Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amino acid-assisted synthesis of In2S3 hierarchical architectures for selective oxidation of aromatic alcohols to aromatic aldehydes†
Tongtong Lia,
Sujuan Zhanga,
Sugang Meng *a,
Xiangju Ye*b,
Xianliang Fu
*a,
Xiangju Ye*b,
Xianliang Fu a and
Shifu Chen*ab
a and
Shifu Chen*ab
aDepartment of Chemistry, Huaibei Normal University, Huaibei, Anhui 235000, People's Republic of China. E-mail: chshifu@chnu.edu.cn; Fax: +86-561-3090518; Tel: +86-561-3806611
bDepartment of Chemistry, University of Science and Technology of Anhui, Fengyang, Anhui 233100, People's Republic of China
First published on 19th January 2017
Abstract
In this work, we report a facile and bioinspired synthesis of mesoporous indium sulfide nanoparticles with flowerlike structures controlled by amino acids. During the synthesis procedure, indium sulfide precipitate was prepared by a simple solvothermal method with different amino acids as the directing and assembling agent. The resulting photocatalyst was characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), UV-vis diffuse reflection spectroscopy (DRS), transmission electron microscopy (TEM), Brunauer–Emmett–Teller (BET) specific surface area, electron spin resonance (ESR) and X-ray photoelectron spectroscopy (XPS). Depending on different amino acids as the crystallization modifiers, the indium sulfide samples exhibit different morphologies. The photocatalytic test of selective oxidation of aromatic alcohols to aromatic aldehydes under visible light irradiation shows that indium sulfide nanoparticles synthesized with addition of amino acids exhibit an increased photoactivity compared to that of In2S3 nanocatalyst without amino acids, which can be attributed to both the relatively high BET surface area and effective separation of photogenerated charge carriers. The effect of various radical scavengers is also investigated. ˙O2− radicals and holes are the main reactive species for photocatalytic oxidation of benzyl alcohol (BA) to benzaldehyde (BAD).
1. Introduction
Selective oxidation of alcohols into corresponding aldehydes and ketones is a vital conversion from the perspective of organic synthesis and industrial manufacturing due to their extensive utilization in manufacture of many spices, vitamins and fragrances.1–4 Many oxidations of this type are carried out using stoichiometric amounts of toxic or corrosive oxidizing reagents (i.e., KMnO4, MnO2, CrO3, Br2, etc.) with considerable demerits such as high energy consumption, redundant byproducts, and serious contamination of the environment.5–9 By contrast, molecular oxygen may serve as superior oxidant with lower cost, greater abundance, and improved safety.10,11 Furthermore, the side reaction can be reduced to the minimum owing to the brief reaction path provided by the alcohol oxidation with unique reaction mechanism.12 Consequently, selective oxidation of alcohols into corresponding aldehydes provides a promising scheme for organic synthesis and commercial manufacture.In recent years, indium sulfide (In2S3), as a typical III–VI group chalcogenide with a band gap of 2.0–2.3 eV, has been the focus of intensive research owing to its photoconductive and luminescence properties. At atmospheric pressure, In2S3 can exhibit three different structure forms, i.e. α-In2S3 (defect cubic structure), β-In2S3 (defect spinel structure obtained in either cubic or tetragonal form), and γ-In2S3 (layered hexagonal structure). Among them, β-In2S3 as an n-type semiconductor plays an increasingly important position owning to its defect spinel structure and corresponding optical, acoustic and electronic properties. It has already inspired many applications in the preparation of green and red phosphors in the manufacture of picture tubes for color televisions, cells, and heterojunction for use in photovoltaic electric generators, photocatalyst for dye degradation, water splitting and selective oxidation.13–17 Many efforts have been devoted to synthesizing β-In2S3 with different morphologies to make better use of the properties for β-In2S3 in photocatalysis.
