Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A zeolite modified carbon paste electrode based on copper exchanged clinoptilolite nanoparticles for voltammetric determination of metronidazole†
Elahe Shahnazari-Shahrezaieab and
Alireza Nezamzadeh-Ejhieh*abc
aDepartment of Chemistry, Shahreza Branch, Islamic Azad University, P.O. Box 311-86145, Shahreza, Isfahan, Iran. E-mail: arnezamzadeh@iaush.ac.ir; Fax: +98 31 53291018; Tel: +98 31 53292515
bYoung Researchers and Elite Club, Shahreza Branch, Islamic Azad University, Shahreza, Iran
cRazi Chemistry Research Center (RCRC), Shahreza Branch, Islamic Azad University, Isfahan, Iran. Tel: +98 31 53292500
First published on 3rd March 2017
Abstract
A simple and effective zeolite modified electrode (ZME) was constructed from Cu(II)-exchanged clinoptilolite nanoparticles (Cu(II)-CNP). The modifier was characterized by FTIR, BET, XRD and TEM techniques. The modified carbon paste electrode (Cu(II)-CNP/CPE) was used for the voltammetric determination of metronidazole (MNZ). The best voltammetric response was obtained by the electrode containing 20% of the modifier in NaCl 0.4 mol L−1 at pH 6. The electrode showed a linear response in the concentration range of 2.0 × 10−8 to 1.6 × 10−6 mol L−1 MNZ with a detection limit of 4.1 × 10−9 mol L−1 from square wave voltammetry. The electrode showed good repeatability, reproducibility, and a long lifetime based on statistical tests. The electrode also has good selectivity and good applicability in the determination of MNZ in some pharmaceutical samples.
1. Introduction
Metronidazole (MNZ), with a broad spectrum of activity against parasitic and bacterial infections and widely use for the treatment of different diseases (trichomonas, giardias, Vincent's organisms, anaerobic bacteria and amebiasis) can cause cancer in animals. Although, there is not enough evidence to confirm its carcinogenic effect on humans, MNZ is banned from veterinary use in the feed of animals.1–3 Accordingly, developing novel methods for the determination of low levels of MNZ is very important in food security, human health and the study of biological toxicity of MNZ. Among the different spectrophotometric, chromatographic and electrochemical methods used for the determination of MNZ,4–7 electrochemical methods are more famous due to their lower cost, higher sensitivity and faster response than other methods. Nevertheless, the nitro group of MNZ, as an electroactive reducible center, has shown poor reproducibility and sensitivity at the bare electrodes.8 In general, modification of electrode surfaces with suitable modifier overcomes to such problems. In recent decades, zeolite-modified electrodes (ZMEs) have been widely used in determination of different organic, inorganic and pharmaceutical compounds. The ZMEs, compared to other chemically-modified electrodes, have the unique size, shape and charge selectivity, due to molecular sieve property of zeolites, with a high cation-exchange capacity (CEC).9–13 High CEC of zeolites permit to load some transition metals with catalytic property into the zeolite for using them in electrocatalytical purpose.In this work, ball-mill clinoptilolite nanoparticles (CNPs) were ion exchanged in Cu(II) aqueous solution and the obtained Cu(II)-CNP was used for modification of carbon paste electrode (CPE). The prepared Cu(II)-CNP/CPE was then used for voltammetric determination of MNZ. Effect of some key operating parameters on the electrode response was studied and optimized.
2. Experimental
2.1. Reagents, preparation and solutions
All chemicals were analytical grade from Merck chemical company and used without further purification. Metronidazole (Batch no. = TM2012127) was purchased from Zhonghan Tianhin Company. Natural Iranian clinoptilolite (Semnan region in the north-east of Iran) was purchased from Afrandtooska Company (Iran). Typical procedure for preparation of and pretreatment of CNPs for removing magnetic and water soluble impurities was illustrated in our previous work.11All solutions were prepared in triply distilled water. Stock solutions of 0.005 mol L−1 MNZ were freshly prepared in water. MNZ pharmaceutical tablets (250 mg) were purchased from Amin, Tehran Chemie, Abidie, Alborz Companies (Iran). Adequate amount of each tablet was well powdered and dissolved in water. The solution was filtered and diluted with water in volumetric flask.
