
60 | 89 | 137–139 |
| 11 | 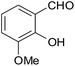 |
–CN | 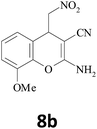 |
50 | 89 | 182–184 |
| 12 | 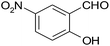 |
–CN | 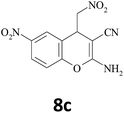 |
45 | 91 | 169–170 |
| 13 | 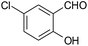 |
–CN | 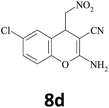 |
45 | 91 | 164–166 |
| 14 | 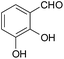 |
–CN | 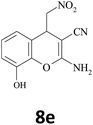 |
50 | 89 | 146–147 |
| 15 | 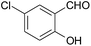 |
–COOEt | 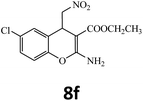 |
45 | 90 | 139–141 |
| 16 | 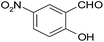 |
–COOEt | 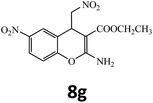 |
30 | 91 | 161–162 |
| 17 | 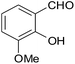 |
–COOEt | 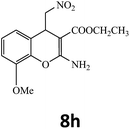 |
50 | 89 | 118–120 |
Recycling of the catalyst
The recyclability of BFE-catalyst was studied for synthesis of 2-amino-4-(4-chlorophenyl)-4H-[f]chromene-3-carbonitrile (4d) under the optimized conditions. After completion of the reaction, the catalyst was separated from reaction mixture by filtration and the filtrate was extracted with ethyl acetate and then applied for repeated reactions under the same reaction conditions (Table 4). To our delight, the catalyst can be reused at least five times with little deactivation.| Run | 1 | 2 | 3 | 4 | 5 |
| Yield | 94 | 94 | 93 | 93 | 91 |
Table 5 compares the efficiency of our method for the synthesis of 2-amino-4H-benzochromene with other reported works. Each of these methods has its own advantages, but some of them suffer from disadvantages such as poor yield, long reaction time, and use of organic solvents and employment of expensive catalyst. So the present method furnishes green reaction medium, takes shorter reaction time, and a small quantity of this inexpensive and readily available catalyst is sufficient to get good yield of the expected product.
| Entry | Catalyst | Solvent (mL) | Temp. (°C) | Time | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| a Present method. | ||||||
| 1 | p-TSA | CH3CN | Reflux | 04 h | 91 | 16 |
| 2 | Me-sulfonic acid | CH3CN | Reflux | 04 h | 91 | 11 |
| 3 | CTACl | H2O | 110 °C | 06 h | 74 | 27 |
| 4 | H14[NaP5W30O110] | H2O | Reflux | 04 h | 91 | 15 |
| 5 | [bmim]OH | H2O | Reflux | 05 min | 96 | 23 |
| 6 | γ-Alumina | H2O | Reflux | 03 h | 84 | 17 |
| 7 | BFEa | EtOH | RT | 30 min | 94 | — |
The dramatic acceleration of 4H-chromenes reaction in BFE is currently not well understood. Literature reports reveal that bael fruit contains calcium, potassium, sodium as major constituents along with a host of other trace elements. Therefore, it is believed that carbonates of calcium, sodium and potassium of bael fruit get transformed into corresponding oxides on thermal treatment and then into hydroxides during water extract which may act as a base catalyst here for 4H-chromenes reactions.
Thus, the alkaline nature of BFE in combination with catalytic activity due to various oxides of metals present in bael fruit,55 proceeded reaction rapidly within short time. To compare the catalytic activity of BFE, we also carried out the model reaction using aqueous solution of above metals oxides (Table 6, entries 1–6). From results, it reveals that, catalyst obtained from bael fruit was found to be excellent with respect to time as well as yield of the product (Table 6, entry 6) suggesting that the strong alkalinity of Bael Fruit Extract (BFE) is essential to promote the reaction efficiency.