Recently, with more emphasis paid to green chemistry, the nanotechnology of biosynthesis has attracted considerable attention in materials science due to their effectiveness, flexibility and environmentally friendly pathways for nanoparticle synthesis.18–20 The biomaterial templating technique has been verified to be an efficient approach for directing the synthesis and manufacture of crystalline inorganic materials under environmentally benign conditions.21 Many organisms (such as DNA, viruses, amino acids and sugars) use functional organic biopolymers to aid sequestration of metal species from their environment and direct mineralization of an inorganic phase.22–24
It is known that amino acids, as the basic structural units of proteins, are of particular interest to scholars due to its multifunctional groups (–NH2, and –COOH).25–28 And it can be used for the conjugation of metallic ions or other functional groups. They contain hydrophilic functional groups and have the ability of complex formation with metal ions, which may serve as precursors of inorganic nanomaterials. Therefore, a directing template can be used to control the growth orientation of the nanocrystals, and then the unique morphology of the product may be obtained.29,30 Furthermore, amino acid is easily available and stable.31 Significant efforts have been made in order to develop catalytic systems that are able to efficiently control inorganic crystal growth, especially a crystal growth modifier for semiconductors.32,33 However, up to now, except for cysteine, it is rarely reported that the other different kinds of amino acids are utilized to control crystal growth of semiconductor.
Herein, we report a facile and tunable method for synthesizing mesoporous In2S3 with hierarchical nanoarchitectures through the addition of a small amount of amino acids as the crystal growth modifier in the reaction system, without the introduction of any macromolecular surfactants or capping agents. And this green and simple method would be expected to be a general method for the fabrications of mesoporous metal sulfides with hierarchical nanoarchitectures.
2. Experimental
2.1. Materials
Indium chloride tetrahydrate (InCl3·4H2O), thioacetamide (TAA), amino acids (asparatic acid, serine and glycine), hydrochloric acid (HCl), n-butanol and other chemicals used in the experiments are commercially available. Deionized water was used throughout this study.2.2. Preparation of photocatalysts
