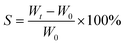Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile fabrication of a magnetic self-healing poly(vinyl alcohol) composite hydrogel
Mingsen Chen,
Guisheng Gong,
Li Zhou* and
Faai Zhang *
*
Guangxi Ministry-Province Jointly-Constructed Cultivation Base for State Key Laboratory of Processing for Nonferrous Metal and Featured Materials, College of Material Science and Engineering, Guilin University of Technology, No. 12 Jiangan Road, Guilin 541004, P. R. China. E-mail: zhangfaai@163.com; zhouli@glut.edu.cn; Fax: +86-773-5897772
First published on 18th April 2017
Abstract
This study proposes a simple method to fabricate a magnetic self-healing poly(vinyl alcohol) (ms-PVA) composite hydrogel. The obtained ms-PVA hydrogel was characterized by vibrating sample magnetometery and transmission electron microscopy. The self-healing property was investigated using a rheometer and tensile strength tests. Results showed that the ms-PVA hydrogel exhibited an excellent self-healing property and controllable magnetic intensity by adjusting the Fe3O4 particle content. The added Fe3O4 particles did not participate in hydrogen bond interactions but did cause the magnetic properties of the hydrogel. The hydrogel showed the best self-healing performance when the amount of Fe3O4 particles was 5 wt% (based on ms-PVA). The ms-PVA composite hydrogel presents a new application of the PVA hydrogel given its facile fabrication process and excellent performance.
1. Introduction
Hydrogels are 3D crosslinked polymeric networks that immobilize large quantities of water (20% to up to a thousand times their dry weight) and remain insoluble because of the presence of crosslinking,1,2 and have been widely applied in a variety of fields, such as biomaterials in wound dressings,3 implants and soft contact lenses,4 and microspheres for controlled drug release.5 However, with the development of functional materials, common hydrogels still cannot meet some specific needs. As a result, functional hydrogels are currently a hot research topic, and a large number of functional hydrogels have been developed,6–9 such as ternary interpenetrating microvascular networks,10 metallo-hydrogels,11 and peptide based hydrogels.12Magnetic hydrogels are a type of promising functional hydrogel with magnetic properties and can be prepared by two main methods:13,14 one is to blend pre-synthesized magnetic iron oxide nanoparticles (MIONs) with hydrogels in solution and to crosslink the MIONs with the polymer, the other is in situ generation of MIONs in the preparation of hydrogels. Magnetic hydrogels have been widely used in biological fields such as magnetic resonance imaging,15 magnetically targeted hyperthermia,16 drug delivery,17,18 and immobilization of proteins,19 which have intrinsic magnetic property and favorable biocompatibility. However, magnetic hydrogel is easily damaged during its application, which may affect the normal use of related products and lead to a short service life.20 Therefore, developing a new types of magnetic hydrogels that automatically heal is needed.
The self-healing concept is derived from the self-healing ability of biology. The human skin could self-heal and regenerate after injury. A material that possesses the self-healing properties can significantly prolong service life and save repair cost.21 However, traditional hydrogel does not possess self-healing properties. Many researchers have reported the preparation of self-healing hydrogels,22,23 which have widely attracted the interests of scientists in designing the internal structure of hydrogel with reversible covalent bonding systems (Diels Alder chemistry24 and acylhydrazone bonds25) and non-covalent bonds (π–π stacking,26 hydrogen bond,27,28 electrostatic interactions,29,30 and hydrophobic interactions31,32). Zhang et al.33 determined that poly(vinyl alcohol) (PVA) hydrogel prepared using the freezing thawing method could self-repair at room temperature without a stimulus or healing agent. The hydrogel exhibited the highest fracture stress reported so far (approximately 200 kPa). Zhang first reported a straightforward method to prepare a novel magnetic hydrogel with healing ability by facilely mixing carboxy-modified Fe3O4 nanoparticles in a dynamic hydrogel composed of chitosan and telechelic difunctional poly(ethylene glycol).20 The results indicated that introducing the self-healing function into magnetic hydrogel was meaningful.