| Entry | Catalyst (mL) | pHb | Time (min) | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1.0 mmol), malononitrile (1.0 mmol) and β-naphthol (1.0 mmol) in presence of catalyst (3 mL) in ethanol (5 mL) at room temperature.b pH of 5% solution of alkaline salts.c Isolated yield based on β-naphthol. | ||||
| 1 | CaO | 13.07 | 60 | 56 |
| 2 | ZnO | 12.08 | 60 | 51 |
| 3 | CaCO3 | 11.07 | 60 | 23 |
| 4 | K2CO3 | 11.58 | 60 | 61 |
| 5 | Na2CO3 | 11.86 | 60 | 51 |
| 6 | BFE | 12.6 | 30 | 94 |
A plausible mechanism for the BFE catalyzed synthesis of 2-amino-4H-chromene derivatives has been presented in Scheme 3. The formation of the 2-amino-4H-chromenes proceeds through the simultaneous formation of cyanocinnamonitrile (I) intermediate via Knoevenagel condensation. Subsequently, the intermediates (I) undergo heterocyclization to give the intermediate (III) which on Michael type addition with enolates of active methylene compounds and nitromethane yields corresponding chromene derivatives 6 and 7 respectively.
Conclusion
In conclusion, it is strongly claimed that a very simple, green, and energy-efficient protocol has been developed by us for the synthesis of 4H-benzochromenes and 4H-chromenes with natural sourced base catalyst namely BFE in ethanol at ambient temperature. The advantage of the present protocol is the elimination of corrosive catalysts and toxic reagents. The other benefits include clean reaction profiles, low cost, biodegradable and highly efficient catalyst obtained from renewable resources, reuse of catalyst for several times, excellent yield of product in a very short reaction time. We except the methodology presented will find great utility in academic and industrial applications in the near future.Experimental
Preparation of catalyst
For the preparation of BFE, rind dry Aegle marmelos (bael) fruits were obtained from the local area and species were authenticated by the Department of Botany. The dry rinds (100 g) were broken into small pieces manually and thermally treated at 900 °C to obtain fine soft ash (5.3 g). This ash was then suspended in distilled water (25 mL) in conical flask and carefully stirred for 1 h at room temperature. The mixture was then filtered to get clear extract which was denominated as BFE. The pH of extracts was measured using pH-meter (ProLab 3000 laboratory pH meter) and it was found to be strongly alkaline with pH 12.6.Typical procedure for the preparation of 2-amino-4-(4-chlorophenyl)-4H-[h]chromene (4d) (Table 2, entry 4)
In a typical procedure, 25 mL round-bottom flask was charged with a mixture of 4-chlorobenzaldehyde (0.140 g, 1 mmol), malononitrile (0.066 g, 1 mmol), β-naphthol (0.144 g, 1 mmol) and ethanol (5 mL) and was stirred thoroughly in the presence of BFE (3 mL) at ambient temperature till the completion of reaction as indicated by TLC (ethylacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 2
hexane 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). After completion of the reaction, the reaction mixture was filtered, and the crude product was further purified by recrystallization from 96% ethanol. The aqueous layer of filtrate was separated and extracted with ethyl acetate to check its utility for recyclability of catalyst under the same reaction conditions. The identity of the compound was ascertained based on 1H NMR, 13C NMR, and FT-IR spectroscopy.
8). After completion of the reaction, the reaction mixture was filtered, and the crude product was further purified by recrystallization from 96% ethanol. The aqueous layer of filtrate was separated and extracted with ethyl acetate to check its utility for recyclability of catalyst under the same reaction conditions. The identity of the compound was ascertained based on 1H NMR, 13C NMR, and FT-IR spectroscopy.
Typical procedure for the preparation of (2-amino-3-cyano-4H-benzochromen-4-yl) propane dinitrile (7d) (Table 3, entry 4)
In a typical procedure, 25 mL round-bottom flask was charged with a mixture of 5-chlorosalialdehyde (0.156 g, 1 mmol), malononitrile (0.132 g, 2 mmol) and ethanol (5 mL) and was stirred thoroughly in the presence of BFE (3 mL) at room temperature till the completion of reaction as indicated by TLC (ethylacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 2
hexane 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). After completion of the reaction, the reaction mixture was filtered, and the crude product was further purified by recrystallization from 96% ethanol. The identity of the compound was ascertained based on 1H NMR, 13C NMR, and FT-IR spectroscopy.