To prepare Cu(II)-CNP, 1 g of CNP was added to 25 mL 0.1, 0.25, 0.4, 0.5, 0.6, 0.8 and 1.0 mol L−1 CuSO4 solutions. The resulted suspensions were shaken on a magnetic stirrer for 12. This was repeated two times to complete ion exchange process and finally centrifuged at 6000 rpm. The filtrate was re-suspended in 2% HCl solution to remove adhered impurities. Typical procedure for digestion of Cu(II)-CNP by HF + HClO4 + HNO3 for atomic spectroscopic determination of Cu(II) was illustrated in our previous work.11
Typical procedure for preparation of the raw and modified CPEs using insulin syringe was illustrated in our previous work.11 The electrode surface was smoothed on a piece of weighing paper and after using it was regenerated by pushing an excess of paste out of the tube, removing the excess, and polishing again mechanically the electrode surface.
For preparation of real samples (tablet, vaginal suppository and ampoule) applied as follows. (A) Each 250 mg metronidazole tablet (obtained from Amin, Abidi, Alborz daru and Tehran Shimi companies, Iran) was dissolved in water and filtered in 250 mL volumetric flask. (B) Metronidazole ampoule was diluted 10 folds and used. (C) 5 g of vaginal gel was dissolved in 50 mL water and filtered in 250 mL volumetric flask. In voltammetric measurements aliquots of each samples was added to the voltammetric cell to achieve desired concentration.
2.2. Apparatus
XRD Bruker diffractometer (D8 Advance, Ni-filtered copper radiation at Kα = 1.5406 Å), Transmission Electron Microscope S-3500 N with Absorbed Electron Detector S-6542 (Hitachi Science System Ltd) and BET (Belsorp Adsorption/Aesorption Data Analysis Software, Japan) are the instruments were used for characterization of samples. The electrochemical experiments were performed with an Autolab PGSTAT101 (Netherland). All voltammograms were recorded with a three-electrode system including an Ag/AgCl (containing 3.5 mol L−1 KCl) reference electrode, a platinum wire as the counter electrode, and the Cu(II)-CNP/CPE as working electrode. The pH of solutions was adjusted using a digital pH meter (Jenway 370).2.3. Voltammetric experiments
Adequate volume of the standard or the measuring MNZ solution was added into voltammetric cell containing 20 mL of NaCl (0.4 mol L−1) as the supporting electrolyte and immediately used after deaerated with N2 for 5 min. The modified electrode, the reference and the counter electrodes were immersed into the measuring solution and CV voltammograms were recorded in the range of 0 to −1 V vs. Ag/AgCl, while N2 was passed over the test solutions. The modified electrode was kept in open air when not in use.3. Results and discussion
3.1. Characterizations studies
The X-ray diffraction (XRD) pattern of the used zeolite nanoparticles is shown in Fig. 1A. This pattern showed good agreement with crystallite data of clinoptilolite (JCPDS no. 39-1383) and hence the most important hkl planes of clinoptilolite were assigned in the pattern.14 Average crystallite size of the raw CNP was estimated about 37 nm by using the Scherrer equation.15 To confirm that nano dimension of zeolite retained after ion exchange process, TEM image of Cu(II)-CNP was recorded and particles size distribution was estimated by applying the image-j software. As shown in Fig. 1B average size of Cu(II)-CNP was about 46 nm.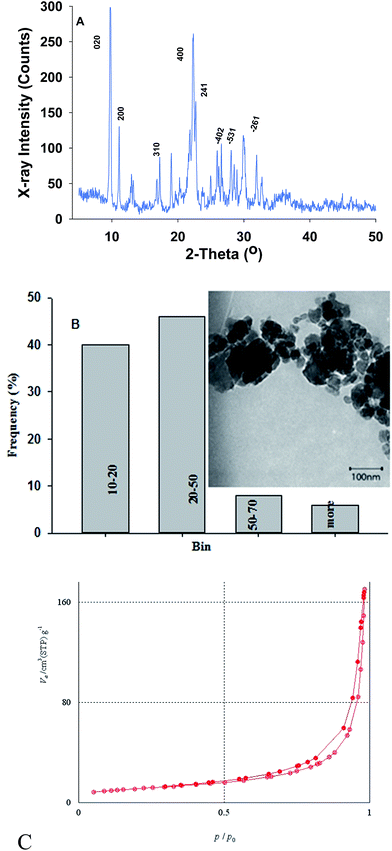 | ||
| Fig. 1 (A) XRD pattern of parent CNP; (B) particles size distribution of the raw CNP (inset: TEM image); (C) adsorption/desorption plot of the modified CNP (Cu(II)0.5-CNP). | ||
The Brunauer–Emmett–Teller (BET) method was used to study of surface properties of the modified CNP sample which of results are shown in Fig. 1C. By using the BET equation surface properties of the samples were estimated from isotherms, which are in accordance with type III according to the IUPAC's rules for the mesoporous structures.16 Comparison of the results with the obtained results for the raw CNP reported in our previous work,11 confirms that SBET (49.0 cm2 g−1), Vp (3.94 cm3 g−1) and dp (28.3 nm) of the raw CNP were changed to 39.7 cm2 g−1, 2.6 cm3 g−1 and 32.8 nm in the Cu(II)-CNP sample, respectively.