Thus, this study proposes a facile method to prepare magnetic hydrogel with self-healing ability by facilely mixing Fe3O4 particles in the self-healing PVA hydrogel. The effects of Fe3O4 particle content on the self-healing ability, mechanical and swelling, and magnetic properties of the PVA hydrogels are investigated.
2. Experimental
2.1 Materials and methods
Poly(vinyl alcohol) (PVA, Mw = 240 kDa), iron(III) chloride hexahydrate (FeCl3·6H2O) powder, iron(II) ferrous sulfate (FeSO4·7H2O) and ammonia solution (25 wt%) were purchased from Aladdin Chemistry Co. All other reagents were analytical grade and used as received without further purification. Deionized water was used throughout the experiments. The crystal structures of Fe3O4 were characterized using an X-ray diffractometer (XRD, PANalytical. B.V.) at a scanning range 2θ of 10° to 90° and rad of 0.04°. The tensile strength was tested on a universal testing machine (DCS-5000, Shimadzu) according to ISO 527-2:1993 at a constant crosshead speed of 100 mm min−1. The magnetic moment was recorded at 300 K on an MPMS XL-7 vibrating-sample magnetometer (VSM). The thermal decomposition behavior of the polymers was examined by thermogravimetry with a heating rate of 10 K min−1 in nitrogen atmosphere on a model TA Q500 (USA). Rheological date were obtained from an AR1500EX strain-controlled rheometer using a 25 mm parallel plate geometry at 25 °C and analyzed with the rheology advantage data analysis software. Transmission electron microscope (TEM, JEM-2100F from JEOL) with an accelerating voltage of 200 kV was used. The samples were thin-sectioned after drying at room temperature for 3 d.The swelling ratio (S) of ms-PVA hydrogels was calculated as follows:
2.2 Synthesis of magnetic Fe3O4 particles
The Fe3O4 nanoparticles are prepared as follows: first, 12.5 g (0.04 mol) of FeSO4·7H2O and 21.7 g (0.08 mol) of FeCl3·6H2O were dissolved in 50 mL distilled water. Then, ammonia solution (50 mL) was added to the solution with a stirring speed of 1000 rpm under the protection of dry nitrogen at 40 °C to form a homogeneous solution. The color of the solution changed from light brown to black after mixing the solution, which indicated the formation of Fe3O4 nanoparticles. This process was maintained for 30 min, and the temperature was increased to 80 °C to crystallize completely for another 60 min. The precipitate Fe3O4 particles were washed by repeating cycles of centrifugation and redispersion in distilled water. Washing was performed five times in distilled water. The final products were dried in a vacuum oven at 60 °C for 24 h, and the Fe3O4 nanoparticles were finally obtained.2.3 Synthesis of magnetic self-healing poly(vinyl alcohol) (ms-PVA) composite hydrogels
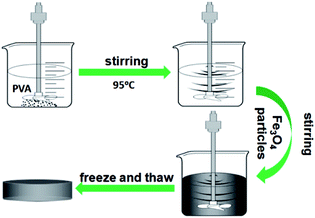
A series of ms-PVA samples was prepared by adjusting the feed amounts of Fe3O4 nanoparticles to investigate the effect of Fe3O4 nanoparticle content on the properties of ms-PVA composite gels (see Table 1). In a typical procedure, 30 g PVA was dissolved in 100 mL distilled water at 95 °C with a stirring speed of 25 rpm for 30 min. A certain amount of Fe3O4 particles was added, and the solution was stirred for 1 h. Then, the homogeneous solution was into a mold of the desired dimension and cooled at −25 °C for 1 h, followed by thawing at room temperature for 1 h. Finally, the ms-PVA composite hydrogels were obtained. Unless otherwise stated, the hydrogel used in this work was prepared with a PVA concentration of 30 wt% and subjected to one freezing thawing cycle.| Sample | PVA (g) | Fe3O4 (g) | H2O (g) |