In a typical synthesis procedure, 2 mmol InCl3·4H2O and 3.4 mmol amino acid were dissolved in the mixture of 30 mL deionized water and 30 mL butanol in a 100 mL Teflon-lined autoclave. Next, drops of HCl solution were added to adjust the pH value of the solution to about 3. And then, 10 mmol TAA was added into the above solution. After stirring for 30 min, the autoclave was sealed and maintained at 150 °C for 30 h. The orange-red precipitates were collected and washed by deionized water and absolute ethyl alcohol for several times, respectively. The final products were dried in air at 60 °C. Additionally, three different kinds of amino acids (aspartic acid, serine, glycine) have been chosen as additives under the same conditions which were labeled as IS-A, IS-S, and IS-G, respectively. For comparison, In2S3 sample was also synthesized without amino acid, and labeled as IS-N.2.3. Characterization
The phase structures and the crystallite size of the photocatalysts were identified by power X-ray diffraction (XRD) on a Bruker D8 advance X-ray powder diffractometer, with Cu Kα radiation (λ = 1.5406 Å) at a scanning rate of 3° min−1. The accelerating voltage and emission current were 40 kV and 40 mA. The morphology and particle sizes of the photocatalyst were investigated using field-emission scanning electron microscope (FESEM, Carl Zeiss SUPRA 55SAPPHIRE) and the analysis of the composition of the samples was performed with an energy dispersive X-ray (EDX) spectroscope coupled to FESEM. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) images were collected on a Field Emission Transmission Electron Microscope (Tecnai G2 F20 S-Twin, FEI), using an accelerating voltage of 200 kV. UV-vis diffuse reflectance spectroscopy (DRS) were measured with a Hitachi UV-365 spectrophotometer equipped with an integrating sphere assembly, using BaSO4 as a reference material. The data were collected ranging from 400 nm to 800 nm. The Brunauer–Emmett–Teller (BET) surface areas were carried out by N2 adsorption–desorption at 77 K using a Micromeritics ASAP 2460 instrument. XPS experiments were carried out on a RBD upgraded PHI-5000C ESCA system with a Mg Kα radiation (hν = 1253.6 eV) or Al Kα radiation (hν = 1486.6 eV). Binding energies were calibrated by using the containment carbon (C 1s = 284.6 eV). Electron spin resonance (ESR) signals of spin-trapped paramagnetic species with 5,5-dimethyl-L-pyrroline N-oxide (DMPO) were examined using a Bruker E500 spectrometer with a light source of a 300 W xenon lamp (λ > 420 nm).2.4. Photoelectrochemical experiment
The photoelectrochemical analysis was performed in a conventional three-electrode system (CHI-660E, Chenhua Instruments Co., Shanghai, China) using a Pt wire as the counter electrode, a Ag/AgCl electrode as the reference electrode, and the sample as the working electrode with an active area of 0.25 cm2. A 300 W xenon lamp (PLS-SXE 300C, Beijing Perfect light) with a UV cutoff filter (λ > 420 nm) was used as the visible light source.2.5. Evaluation of photocatalytic activity