The modified CNPs prepared by ion exchanging in solutions containing different Cu(II) concentrations ranging from 0.1 to 0.6 mol L−1 were analyzed by atomic absorption spectroscopy and the corresponding results are summarized in Table 1. Effect of concentration on ion exchange extent of zeolite has illustrated in literature.17 As shown later, the modified CNP prepared in 0.5 mol L−1 Cu(II) solution showed the best voltammetric response and hence it has used in all above characterization techniques.
| Sam. abbreviation | CCu (ion exch. sol.: mol L−1) | Loaded Cu (meq g−1) |
|---|---|---|
| Cu(II)0.1-CNP | 0.10 | 0.25 |
| Cu(II)0.25-CNP | 0.25 | 0.73 |
| Cu(II)0.4-CNP | 0.40 | 1.23 |
| Cu(II)0.5-CNP | 0.50 | 1.73 |
| Cu(II)0.6-CNP | 0.60 | 1.51 |
3.2. Voltammetric measurements
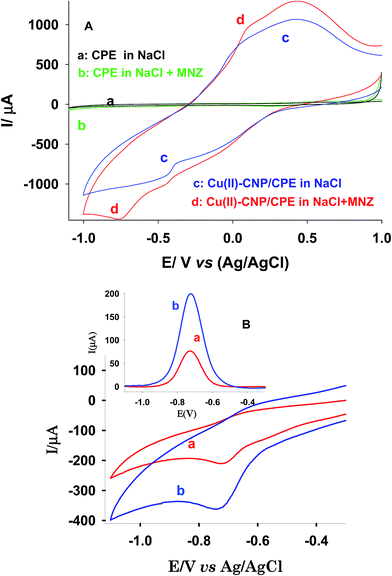
In case of Cu(II)-CNP/CPE in NaCl solution, according to eqn (1), ion exchange between Na(I) cations in supporting electrolyte and Cu(II) cations in the modified zeolite present in CPE, brings Cu(II) cations at the electrode surface. These cations undergo reduction reactions according to reactions (2) and (3). The first reduction begun at 0.304 V and continue till −0.371 V. This reaction show no sharp peak because Cu(II) cations exit from zeolite holes gradually. Produced Cu(I) in reaction (2) can be reduced at more negative potentials (−0.382 V) to metallic Cu.
By addition of MNZ to the solution, peak currents especially the cathodic one increased and hence this change was followed in the next studies. We suggest following phenomena to illustrate our observations. In the first alternative, Cu(I) cations in the electrode–solution interface form a complex with MNZ. On the other hand, produced Cu(I) cations in reaction (2) are instable and immediately form a complex with MNZ. This process favors reactions (1) to (3) hence more Cu(II) cations reach in the electrode–solution interface. Hence, peak current resulted in reactions (2) and (3) belong to free Cu(II) and Cu(I) cations was increased. In addition, produced Cu(I)–MNZ complex can be reduced at more negative potentials about −0.385 V because complexed Cu(I) cations can be reduced more difficult than the free cations. In other alternative, metallic Cu adsorbs some MNZ molecules and form Cu–MNZ complex. In the later step, the internal redox reaction occurs in this complex according to reaction (4). This can produce more Cu(I) species at the electrode–solution interface cause to higher oxidation current in the reverse scan.
| Cu(II)-CNP/CPE + 2Na(s)+ ↔ (Na+)2-CNP/CPE + Cu2+(i) | (1) |
| Cu2+(i) + e− ↔ Cu+(i) | (2) |
| Cu+(i) + e− ↔ Cu(i) | (3) |
| Cu–MNZ(i) ↔ Cu(I)–MNZ(i) | (4) |
In these equations, descriptors z, s and i stand for zeolite, solution, and zeolite–solution interface, respectively.