|---|---|---|---|
| ms-PVA-1 | 30 | 0.75 | 100 |
| ms-PVA-2 | 30 | 1.50 | 100 |
| ms-PVA-3 | 30 | 2.25 | 100 |
| ms-PVA-4 | 30 | 3.00 | 100 |
3. Results and discussion
3.1 Preparation of Fe3O4 particles
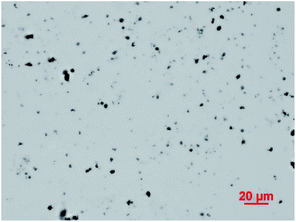
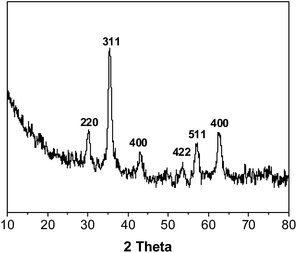
The optical microscope image of Fe3O4 particles dispersed in water is presented in Fig. 1. Fig. 1 shows that magnetic Fe3O4 particles were dispersed uniformly with an average particle size of 1 μm to 5 μm, and a few particles were connected. Fig. 2 shows the XRD pattern of the sample. Six characteristic peaks for Fe3O4 (2θ = 30.1, 35.5, 43.3, 53.4, 57.2 and 62.5) were observed, which indicates the successful preparation of the magnetic Fe3O4 particles.343.2 Preparation of ms-PVA composite hydrogels
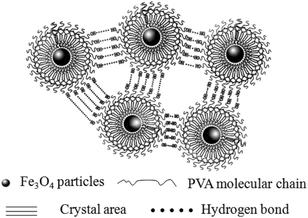
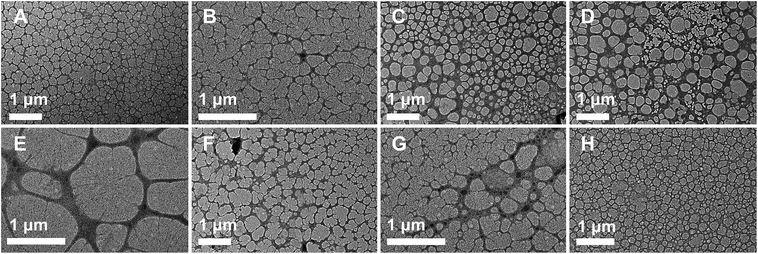
The synthetic route of the preparation of ms-PVA composite hydrogels illustrated in Scheme 1. First, PVA solution and an appropriate amount of Fe3O4 particles were mixed to form a homogeneous solution. Then, the ms-PVA composite hydrogels were fabricated by freezing and thawing the solution. The synthetic protocol was designed based on the following considerations. On one hand, the PVA molecular chain would crosslink each other to form a network structure by intermolecular or intramolecular hydrogen bonding through freezing and thawing; the structure consists of crystalline regions and amorphous region.35 On the other hand, the iron oxide particles could endow PVA with excellent magnetic characteristics and serve as a crosslinker to gelate PVA molecules.36 Moreover, the hydrogels exhibited excellent self-healing property through the reversible hydrogen bond between the hydroxyl groups because of the existence of a large number of hydroxyl groups in the PVA chain (Fig. 3). Fig. 4 shows the TEM images of ms-PVA hydrogels and ones after prolong freezing time. As shown in Fig. 4, PVA hydrogels all display like dried river beds, for neat PVA, a large number of cracks are observed, which provide relatively good swelling properties of the gel. However, with increasing the feeding amounts of Fe3O4 nanoparticles, few cracks are produced, this led to the poor water swelling properties of the hydrogel. In addition, the longer freezing time, the more cracks are formed.3.3 Thermogravimetric analysis
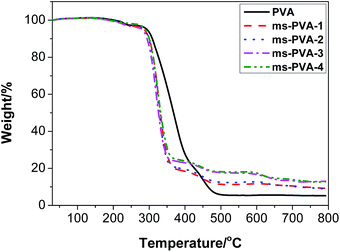
Thermogravimetric analysis was conducted to investigate the stability of the ms-PVA composite hydrogels further as shown in Fig. 5. Neat PVA hydrogel was almost completely decomposed as the temperature approached 500 °C, and its residue weight was 5.4%. However, the composite gels had lower initial decomposition temperatures and higher final decomposition temperatures and residues compared to neat PVA due to the presence of Fe3O4 particles, which resulting in the decrease of the crystallinity of the soft crosslinked gel. The relative differences in the residual weight of composite gels above 500 °C were attributed to the inherently different compositions of Fe3O4 particles.3.4 Magnetic properties