8). After completion of the reaction, the reaction mixture was filtered, and the crude product was further purified by recrystallization from 96% ethanol. The identity of the compound was ascertained based on 1H NMR, 13C NMR, and FT-IR spectroscopy.
Acknowledgements
We acknowledge the UGC, New Delhi for funding the Major Research Project, [F. No. 42-379/2013 (SR)] for the financial assistance.References
- (a) J. H. Clark and D. Macquarrie, in Handbook of Green Chemistry and Technology, Blackwell Publishers, Oxford, 2002 Search PubMed; (b) P. Anastas and N. Rghbali, Chem. Soc. Rev., 2010, 39, 301–310 RSC; (c) P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- (a) R. Srivastava and P. Venkataramani, Synth. Commun., 1988, 18, 1537–1544 CrossRef CAS; (b) M. Shen and T. Driver, Org. Lett., 2008, 10, 3367–3370 CrossRef CAS PubMed; (c) K. Bahrami, M. Khodaci and F. Naali, J. Org. Chem., 2008, 73, 6835–6837 CrossRef CAS PubMed; (d) P. Wender, S. Handy and D. Wright, Chem. Ind., 1997, 765, 767–769 Search PubMed; (e) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS; (f) H. Kiyani and F. Ghorbani, J. Saudi Chem. Soc., 2014, 18, 689–701 CrossRef.
- D. B. Ramachary and M. Kishor, J. Org. Chem., 2007, 72, 5056–5058 CrossRef CAS PubMed.
- L. D. S. Yadav, S. Singh and V. K. Rai, Green Chem., 2009, 11, 878–882 RSC.
- M. Khafagy, A. El-Wahas, F. Eid and A. El-Agrody, Farmaco, 2002, 57, 715–722 CrossRef CAS PubMed.
- A. Martinez and L. Marco, Bioorg. Med. Chem. Lett., 1997, 7, 3165–3170 CrossRef.
- G. Bianchi and A. Tava, Agric. Biol. Chem., 1987, 51, 2001–2002 CAS.
- S. Mohr, M. Chirigos and F. Fuhrman, J. Cancer Res., 1975, 35, 3750–3754 CAS.
- F. Toda, K. Tanaka and K. Hamai, J. Chem. Soc., Perkin Trans. 1, 1990, 3207–3209 RSC.
- S. Prado, H. Ledeit, S. Michel, M. Koch, C. Darbord, T. Cole, F. Tillequin and P. Brodin, Bioorg. Med. Chem., 2006, 14, 5423–5428 CrossRef CAS PubMed.
- M. Elinson, A. Dorofeev, F. Miloserdov, M. Ilovaisky, S. Feducovich, P. Belyakov and G. Nikishin, Adv. Synth. Catal., 2008, 350, 591–601 CrossRef CAS.
- M. Curini, G. Cravotto, F. Epifano and G. Giannone, Curr. Med. Chem., 2006, 13, 199–222 CrossRef CAS PubMed.
- M. Heravi, B. Baghernejad and H. Oskooie, J. Chin. Chem. Soc., 2008, 55, 659–662 CrossRef CAS.
- S. Kumar, N. Srinivasula, H. Udupi, B. Rajitha, T. Reddy, N. Reddy and S. Kumar, J. Heterocycl. Chem., 2006, 43, 1691–1693 CrossRef.
- M. Heravi, K. Bakhtiari, V. Zadsirjan, F. Bamoharram and O. Heravi, Bioorg. Med. Chem. Lett., 2007, 17, 4262–4265 CrossRef CAS PubMed.
- B. Baghernejad, M. Heravi and H. Oskooie, J. Korean Chem. Soc., 2009, 53, 631–634 CrossRef CAS.
- R. Maggi, R. Ballini, G. Sartori and R. Sartorio, Tetrahedron Lett., 2004, 45, 2297–2299 CrossRef CAS.
- M. Reza Naimi-Jamal, S. Mashkouri and A. Sharifi, Mol. Diversity, 2010, 14, 473–477 CrossRef PubMed.
- M. Kidwai, S. Saxena, M. Khan and S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295–4298 CrossRef CAS PubMed.
- Q. Zhuang, L. Rong and D. Shi, Chin. J. Org. Chem., 2003, 23, 671–673 CAS.