Effect of particle size of zeolite on the voltammetric behavior of the modified electrodes in the presence of MNZ was studied in CV and square wave voltammetric (SqW) techniques. The best performances obtained in SqW at step potential of 5 mV, amplitude 55 mV and frequency 25 Hz. Typical voltammograms in Fig. 2B shows significant increase in peak current in case of modified CPE with Cu(II)-CNP and hence it was used in the next steps.
Effect of amount of loaded Cu(II) on the voltammetric response of the modified Cu(II)-CNP/CPE electrode in the presence of MNZ was studied (20% modifier, 0.1 mol L−1 NaCl + 0.005 mol L−1 MNZ at pH 5.5). The Cu(II)-CNP modifiers were obtained via ion exchanging of CNPs in Cu(II) solutions with different concentrations of 0.1, 0.25, 0.4 and 0.5 mol L−1. The best voltammetric currents were obtained by the electrode that its modifier prepared in 0.5 mol L−1 Cu(II) solution (Cu(II)0.5-CNP/CPE) in both CV and SqW techniques (see SD1 in ESI data†). Higher concentration of Cu(II) did not studied because of reducing of activity at higher concentrations. Hence this electrode was used in next studies.
Among the modified electrodes containing different dosages of the Cu(II)0.5-CNP modifier (10, 20, 25 and 30%), the best peak currents obtained by the electrode containing 20% of the modifier (see SD2 in ESI data†). At dosage below the optimum values low amounts of the modifier present in the electrode, while at higher dosages above the optimum value resistant of the modified electrode tend to increase, both resulting in peak current decrease.
The modified electrode containing 20% of the Cu(II)0.5-CNP modifier was then use to study of the effect of nature of the supporting electrolyte at the above mentioned conditions. Among the 0.1 mol L−1 of KCl, KNO3, MgCl2, CaCl2, NaCl and NaNO3 supporting electrolytes, the best voltammetric peak current obtained in NaCl supporting electrolyte. Among the different concentrations of NaCl solutions covering the range from 0.1 to 0.5 mol L−1, the best voltammetric peak current obtained in 0.4 mol L−1 NaCl solution (see SD3 and SD4 in ESI data†). In general, ion exchange extent plays an important role in the voltammetric behavior of ZMEs. Ion exchange of ZMEs (and zeolites, in general) significantly depends to nature and concentration of supporting electrolyte, because size and charge density of the cations present in supporting electrolyte and the zeolite affect the ion exchange of zeolitic materials.9–13
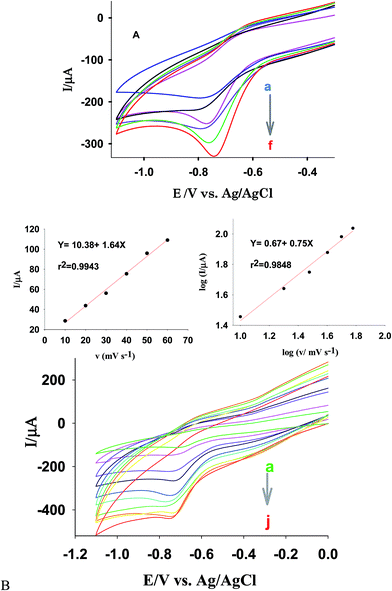
A linear relationship between Ip–ν1/2 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ip–log
Ip–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν in potential scan rate from 10 to 80 mV s−1 was observed with equations of y = 7.5 + 0.7.3x (r2 = 0.9831) and y = 0.65 + 0.58x (r2 = 0.9875), respectively (see SD5†). These confirm the electrode process is controlled by a diffusion process. On the other hand, diffusion of Cu(II) cations from the channels of the zeolite in carbon paste to the electrode surface controls the rate of the electrochemical reaction. It has demonstrated that if the slope of log
ν in potential scan rate from 10 to 80 mV s−1 was observed with equations of y = 7.5 + 0.7.3x (r2 = 0.9831) and y = 0.65 + 0.58x (r2 = 0.9875), respectively (see SD5†). These confirm the electrode process is controlled by a diffusion process. On the other hand, diffusion of Cu(II) cations from the channels of the zeolite in carbon paste to the electrode surface controls the rate of the electrochemical reaction. It has demonstrated that if the slope of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ip–log
Ip–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν plot is 0.5 and 1.0, the electrode reaction is expected to be controlled by diffusion and surface confinement processes, respectively.19–21
ν plot is 0.5 and 1.0, the electrode reaction is expected to be controlled by diffusion and surface confinement processes, respectively.19–21
Similar observations were also obtained in the presence of MNZ (Fig. 3B). So, the linear behavior between Ip–ν (y = 10.38 + 1.65x, r2 = 0.9943) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ip–log
Ip–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν (y = 0.67 + 0.75x, r2 = 0.9848) in the range of 10–60 mV s−1 confirm the electrode response is controlled by an adsorption process. On the other hand in these conditions, MNZ molecules are present at diffusion layer (or adsorbed at the electrode surface) and control electrochemical reaction of Cu(II) cations. While at higher scan rates (70–160 mV s−1) a linear response was observed for plot of Ip–ν1/2 (y = 99.6 + 3.89x, r2 = 0.9928), confirming a diffusion control process at these conditions. On the other hand, at such scan rate region diffusion of MNZ to the electrode–solution interface is controlling factor for the electrode behavior.
ν (y = 0.67 + 0.75x, r2 = 0.9848) in the range of 10–60 mV s−1 confirm the electrode response is controlled by an adsorption process. On the other hand in these conditions, MNZ molecules are present at diffusion layer (or adsorbed at the electrode surface) and control electrochemical reaction of Cu(II) cations. While at higher scan rates (70–160 mV s−1) a linear response was observed for plot of Ip–ν1/2 (y = 99.6 + 3.89x, r2 = 0.9928), confirming a diffusion control process at these conditions. On the other hand, at such scan rate region diffusion of MNZ to the electrode–solution interface is controlling factor for the electrode behavior.
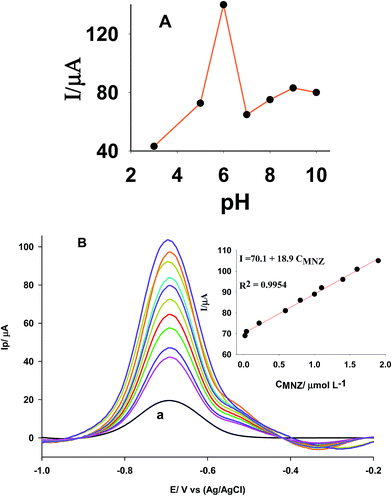
Long term stability of the proposed electrode was investigated by taking its response in a period of 6 months. The electrode was stored in open air when not in use. Voltammetric measurements were performed every week during the mentioned period. The obtained peak currents in each case were averaged based on 5 replicates and the standard deviations compared by statistical g-test. The value of 0.0851 for gexp was smaller than the critical value (g0.05,5,24 = 0.1656), indicating the electrode responses in the mentioned period affected by random errors and no considerable difference is present between the averages at 95% confidence interval.
Good repeatability of the electrode (within electrode variation) was evaluated by the small relative standard deviation of 1.5% in 10 replicate measurements on a single electrode. Similarly, good reproducibility of the electrodes was studied by comparing of the response of 4 similar independent electrodes (between electrode variations). Comparison of gexp of 0.4485 with the critical value (g0.05,5,4 = 0.7212) confirms that the response of these electrodes have not significant difference at 0.95% confidence interval. In long term stability, repeatability and reproducibility experiments the optimized conditions were used (0.4 mol L−1 NaCl + 0.005 mol L−1 MNZ at pH 5.5 20% Cu(II)0.5-CNP modifier, scan rate 70 mV s−1).