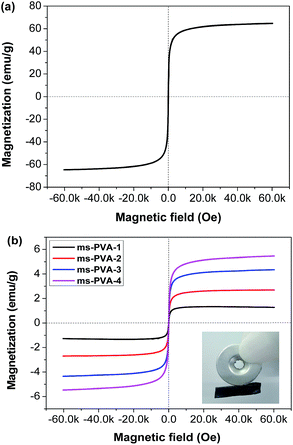
The magnetic properties of Fe3O4 particles (Fig. 6a) and the ms-PVA hydrogels (Fig. 6b) were measured by VSM at 300 K. The magnetization curves showed that Fe3O4 particles and ms-PVA hydrogels were superparamagnetic with no coercivity at room temperature. The actual saturation magnetization values for Fe3O4 particles, ms-PVA-1, ms-PVA-2, ms-PVA-3 and ms-PVA-4 were 64.7, 1.3, 2.7, 4.3 and 5.5 emu g−1, respectively, which were close to the theoretical value (see Table 2). Therefore, the magnetic intensity of the ms-PVA hydrogels can be readily tuned by altering the weight ratio of MIONs. The favorable property of ms-PVA hydrogel promises the remote control property as shown in the inset of Fig. 6b, the circle is the magnet as the external magnetic field, the black is the hydrogel sample, the hydrogel can interact with the magnet under the magnetic effect, so the remote control of the hydrogel can be realized by controlling the shift of the magnet.| Fe3O4 | ms-PVA-1 | ms-PVA-2 | ms-PVA-3 | ms-PVA-4 | |
|---|---|---|---|---|---|
| Saturation magnetization (emu g−1) | 64.7 | 1.3 | 2.7 | 4.3 | 5.5 |
| Fe3O4 content (%, theoretical) | 100 | 2.4 | 4.8 | 7.0 | 9.1 |
| Fe3O4 content (%, actual) | 100 | 2.0 | 4.2 | 6.6 | 8.5 |
3.5 Swelling capacity
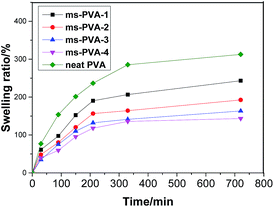
The effect of Fe3O4 content on the swelling absorption of ms-PVA hydrogels was investigated. Fig. 7 shows that Fe3O4 content is an important factor that influences the water absorbency of the composite hydrogels. The increasing Fe3O4 weight ratio from 0.0% to 0.1% caused a decrease in water absorbency because of two factors. First, the interaction of the PVA chains with the Fe3O4 particles may form some low-mobility regions that can act as additional physical crosslinking points, which reduced the swelling ability of the matrix.36 Second, the addition of Fe3O4 particles reduces the content of PVA in hydrogel, which is the main factor influencing the water-absorbing capacity. Therefore, water absorbency decreased with the increase of in Fe3O4 weight ratio.3.6 Rheological analysis
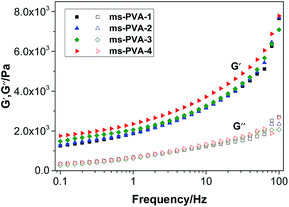
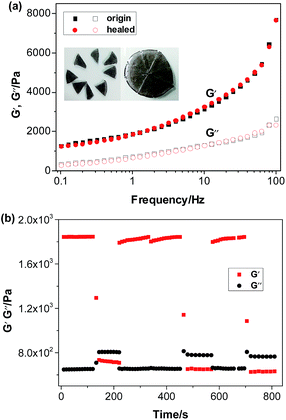
Fig. 8 shows that the value of storage modulus G′ is higher than that of loss modulus G′′, indicating hydrogel formation.37 Moreover, with the increase in frequency (0.1 Hz to 100 Hz, strain (%) = 5) particles, the storage modulus G′ of the hydrogels increased. The polymer chains could not recover from previous movement in high frequency, indicating the formation of the stable structure of the hydrogels, which was not broken down by the mechanical shear force. Furthermore, a higher G′ value was observed for the samples with a high ratio of Fe3O4 particles, indicating that the addition of Fe3O4 particles had a positive effect on the gelation of PVA aqueous solution.