- D. Kumar, B. Reddy, G. Mishra, K. Rana, N. Nadagouda and S. Varma, Tetrahedron, 2007, 63, 3093–3097 CrossRef CAS.
- A. Zhang, M. Zhang and H. Chen, Synth. Commun., 2007, 37, 231–235 CrossRef CAS.
- K. Gong, H. Wang, D. Fang and Z. Liu, Catal. Commun., 2008, 9, 650–653 CrossRef CAS.
- S. Jin, S. Zhang, B. Liu, Q. Wang and S. Li, Synth. Commun., 2006, 36, 2009–2015 CrossRef.
- S. Jin, C. Xiao, J. Wang and S. Li, Ultrason. Sonochem., 2004, 11, 393–397 Search PubMed.
- D. Shi, S. Zhang, Q. Zhuang and X. Wang, Chin. J. Org. Chem., 2003, 23, 1419–1421 CAS.
- R. Ballini, G. Bosica, A. Mazzacani, M. Conforti, P. Righi, R. Maggi and G. Sartori, Tetrahedron, 2001, 57, 1395–1398 CrossRef CAS.
- L. Chen, J. Huang and J. Zheng, Monatsh. Chem., 2009, 140, 45–47 CrossRef CAS.
- (a) A. Sakurai, Y. Motomura and H. Midorikawa, J. Org. Chem., 1972, 37, 1523–1526 CrossRef CAS; (b) C. O'Callaghan, T. McMurry and J. O'Brian, J. Chem. Soc., Perkin Trans. 1, 1995, 417–420 RSC; (c) J. Volmajer, R. Toplak, I. Leban and A. Le Marechal, Tetrahedron, 2005, 61, 7012–7021 CrossRef CAS; (d) F. Fringuelli, O. Piermatti and F. Pizzo, Synthesis, 2003, 15, 2331–2334 Search PubMed.
- (a) A. Fujimoto and A. Sakurai, Synthesis, 1977, 12, 871–872 CrossRef; (b) D. Shi, X. Wang, S. Tu, C. Yao and Y. Wang, Jiegou Huaxue, 2002, 21, 60 CAS.
- J. Roudier and A. Foucaud, Synthesis, 1984, 2, 159–160 CrossRef.
- M. Curini, F. Epifano, S. Chimichi, F. Montanari, M. Nocchetti and O. Rosati, Tetrahedron Lett., 2005, 46, 3497–3499 CrossRef CAS.
- J. Yadav, B. Reddy, M. Gupta, I. Prathap and S. Pandey, Catal. Commun., 2007, 8, 2208–2211 CrossRef CAS.
- M. Elinson, A. Ilovaisky, V. Merkulova, P. Belyakov, A. Chizhov and G. Nikishin, Tetrahedron, 2010, 66, 4043–4048 CrossRef CAS.
- G. Yang, C. Luo, X. Mu, T. Wang and X. Liu, Chem. Commun., 2012, 48, 5880–5882 RSC.
- (a) C. Marta, A. Filipe, A. Luis, V. Armando and P. Fernanda, J. Org. Chem., 2008, 73, 1954–1962 CrossRef PubMed; (b) M. Elinson, A. Dorofeev, S. Feducovich, S. Gorbunov, R. Nasybullin, N. Stepanov and G. Nikishin, Tetrahedron Lett., 2006, 47, 7629–7633 CrossRef CAS.
- (a) F. He, P. Li, Y. Gu and G. Li, Green Chem., 2009, 11, 1768–1773 Search PubMed; (b) M. Li, C. Chen, F. He and Y. Gu, Adv. Synth. Catal., 2010, 352, 519–530 CrossRef CAS; (c) D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC; (d) B. Zhou, J. Yang, M. Li and Y. Gu, Green Chem., 2011, 13, 2204–2211 RSC; (e) Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC; (f) S. Sun, R. Bai and Y. Gu, Chem.–Eur. J., 2014, 20, 549–558 CrossRef CAS PubMed.
- R. S. Makkar and K. J. Rockne, Environ. Toxicol. Chem., 2003, 22, 2280–2292 CrossRef CAS PubMed.
- W. Leitner, Green Chem., 2003, 11, 603 Search PubMed.