Selectivity of the proposed method in the determination of MNZ was studied by measuring MNZ (1.0 × 10−6 mol L−1) in the presence of some various foreign species (see Table 2). Tolerance limit is the maximum concentration of foreign substances that caused relative error about ±5%. Hence, the constructed electrode can be used for determination of MNZ in the presence of used interfering species.
| Species | C (Interf./MNZ) | Interference limit in literature22 |
|---|---|---|
| Mg2+ | 150 | 50 |
| K+ | 460 | 300 |
| Pb2+ | 55 | 20 |
| Zn2+ | 60 | 50 |
| Al3+ | 85 | 20 |
| Ca2+ | 250 | 300 |
| Ni2+ | 250 | — |
| Co2+ | 350 | — |
Practical applicability of the proposed electrode was also tested in determination of MNZ in some pharmaceutical samples. The standard addition method was applied in order to prevent any matrix effects (Table 3). Comparison of t-values with the critical value (t0.05,2 = 4.30) confirms there are no significant differences between the averages obtained by the modified electrode and reference values at 95% confidence interval. Hence, the proposed electrode can be used for the determination of MNZ in real samples.
| MNZ samples | Company | Measured value (n = 3) | Standard values | texp |
|---|---|---|---|---|
| Tablet | Tehran Chemie | 248.8 ± 2.71 mg per tablet | 250 | 0.76 |
| Alborz | 251.2 ± 3.29 mg per tablet | 250 | 0.61 | |
| Amin | 252.6 ± 3.23 mg per tablet | 250 | 1.39 | |
| Abidie | 247.9 ± 3.34 mg per tablet | 250 | 1.08 | |
| Vaginal suppository | Behvazan | 0.75 ± 0.02 g/100 g | 0.75 | 0.91 |
| Injection solution | Iran Samen | 498.4 ± 6.47 mg/100 mL | 500 | 0.42 |
Table 4 shows the characteristics of the proposed modified electrode with respect to some previous modified electrodes in voltammetric determination of MNZ.21–27 As shown, the proposed method has better linear range and detection limit than the most published works.
| Electrode/modifier | Method | LR (mol L−1) | DL (mol L−1) | Ref. |
|---|---|---|---|---|
| Coated GCE | CV | 7 × 10−5 to 8 × 10−4 | 2.3 × 10−6 | 3 |
| DNA/GCE | CV | 1 × 10−7 to 6 × 10−6 | 2 × 10−9 | 7 |
| Gr-IL/GCE | CV | 1 × 10−7 to 2.5 × 10−7 | 1 × 10−8 | 21 |
| Au electrode | CPP | 2 × 10−5 to 8 × 10−4 | 1.5 × 10−7 | 22 |
| 3D GNE | SWV | 1 × 10−9 to 2 × 10−6 | 1 × 10−10 | 23 |
| MIS-CPE | DPSV | 1 × 10−6 to 1 × 10−4 | 3.6 × 10−9 | 24 |
| Cu-poly(Cys)/GCE | LSV/CV | 5 × 10−7 to 4 × 10−4 | 3.7 × 10−7 | 25 |
| MMIP/MGCE | CV/EIS | 5 × 10−8 to 1 × 10−6 | 1.6 × 10−8 | 26 |
| Activated GCE | LSV | 2 × 10−6 to 6 × 10−4 | 1.1 × 10−6 | 27 |
| Cu(II)0.5-CNP/CPE | SqW | 2 × 10−8 to 1.6 × 10−6 | 4.1 × 10−9 | This work |
4. Conclusion
Modified carbon electrode with Cu(II)-exchanged clinoptilolite showed good voltammetric response in the determination of metronidazole in aqueous solution. This is important because nitro group of MNZ which acts as an electroactive reducible center has shown poor reproducibility and sensitivity at the bare electrodes. The electrode process contains reduction of Cu(II)/Cu which produced Cu adsorbs MNZ to form Cu–MNZ complex. In this complex, the internal redox reaction occurs and the obtained Cu(II)–MNZ can reduce at the electrode–solution interface, causing the peak current at more negative potentials. Hence, solution pH played an important role in formation of complex and ion exchange process at the electrode surface, so the best peak current obtained at pH 6. Corresponding peak current of reduction of Cu(II)–MNZ is proportional to CMNZ in solution and hence the proposed electrode can be used for quantitative determination of MNZ in aqueous solutions.References
- H. B. Ammar, M. Brahim, R. Abdelhédi and Y. Samet, Sep. Purif. Technol., 2016, 157, 9–16 CrossRef CAS.
- E. Roy, S. K. Maity, S. Patra, R. Madhuri and P. K. Sharma, RSC Adv., 2014, 4, 32881–32893 RSC.
- C. Li, B. Zheng, T. Zhang, J. Zhao, Y. Gu, X. Yan, Y. Li, W. Liu, G. Feng and Zh. Zhang, RSC Adv., 2016, 6, 45202–45209 RSC.
- J. Adamovics, J. Chromatogr., 1984, 309, 436–440 CrossRef CAS PubMed.
- M. Palomeque, J. A. Garć Bautista, J. V. Garc Mateo and J. Martínez Calatayud, Anal. Chim. Acta, 1999, 401, 229–236 CrossRef CAS.
- K. A. M. Attia, M. W. I. Nassar and M. B. El-Zeiny, Spectrochim. Acta, Part A, 2016, 154, 232–236 CrossRef CAS PubMed.
- X. Jiang and X. Lin, Bioelectrochem., 2006, 68, 206–212 CrossRef CAS PubMed.
- G. Yanga, F. Zhaoa and B. Zeng, Electrochim. Acta, 2014, 135, 154–160 CrossRef.
- A. Bagheri-Ghomi and V. Ashayeri, Iran. J. Catal., 2012, 2(3), 135–140 Search PubMed.
- M. Shabani-Nooshabadi and F. Karimian-Taheri, RSC Adv., 2015, 5, 96601–96610 RSC.
- S. Sharafzadeh and A. Nezamzadeh-Ejhieh, Electrochim. Acta, 2015, 184, 371–380 CrossRef CAS.
- A. T. Lawal and S. B. Adeloju, Talanta, 2013, 114, 191–203 CrossRef CAS PubMed.
- Y. Zhuang, D. G. Zhang and H. Ju, Analyst, 2005, 130, 534–540 RSC.
- M. M. J. Treacy and J. B. Higgins, Elsevier, 2001, 184–189 Search PubMed.
- S. Aghabeygi, R. Kia Kojoori and H. Vakili Azad, Iran. J. Catal., 2016, 6(3), 275–279 Search PubMed.
- M. Inagaki, H. Orikasa and T. Morishita, RSC Adv., 2011, 1, 1620 RSC.
- A. Nezamzadeh, M. Amini and H. Faghihian, Int. J. Electrochem. Sci., 2007, 2, 583–594 CAS.
- J. Peng, C. Hou and X. Hu, Sens. Actuators, B, 2012, 169, 81–87 CrossRef CAS.
- D. K. Gosser, Cyclic Voltammetry, Simulation and Analysis of Reaction Mechanisms, Wiley-VCH, New York, 1993 Search PubMed.
- E. G. D. Kul, M. Gumustas, B. Uslu and S. A. Ozkan, Talanta, 2010, 82, 286–295 CrossRef PubMed.
- N. Erk, Anal. Biochem., 2003, 323, 48–53 CrossRef CAS PubMed.
- B. Rezaei and S. Damiri, Electrochim. Acta, 2010, 55, 1801–1808 CrossRef CAS.
- Y. B. Mollamahale, M. Ghorbani, M. Ghalkhani, M. Vossoughi and A. Dolati, Electrochim. Acta, 2013, 106, 288–292 CrossRef CAS.
- C. Hu, J. Deng, X. Xiao, X. Zhan, K. Huang, N. Xiao and S. Ju, Electrochim. Acta, 2015, 158, 298–305 CrossRef CAS.
- Y. Gu, X. Yan, W. Liu, C. Li, R. Chen, L. Tang, Zh. Zhang and M. Yang, Electrochim. Acta, 2015, 152, 108–116 CrossRef CAS.
- D. Chen, J. Deng, J. Liang, J. Xie, C. Hu and K. Huang, Sens. Actuators, B, 2013, 183, 594–600 CrossRef CAS.
- S. A. Ozkan, Y. Ozkan and Z. Senturk, J. Pharm. Biomed. Anal., 1998, 17, 299–305 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28603h |
| This journal is © The Royal Society of Chemistry 2017 |