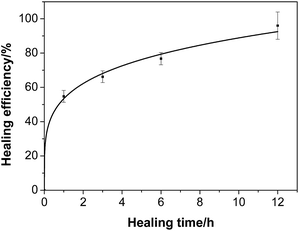
3.7 Self-healing performance
Fe3O4 particles in the hydrogel do not affect the dynamic linkage because the hydrogel network is constructed through a reversible hydrogen bond. Thus, the magnetic hydrogel is self-healable, which is proven by the phenomenon shown in Fig. 12. A piece of the original hydrogels (1) was divided into two pieces using scissors (2) and placed together immediately to insure contact of the freshly created fracture surfaces (3). After 12 h, the self-healing hydrogel was stretched to break with a universal testing machine (4). The hydrogel do not even fracture at the wound (5). Thus, the ms-PVA hydrogels have excellent self-healing performance.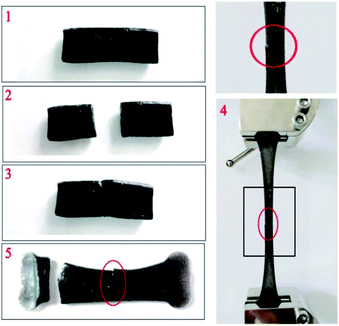 | ||
| Fig. 12 Self-healing process of magnetic hydrogel ((1) original sample; (2) cutting off; (3) healing; (4) stretching; (5) fracture). | ||
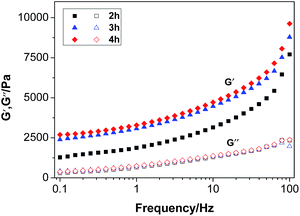
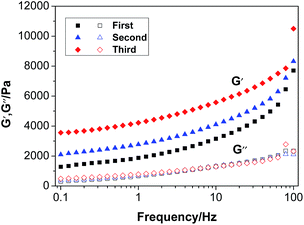
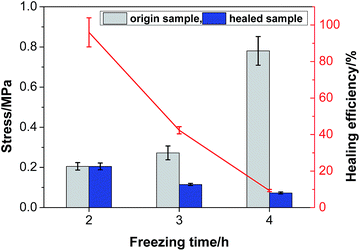
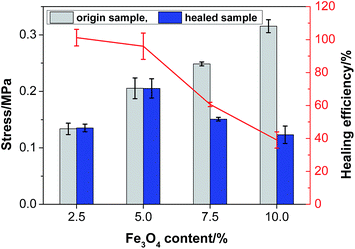
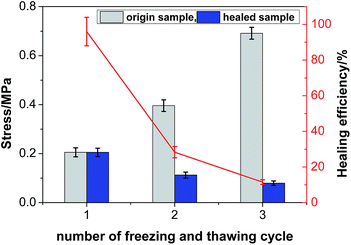
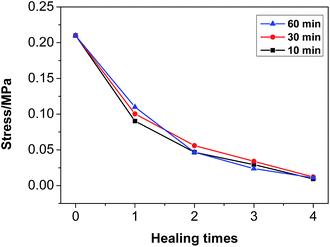
3.8 Mechanical properties
4. Conclusions
In summary, this study proposed an easy and viable strategy to fabricate superparamagnetic self-healing bifunctional PVA composite hydrogels by simply adding magnetic iron oxide particles to physically crosslinked PVA hydrogel by freezing and thawing. The obtained ms-PVA hydrogels show a controllable magnetic property by adjusting the magnetic iron oxide content. Moreover, the addition of magnetic iron oxide particles largely affects the self-healing property of the ms-PVA hydrogel because of its influence on the crosslinked structure of the composite hydrogel. The results highlight the combined advantages of self-healing and magnetic hydrogels, which extend the application of the PVA hydrogel.Acknowledgements
The authors greatly acknowledgement the financial support from the Natural Science Foundation of China (21364003, 51263004).References
- J. Jagur-Grodzinski, Polym. Adv. Technol., 2010, 21, 27–47 CAS.