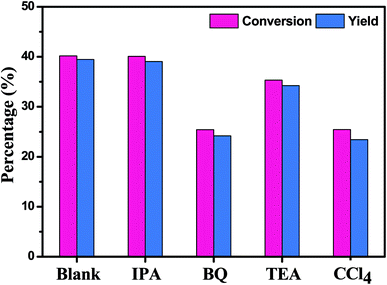
Due to its inertness to oxidation and high solubility for molecular oxygen, benzotrifluoride (BTF) was chosen as the solvent in the experiment.34 The main steps of the photocatalytic selective oxidation of alcohols are as follows: 0.5 mmol alcohol and 80 mg of catalyst were dispersed in 15 mL BTF, and then loaded into a 100 mL of Teflon-lined stainless steel autoclave filled with molecular oxygen at a pressure of 0.1 MPa. A 300 W xenon lamp (PLS-SXE 300C, Beijing Perfect light) with a UV-cut filter (λ > 420 nm, Instrument Company of Nantong, China) was used as a visible light source. In order to maintain the temperature of the reaction solution at about 50 °C, the circulating water was introduced in the reaction system during the illumination. After the reaction, the mixture was centrifuged to remove the photocatalyst particles. The obtained solution was analyzed by a Gas Chromatograph (FuLi-9790) equipped with a SE-30 capillary column (30 m, 0.53 mm, Lanzhou Atech Technologies Co. Ltd). Conversion of alcohol, yield of aldehyde, and selectivity for aldehyde were defined as the follows:| Conversion (%) = [(C0 − Calcohol)/C0] × 100 | (1) |
| Yield (%) = Caldehyde/C0 × 100 | (2) |
| Selectivity (%) = [Caldehyde/(C0 − Calcohol)] × 100 | (3) |
3. Results and discussion
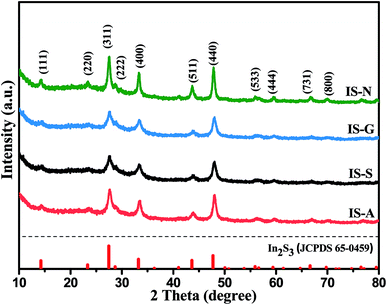
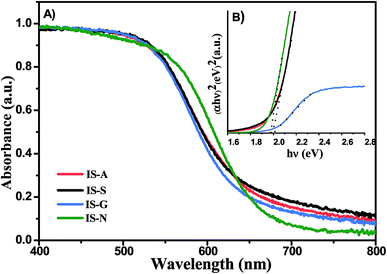
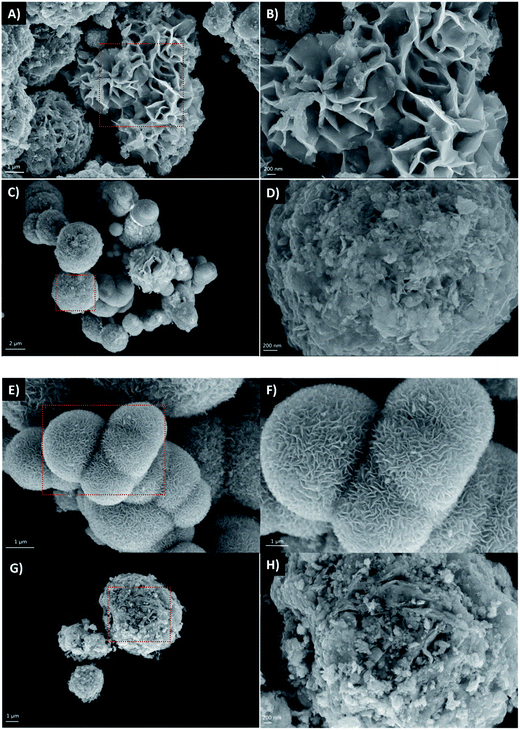
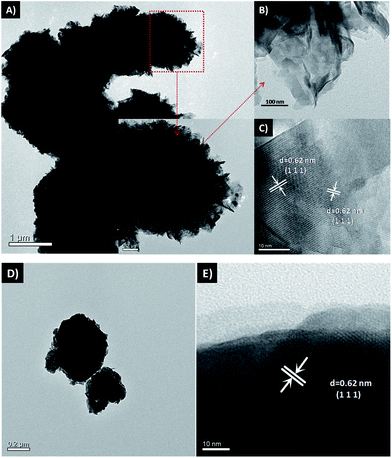
3.1. Characterization of photocatalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
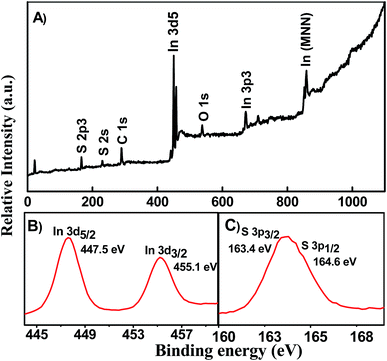
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8, respectively. These results indicate that the In and S are present as In3+ and S2−.39
2.8, respectively. These results indicate that the In and S are present as In3+ and S2−.39
3.2. Evaluation of photocatalytic activity
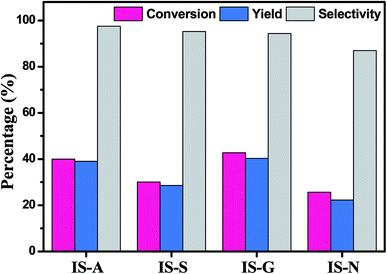 | ||
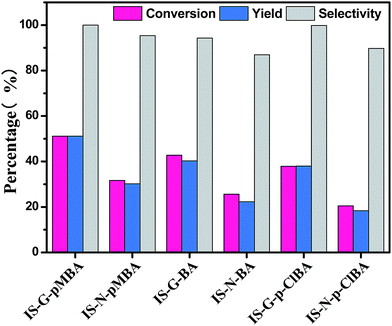
| Fig. 6 Photocatalytic performance for selective oxidation of benzyl alcohol to benzaldehyde using In2S3 (IS-A, IS-S, IS-G, IS-N) samples under visible light irradiation for 2 h. | ||
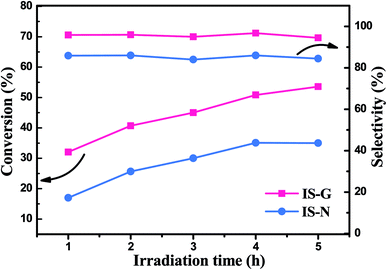
Furthermore, IS-G and IS-N were selected as representative photocatalysts, time-online profile has been shown in Fig. 7. On the whole, it can be clearly seen that the conversion of BA over IS-G and IS-N increases gradually with the extension of time and the former exhibit a better photocatalytic activity at each time point. Besides, the reactive selectivity of the IS-G is also slightly higher than IS-N (approximately maintained at 96 and 86%, respectively) in general.
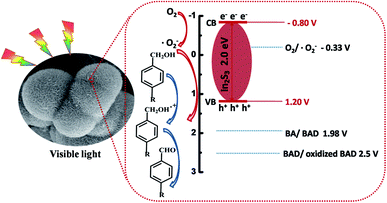
3.3. Proposed photocatalytic mechanism
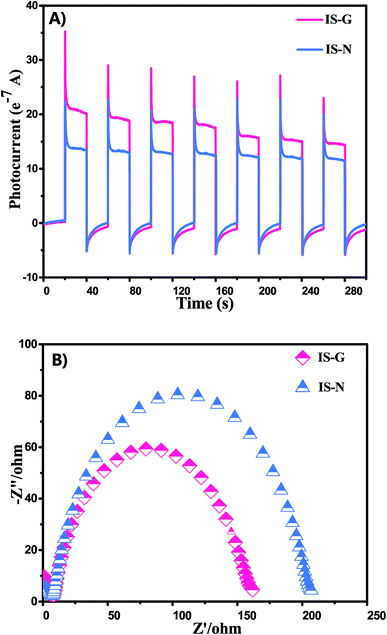
In addition, electrochemical impedance spectroscopy (EIS) has been carried out to further investigate the migration of charge carriers in the electrode materials. Under the similar preparation of the electrodes and electrolyte, the high frequency arc corresponds to the charge transfer limiting process and can be attributed to the charge transfer resistance at the contact interface between the electrode and electrolyte solution.47,48 As shown in Fig. 9B, the IS-G electrode exhibits decreased arc at high frequency under visible light irradiation compared IS-N electrodes. It manifests that the more efficient transfer of charge carriers is obtained over IS-G than that of IS-N.
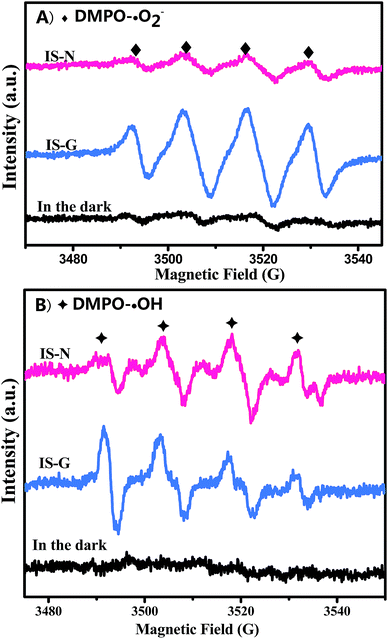
 ) and ˙OH (DMPO–˙OH, labeled with
) and ˙OH (DMPO–˙OH, labeled with  ) signals with irradiation time are shown in Fig. 11A and B, respectively. The characteristic peaks of DMPO–˙O2− adducts50–52 and DMPO–˙OH adducts53 are detected both on IS-G and IS-N systems after visible irradiation for 140 s. The intensity of the formed superoxide radical species in IS-G sample is stronger than that of IS-N, which is in line with a higher photocatalytic performance of IS-G than IS-N on the selective oxidation of benzyl alcohol. Additionally, both of the intensities of the formed hydroxyl radical species in IS-G and IS-N are quite weak, which indicates that there is a slight amount of ˙OH produced in the reaction system. And this result coincides with the former controlled experiments.
) signals with irradiation time are shown in Fig. 11A and B, respectively. The characteristic peaks of DMPO–˙O2− adducts50–52 and DMPO–˙OH adducts53 are detected both on IS-G and IS-N systems after visible irradiation for 140 s. The intensity of the formed superoxide radical species in IS-G sample is stronger than that of IS-N, which is in line with a higher photocatalytic performance of IS-G than IS-N on the selective oxidation of benzyl alcohol. Additionally, both of the intensities of the formed hydroxyl radical species in IS-G and IS-N are quite weak, which indicates that there is a slight amount of ˙OH produced in the reaction system. And this result coincides with the former controlled experiments.
| EVB = X + 0.5Eg − Ee | (4) |
| ECB = EVB − Eg | (5) |
4. Conclusions
In conclusion, amino acids with different functionalities and structural conformation have been used as good crystal growth modifiers for controlled growth of indium sulfide particles by a solvothermal approach. Compared with In2S3 synthesized without amino acids, the flower-like IS-G photocatalyst with nanosheets exhibits better photocatalytic activity toward the selective oxidation of aromatic alcohols to aromatic aldehydes under light irradiation. The highly selective photoactivity is attributed to its big surface area and pore volume, morphology, and highly effective separation of photogenerated charge carriers. According to the results of radical scavenger experiments, the reactive holes and superoxide radicals were suggested to play a critical role in the selective oxidation system. Moreover, the facile solvothermal approach by the introduction of amino acid with a suitable combination of functional groups, such as –NH2 and –COOH, presents a means of achieving well-ordered nanomaterials in a cost-effective and scalable manner.Acknowledgements
This study was supported by the Natural Science Foundation of China (NSFC, grant No. 51472005, 51272081, 51172086, 21603002 and 21473066) and the Natural Science Foundation of Anhui Province (Grant No. 1608085QB37 and 1408085QB38).References
- P. L. Anelli, C. Biffi, F. Montanari and S. Quici, J. Org. Chem., 1987, 52, 2559–2562 CrossRef.
- S. Velusamy and T. Punniyamurthy, Org. Lett., 2004, 6, 217–219 CrossRef CAS PubMed.
- R. V. Jagadeesh, H. Junge, M. M. Pohl, J. Radnik, A. Brückner and M. Beller, J. Am. Chem. Soc., 2013, 135, 10776–10782 CrossRef CAS PubMed.
- X. H. Li, X. C. Wang and M. Antonietti, ACS Catal., 2012, 2, 2082–2086 CrossRef CAS.
- D. I. Enache, J. K. Edwards, P. Landon, B. S. Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362–365 CrossRef CAS PubMed.
- A. Tanaka, K. Hashimoto and H. Kominami, J. Am. Chem. Soc., 2012, 134, 14526–14533 CrossRef CAS PubMed.
- E. Fritz-Langhals, Org. Process Res. Dev., 2005, 9, 577–582 CrossRef CAS.
- N. Jiang and A. J. Ragauskas, J. Org. Chem., 2007, 72, 7030–7033 CrossRef CAS PubMed.
- F. Arena, B. Gumina, A. F. Lombardo, C. Espro, A. Patti, L. Spadaro and L. Spiccia, Appl. Catal., B, 2015, 162, 260–267 CrossRef CAS.
- R. V. Jagadeesh, H. Junge, M. M. Pohl, J. Radnik, A. Brückner and M. Beller, J. Am. Chem. Soc., 2013, 135, 10776–10782 CrossRef CAS PubMed.
- X. H. Li, X. C. Wang and M. Antonietti, ACS Catal., 2012, 2, 2082–2086 CrossRef CAS.
- F. Arena, B. Gumina, A. F. Lombardo, C. Espro, A. Patti, L. Spadaro and L. Spiccia, Appl. Catal., B, 2015, 162, 260–267 CrossRef CAS.
- X. L. Fu, X. X. Wang, Z. X. Chen, Z. Z. Zhang, Z. H. Li, D. Y. C. Leung, L. Wu and X. Z. Fu, Appl. Catal., B, 2010, 95, 393–399 CrossRef CAS.
- Y. H. He, D. Z. Li, G. C. Xiao, W. Chen, Y. B. Chen, M. Sun, H. J. Huang and X. Z. Fu, J. Phys. Chem. C, 2009, 113, 5254–5262 CAS.
- C. Herzog, A. Belaidi, A. Ogacho and T. Dittrich, Energy Environ. Sci., 2009, 2, 962–964 CAS.
- R. Lucena, F. Fresno and J. C. Conesa, Catal. Commun., 2012, 20, 1–5 CrossRef CAS.
- X. Z. Li, B. Weng, N. Zhang and Y. J. Xu, RSC Adv., 2014, 4, 64484–64493 RSC.
- H. J. Liang, T. E. Angelini, J. Ho, P. V. Braun and G. C. L. Wong, J. Am. Chem. Soc., 2003, 125, 11786–11787 CrossRef CAS PubMed.
- C. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, Science, 2004, 303, 213–217 CrossRef CAS PubMed.
- J. L. Zhang, J. M. Du, B. X. Han, Z. M. Liu, T. Jiang and Z. F. Zhang, Angew. Chem., 2006, 45, 1116–1119 CrossRef CAS PubMed.
- A. D. Pomogailo and G. I. Dzhardimalieva, Nanostructured Materials Preparation via Condensation Ways, Springer, 2014, pp. 389–447 Search PubMed.
- Z. Schnepp, Angew. Chem., Int. Ed., 2013, 52, 1096–1108 CrossRef CAS PubMed.
- J. H. Xiang, H. Q. Cao, Q. Z. Wu, S. C. Zhang, X. R. Zhang and A. A. R. Watt, J. Phys. Chem. C, 2008, 112, 3580–3584 CAS.
- B. Zhang, X. C. Ye, W. Y. Hou, Y. Zhao and Y. Xie, J. Phys. Chem. B, 2006, 110, 8978–8985 CrossRef CAS PubMed.
- K. Chang and W. X. Chen, ACS Nano, 2011, 6, 4720–4728 CrossRef PubMed.
- G. J. Zhang, Z. R. Shen, M. Liu, C. H. Guo, P. C. Sun, Z. Y. Yuan, B. H. Li, D. T. Ding and T. H. Chen, J. Phys. Chem. B, 2006, 110, 25782–25790 CrossRef CAS PubMed.
- Z. B. He, S. H. Yu and J. P. Zhu, Chem. Mater., 2005, 17, 2785–2788 CrossRef CAS.
- Q. Z. Wu, H. Q. Cao, Q. Y. Luan, J. Y. Zhang, Z. Wang, J. H. Warner and A. A. R. Watt, Inorg. Chem., 2008, 47, 5882–5888 CrossRef CAS PubMed.
- Q. Z. Wu, H. Q. Cao, Q. Y. Luan, J. Y. Zhang, Z. Wang, J. H. Warner and A. A. R. Watt, Inorg. Chem., 2008, 47, 5882–5888 CrossRef CAS PubMed.
- E. R. Goldman, I. L. Medintz, A. Hayhurst, G. P. Anderson, J. M. Mauro, B. L. Iverson, G. Georgiou and H. Mattoussi, Anal. Chim. Acta, 2005, 534, 63–67 CrossRef CAS.
- F. Jones, M. Mocerino, M. I. Ogden, A. Oliveira and G. M. Parkinson, Cryst. Growth Des., 2005, 5, 2336–2343 CAS.
- C. A. Orme, A. Noy, A. Wierzbicki, M. T. McBride, M. Grantham, H. H. Teng, P. M. Dove and J. J. DeYoreo, Nature, 2001, 411, 775–779 CrossRef CAS PubMed.
- Z. B. He, S. H. Yu and J. P. Zhu, Chem. Mater., 2005, 17, 2785–2788 CrossRef CAS.
- J. Xu, L. Luo, G. Xiao, Z. Zhang, H. Lin, X. Wang and J. Long, ACS Catal., 2014, 4, 3302–3306 CrossRef CAS.
- S. Sakthivel and H. Kisch, Angew. Chem., Int. Ed., 2003, 42, 4908–4911 CrossRef CAS PubMed.
- K. H. Park, K. Jang and S. U. Son, Angew. Chem., Int. Ed., 2006, 45, 4608–4612 CrossRef CAS PubMed.
- S. Rengaraj, S. Venkataraj, C. W. Tai, Y. Kim, E. Repo and M. Sillanpaa, Langmuir, 2011, 27, 5534–5541 CrossRef CAS PubMed.
- J. Shen, B. Yan, M. Shi, H. Ma, N. Li and M. Ye, J. Mater. Chem., 2011, 21, 3415–3421 RSC.
- W. W. Gao, W. X. Liu, Y. H. Leng, X. W. Wang, X. Q. Wang, B. Hu, D. H. Yu, Y. H. Sang and H. Liu, Appl. Catal., B, 2015, 176, 83–90 CrossRef.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- L. Nie, A. Meng, J. Yu and M. Jaroniec, Sci. Rep., 2013, 3, 3215 Search PubMed.
- T. Gao, Q. Li and H. T. H. Wang, Chem. Mater., 2005, 17, 887–892 CrossRef CAS.
- J. Feng, Z. F. Wang, B. Shen, L. M. Zhang, X. Yang and N. Y. He, RSC Adv., 2014, 4, 28683–28690 RSC.
- Y. Liu, P. Zhang, B. Z. Tian and J. L. Zhang, ACS Appl. Mater. Interfaces, 2015, 25, 13849–13858 Search PubMed.
- N. Zhang, S. Q. Liu, X. Z. Fu and Y. J. Xu, J. Mater. Chem., 2012, 22, 5042–5052 RSC.
- Z. R. Tang, Y. H. Zhang and Y. J. Xu, ACS Appl. Mater. Interfaces, 2012, 4, 1512–1520 CAS.
- N. Zhang, Y. Zhang, X. Pan, X. Fu, S. Liu and Y.-J. Xu, J. Phys. Chem. C, 2011, 115, 23501–23511 CAS.
- S. Liu, Z. Chen, N. Zhang, Z. R. Tang and Y. J. Xu, J. Phys. Chem. C, 2013, 117, 8251–8261 CAS.
- L. Q. Ye, J. Y. Liu, C. Q. Gong, L. H. Tian, T. Y. Peng and L. Zan, ACS Catal., 2012, 2, 1677–1683 CrossRef CAS.
- V. Brezova, P. Tarabek, D. Dvoranova, A. Stasko and S. Biskupic, J. Photochem. Photobiol., A, 2003, 155, 179–198 CrossRef CAS.
- F. Chen, Y. Xie, J. He and J. Zhao, J. Photochem. Photobiol., A, 2001, 138, 139–146 CrossRef CAS.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- Y. Li, B. Wen, C. Yu, C. Chen, H. Ji, W. Ma and J. Zhao, Chem.–Eur. J., 2012, 18, 2030–2039 CrossRef CAS PubMed.
- Z. W. Mei, S. X. Ouyang, D. M. Tang, T. Kako, D. Golbergc and J. H. Ye, Dalton Trans., 2013, 42, 2687–2690 RSC.
- X. Y. Xiao, J. Jiang and L. Z. Zhang, Appl. Catal., B, 2013, 142, 487–493 CrossRef.
- N. Zhang, X. Z. Fu and Y. J. Xu, J. Mater. Chem., 2011, 21, 8152–8158 RSC.
- N. Zhang, S. Q. Liu, X. Z. Fu and Y. J. Xu, J. Mater. Chem., 2012, 22, 5042–5052 RSC.
- Y. H. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, Chem. Sci., 2012, 3, 2812–2822 RSC.
- T. Y. Zhao, Z. Y. Liu, K. Nakata, S. Nishimoto, T. Murakami, Y. Zhao, L. Jiang and A. Fujishima, J. Mater. Chem., 2010, 20, 5095–5099 RSC.
- B. Z. Fang, Y. L. Xing, A. Bonakdarpour, S. C. Zhang and D. P. Wilkinson, ACS Sustainable Chem. Eng., 2015, 5, 2381–2388 CrossRef.
- B. Z. Fang, A. Bonakdarpour, K. Reilly, Y. L. Xing, F. Taghipour and D. P. Wilkinson, ACS Appl. Mater. Interfaces, 2014, 6, 15488–15498 CAS.
- L. Y. Chen and Z. D. Zhang, J. Phys. Chem. C, 2008, 112, 18798–18803 CAS.
- L. Y. Chen, Y. G. Zhang, W. Z. Wang and Z. D. Zhang, Eur. J. Inorg. Chem., 2008, 9, 1445–1451 CrossRef.
- K. Chang and W. X. Chen, ACS Nano, 2011, 5, 4720–4728 CrossRef CAS PubMed.
- K. Chang, W. X. Chen and H. Li, Electrochim. Acta, 2011, 56, 2856–2861 CrossRef CAS.
- L. L. Kang, P. Xu, D. T. Chen, B. Zhang, Y. C. Du, X. J. Han, Q. Li and H. L. Wang, J. Phys. Chem. C, 2013, 19, 10007–10012 Search PubMed.
- Z. B. He, S. H. Yu and J. P. Zhu, Chem. Mater., 2005, 11, 2785–2788 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28560k |
| This journal is © The Royal Society of Chemistry 2017 |