- (a) S. T. Morbale, S. D. Jadhav, M. B. Deshmukh and S. S. Patil, RSC Adv., 2015, 5, 84610–84620 RSC; (b) M. Deshmukh, S. Patil, S. Jadhav and P. Pawar, Synth. Commun., 2012, 42, 1177–1183 CrossRef CAS.
- (a) P. C. Sharma, V. Bhatia, N. Bansal and A. Sharma, Indian J. Nat. Prod. Resour., 2007, 6(2), 171–178 Search PubMed; (b) J. Morton, Bael Fruit, in Fruits of warm climates, Miami, FL, 1987, pp. 187–190 Search PubMed; (c) S. Brijesh, P. Daswani, P. Tetali, N. Antia and T. Birdi, BMC Complementary Altern. Med., 2009, 9, 47 CrossRef CAS PubMed.
- (a) I. Lamproti, D. Martello, N. Bianchi, M. Borgatti, E. Lambertini, R. Piva, S. Jabbar, M. S. Choudhari, M. T. Khan and R. Gambhari, Phytomedicine, 2003, 10(4), 300–308 CrossRef; (b) F. D. Shobha and M. Thomos, J. Ethnopharmacol., 2001, 76(1), 73–76 CrossRef.
- S. K. Roy and R. N. Singh, Punjab Hortic. J., 1980, 20(3–4), 190–197 Search PubMed.
- R. D. Gaur and J. K. Tiwari, Indigenous medicinal plants of Garhwal Himalaya (India): An Ethonobotanical study, in Medicinal and poisonous plant of tropics, ed. A. J. M. Leeuwenberg, International Book Distributors, Dehra Dun, 1988, pp. 139–143 Search PubMed.
- C. P. Kala, Indian J. Tradit. Know., 2006, 5, 537–540 Search PubMed.
- S. Farooq, in 555 Medicinal Plants: Field and Laboratory Mannual, International Book Distributors, Dehara Dun, 2005, pp. 40–42 Search PubMed.
- B. K. Rana, U. P. Singh and V. Tanjeva, J. Ethnopharmacol., 1997, 57(1), 29–34 CrossRef CAS PubMed.
- A. K. Dhiman, in Discussion of Plants, Sacred Plants and their Medicinal Uses, Daya Publication House, New Delhi, 2003, pp. 18–21 Search PubMed.
- V. S. Agarwal, Rural Economics of Medicinal Plants: Vegetation in the Forest, in Drug Plants of India, Kalyani Publishers, New Delhi, 1997, vol. 1, pp. 1, 6, 44, 45, 12, 103, 129, 160 Search PubMed.
- V. S. Agarwal, Economic Plants of India, Kailash Prakashan, Culcutta, 1990, pp. 3–9 Search PubMed.
- R. K. Goel, R. N. Maiti, M. Manickan and A. B. Ray, Indian J. Exp. Biol., 2000, 35(10), 1080–1083 Search PubMed.
- M. Banerji, M. S. Sem and P. G. Datta, Acta Pharm. Hung., 1982, 52(3), 97–101 Search PubMed.
- S. Paricha, Orissa Rev., 2004, 16–17 Search PubMed.
- K. N. Reddy, C. S. Reddy and S. Trimurthulu, Ethonobotanical survey on respiratory disorders in Eastern Ghats of Andhra Pradesh, India, http://www.sis.edu/%7Eebl/leaflets/reddy.htm dated 05/12/2006 Search PubMed.
- (a) P. Piste, S. Didwagh and A. Mokashi, Calcium, and its role in human body, Int. J. Res. Pharm. Biomed. Sci., 2013, 4, 659–668 Search PubMed; (b) C. S. Laddha, S. G. Kunjalwar, P. R. Itankar and M. Tauqeer, Asian J. Pharm. Clin. Res., 2015, 8(1), 76–78 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Complete experimental procedures are provided, including preparation of catalyst, general procedure for synthesis of 2-amino-4H-chromenes and 2-amino-4H-benzochromenes, IR, 1H NMR, and 13C NMR of some representative compounds. See DOI: 10.1039/c6ra28779d |
| This journal is © The Royal Society of Chemistry 2017 |