- M. Ahmad, S. M. Rai and A. Mahmood, Adv. Polym. Technol., 2016, 35, 121–128 CrossRef CAS.
- H.-J. Gwon, Y.-M. Lim, S.-J. An, M.-H. Youn, S.-H. Han, H.-N. Chang and Y.-C. Nho, Korean J. Chem. Eng., 2009, 26, 1686–1688 CrossRef CAS.
- B. S. Fazly Bazzaz, B. Khameneh, M.-m. Jalili-Behabadi, B. Malaekeh-Nikouei and S. A. Mohajeri, Cont. Lens Anterior Eye, 2014, 37, 149–152 CrossRef PubMed.
- M. Montiel-Herrera, A. Gandini, F. M. Goycoolea, N. E. Jacobsen, J. Lizardi-Mendoza, M. Recillas-Mota and W. M. Argüelles-Monal, Carbohydr. Polym., 2015, 128, 220–227 CrossRef CAS PubMed.
- M. Liu, Y. Ishida, Y. Ebina, T. Sasaki, T. Hikima, M. Takata and T. Aida, Nature, 2015, 517, 68–72 CrossRef CAS PubMed.
- F. Song, X. Li, Q. Wang, L. Liao and C. Zhang, J. Biomed. Nanotechnol., 2015, 11, 40–52 CrossRef CAS PubMed.
- Y. J. Che, D. P. Li, Y. L. Liu, Q. L. Ma, Y. B. Tan, Q. Y. Yue and F. J. Meng, RSC Adv., 2016, 6, 106035–106045 RSC.
- M. Criado, J. M. Rey, C. Mijangos and R. Hernandez, RSC Adv., 2016, 6, 105821–105826 RSC.
- C. J. Hansen, S. R. White, N. R. Sottos and J. A. Lewis, Adv. Funct. Mater., 2011, 21, 4320 CrossRef CAS.
- S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2014, 50, 2356–2359 RSC.
- K. Basu, A. Baral, S. Basak, A. Dehsorkhi, J. Nanda, D. Bhunia, S. Ghosh, V. Castelletto, I. W. Hamley and A. Banerjee, Chem. Commun., 2016, 52, 5045–5048 RSC.
- L. Zhou, B. He and F. Zhang, ACS Appl. Mater. Interfaces, 2011, 4, 192–199 Search PubMed.
- G. Gong, F. Zhang, Z. Cheng and L. Zhou, Int. J. Biol. Macromol., 2015, 81, 205–211 CrossRef CAS PubMed.
- A. Fernández-Ferreiro, M. González Barcia, M. Gil-Martínez, A. Vieites-Prado, I. Lema, B. Argibay, J. Blanco Méndez, M. J. Lamas and F. J. Otero-Espinar, Eur. J. Pharm. Biopharm., 2015, 94, 342–351 CrossRef PubMed.
- M. Häring, J. Schiller, J. Mayr, S. Grijalvo, R. Eritja and D. D. Díaz, Gels, 2015, 1, 135–161 CrossRef.
- G. R. Mahdavinia, H. Etemadi and F. Soleymani, Carbohydr. Polym., 2015, 128, 112–121 CrossRef CAS PubMed.
- N. Zhang, J. Lock, A. Sallee and H. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 20987–20998 CAS.
- F.-Y. Chou, C.-M. Shih, M.-C. Tsai, W.-Y. Chiu and S. J. Lue, Polymer, 2012, 53, 2839–2846 CrossRef CAS.
- Y. Zhang, B. Yang, X. Zhang, L. Xu, L. Tao, S. Li and Y. Wei, Chem. Commun., 2012, 48, 9305–9307 RSC.
- S. R. White, N. Sottos, P. Geubelle, J. Moore, M. R. Kessler, S. Sriram, E. Brown and S. Viswanathan, Nature, 2001, 409, 794–797 CrossRef CAS PubMed.
- D. L. Taylor and M. In Het Panhuis, Adv. Mater., 2016, 28, 9060–9093 CrossRef CAS PubMed.
- Y. Zhang, B. Yang, L. Xu, X. Zhang, L. Tao and Y. Wei, Acta Phys.-Chim. Sin., 2013, 71, 485–492 CrossRef CAS.
- Z. Wei, J. H. Yang, X. J. Du, F. Xu, M. Zrinyi, Y. Osada, F. Li and Y. M. Chen, Macromol. Rapid Commun., 2013, 34, 1464–1470 CrossRef CAS PubMed.
- G. Deng, F. Li, H. Yu, F. Liu, C. Liu, W. Sun, H. Jiang and Y. Chen, ACS Macro Lett., 2012, 1, 275–279 CrossRef CAS.
- Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS PubMed.
- A. Phadke, C. Zhang, B. Arman, C.-C. Hsu, R. A. Mashelkar, A. K. Lele, M. J. Tauber, G. Arya and S. Varghese, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4383–4388 CrossRef CAS PubMed.
- M. M. C. Bastings, S. Koudstaal, R. E. Kieltyka, Y. Nakano, A. C. H. Pape, D. A. M. Feyen, F. J. van Slochteren, P. A. Doevendans, J. P. G. Sluijter, E. W. Meijer, S. A. J. Chamuleau and P. Y. W. Dankers, Adv. Healthcare Mater., 2014, 3, 70–78 CrossRef CAS PubMed.
- X. Wang, F. Liu, X. Zheng and J. Sun, Angew. Chem., Int. Ed., 2011, 50, 11378–11381 CrossRef CAS PubMed.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339–343 CrossRef CAS PubMed.
- U. Gulyuz and O. Okay, Soft Matter, 2013, 9, 10287–10293 RSC.
- G. Jiang, C. Liu, X. Liu, G. Zhang, M. Yang, Q. Chen and F. Liu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2010, 47, 335–342 CrossRef CAS.
- H. Zhang, H. Xia and Y. Zhao, ACS Macro Lett., 2012, 1, 1233–1236 CrossRef CAS.
- Y. Zhou, T. Fan, R. Yang, H. Sun and Q. Xia, J. Pharm. Sci., 2007, 16, 187 Search PubMed.
- H. Hong, H. Liao, S. Chen and H. Zhang, Mater. Lett., 2014, 122, 227–229 CrossRef CAS.
- J. S. Gonzalez, C. E. Hoppe, D. Muraca, F. H. Sanchez and V. A. Alvarez, Colloid Polym. Sci., 2011, 289, 1839–1846 CAS.
- S. Li, H.-Y. Lu, Y. Shen and C.-F. Chen, Macromol. Chem. Phys., 2013, 214, 1596–1601 CrossRef CAS.
- P. Sahoo, R. Sankolli, H.-Y. Lee, S. R. Raghavan and P. Dastidar, Chem.–Eur. J., 2012, 18, 8057–8063 CrossRef CAS PubMed.
- N. A. Peppas and S. R. Stauffer, J. Controlled Release, 1991, 16, 305–310 CrossRef CAS.
- M. Lee, H. Bae, S. Lee, N.-o. Chung, H. Lee, S. Choi, S. Hwang and J. Lee, Macromol. Res., 2011, 19, 130–136 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |