Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two impurity energy level regulation leads to enhanced thermoelectric performance of Ag1−xCdxIn5Se8
Xingchen Shena,
Nusrat Shaheena,
Aijuan Zhanga,
Dingfeng Yanga,
Wei Yaoa,
Guoyu Wangb,
Xu Lu*a and
Xiaoyuan Zhou *a
*a
aDepartment of Applied Physics, Chongqing University, Chongqing 400044, P. R. China. E-mail: xiaoyuan2013@cqu.edu.cn; luxu@cqu.edu.cn
bChongqing Institute of Green and Intelligent Technology, Chinese Academy of Science, Chongqing 400714, P. R. China
First published on 22nd February 2017
Abstract
The tetrahedrally bonded diamond-like compound AgIn5Se8 is proved to be a promising thermoelectric material due to its intrinsically low thermal conductivity. For the pristine AgIn5Se8 compound, however, the inferior electrical properties generally result in a limited zT value, owing to its wide band gap, up to 1.1 eV. Here, we report on the synthesis of AgIn5Se8 compound through a quick and convenient solid-state reaction route. Furthermore, the two impurity energy levels are regulated in those doped samples by incorporating Cd2+ ions at Ag+ lattice sites; the electron concentration dramatically increases, resulting in greatly enhanced electrical conductivity and thus a thermoelectric power factor over the entire temperature range. Combined with the intrinsically low lattice thermal conductivity, Cd-doped Ag1−xCdxIn5Se8 with x = 0.05 reaches the highest zT value of 0.63 at 883 K, favorably improved by 40% compared with that of the pristine AgIn5Se8.
1. Introduction
Thermoelectric (TE) materials can convert heat into useful electricity through the Seebeck effect, showing great potential for waste recovery or powering deep-space missions.1–4 The fundamental physics and applications of TE materials have motivated a variety of relevant research areas in physics, chemistry and engineering for decades. The overall performance of TE material is determined by the dimensionless thermoelectric figure of merit zT, which can be defined as zT = S2σT/κ, where S is the Seebeck coefficient, σ the electrical conductivity, T the absolute temperature and κ the thermal conductivity.Given the strong interrelation among the three transport parameters, S, σ and κ, it is difficult to optimize zT values by tuning one of these parameters alone without degrading the other two. The total thermal conductivity is composed of a lattice part and electronic part; fortunately, the lattice part of thermal conductivity is independent of electrical transport properties or power factor σS2, which, to some extent, allows us optimize the electrical transport and lattice thermal conductivity separately. The most common and convenient way to enhance the power factor is to optimize carrier concentration while lattice thermal conductivity can be reduced by solid solution or directly choosing material with high disordered structure.5–7
Traditionally, a good TE material should be a narrow gap semiconductor with band gap around 6–10KBT depending on the operation temperature in order to attain good electrical conductivity and avoid the bipolar effect at high temperature. However, there is no theoretical upper limit for the band gap of TE materials. The preference of narrow band semiconductors for TE application relies on the experimental observation that wide band semiconductors typically have limited solubility for dopants, high lattice thermal conductivity or low carrier mobility. Most recently, the ternary wide gap family AIBIIIC2VI compounds with diamond-like structure (A = Cu, Ag; B = Al, Ga, In; C = S, Se, Te) have been reported to show promising TE properties.8 The lattice thermal conductivity (κl) of those compounds is fairly low due to the strong phonon scatting by the ordered vacancy defects or highly distorted crystal structures.8–15 Besides, the three constituent elements give numerous choices of dopants, providing great opportunity to tune electrical transport properties.8,16,17
The ternary compound AgIn5Se8 belongs to the AIB5IIIC8VI – type wide band gap semiconductor family, which is regarded as being composed of an In2Se3-based (In2Se3)2(AgInSe2) or (In2Se3)2.5(Ag2Se)0.5 pseudo binary alloy.18 Besides, the structure of these ternary compound is analogous to B2IIIC3VI – type crystalline structures,19,20 which allows the incorporated foreign elements to take the most stable valence state,20 resulting in the reduction in band gap. This is caused by the preference of Ag or Cu incorporation in α-In2Se3, favoring the formation of Ag2Se or Cu2Se slab.18,21 The crystal structure and thermoelectric properties of the novel ternary AgIn5Se8 were first reported by Cui et al.,18 who investigated the thermoelectric performance of the pristine AgIn5Se8 compounds with different annealing time. In this study, in order to explore the possibility of further enhancing the electrical conductivity, we investigate and regulate the thermoelectric properties of ternary compound Ag1−xCdxIn5Se8 through Cd doping in addition to the intrinsic Se vacancy. The highest zT value of 0.63 at 883 K was achieved for the x = 0.05 sample, which is greatly improved by 40% in contrast with that of the pristine AgIn5Se8.
2. Experimental procedure
Alloys of composition Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05) samples were synthesized by melting and annealing procedure. Firstly, high purity elements Ag (granular, 99.99999%), In (silk, 99.99999%), Se (granular, 99.99999%), Cd (granular, 99.99999%) were weighed in stoichiometric quantities, followed by mixing and putting into quartz tubes. Secondly, all the tubes were evacuated by vacuum pump to 10−4 Pa, and then quickly were sealed. Thirdly, the tubes were put into the muffle furnace, slowly heated from room temperature to 1273 K in 14 hours, and kept at this temperature for 15 hours. Subsequently, the samples were cooled down to 873 K in 6 hours and held for 20 hours. Thereafter, the samples were left for natural cooling. The obtained ingots were grinded into fine powders and annealed for 30 hours at 923 K. Finally, these annealed fine powders were consolidated by Spark Plasma Sintering (SPS) at 873 K for 5 minutes under the pressure of 45 MPa in an Ar-flow atmosphere. The final products were confirmed to have 98% of theoretical density by using the Archimedes method.The composition phase analysis was performed by using X-ray diffraction (XRD). Powder X-ray diffraction (XRD) patterns of all samples after SPS were collected using a PANalytical X'Pert apparatus with Cu Kα radiation. The XRD data was analyzed by the commercial software JADE 8.0.
The thermoelectric properties of the samples were measured in the temperature range of 300–883 K. The electrical conductivity (σ) and Seebeck coefficient (σ) were measured by Linseis LSR-3 system, while the thermal conductivity were calculated from the formula κ = λρCp. The thermal diffusivity (λ) was gained by Netzsch LFA 457 lasher flash system, and the heat capacity (Cp) value was measured by a differential scanning calorimeter, Netzsch, 404 F3. The Hall carrier concentration (nH) and Hall mobility (uH) were calculated from Hall coefficient (RH) at room temperature based on the equations  and uH = RHσ, which was characterized by a home-made Hall system. The absorption coefficients were obtained by using a UV-vis spectrophotometer apparatus (Shimadzu UV-3600), and a strong absorption is observed at about 1127 nm when the photon wavelength is scanned from 300 nm to 1500 nm.
and uH = RHσ, which was characterized by a home-made Hall system. The absorption coefficients were obtained by using a UV-vis spectrophotometer apparatus (Shimadzu UV-3600), and a strong absorption is observed at about 1127 nm when the photon wavelength is scanned from 300 nm to 1500 nm.
3. Results and discussions
3.1 Crystal structure and phase analysis
AgIn5Se8 crystallizes in the space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m with unit cell parameters: a = 6.1952 Å, b = 6.1952 Å, c = 12.419 Å, V = 390.08 Å3. The cation Ag+ and In3+ are orderly positioned in the network of Se2− lattices, in which both In3+ (In1, In2) and Se2− (Se1, Se2) occupy two inequivalent sites. The whole structure can be well described as a frame work of distorted AgSe4 and InSe4 tetrahedrons connected by common faces. As an ordered vacancy compound in chalcopyrite family, AgIn5Se8 possesses a cation deficiency. Namely, each anion is coordinated by two In atoms, one Ag atom while each cation is coordinated by four anions. Fig. 1 shows atomic positions of AgIn5Se8 projected along the b axis. As can be seen, the structure is characterized by a close packed and alternatively arranged Se12− and Se22− anion lattice, completed by the Ag, In1 and In2 cations. The atomic sites on In1 planes are totally occupied while those on the Ag and In2 planes are partially occupied, leaving voids along the 010 direction, as shown in Fig. 1.22
2m with unit cell parameters: a = 6.1952 Å, b = 6.1952 Å, c = 12.419 Å, V = 390.08 Å3. The cation Ag+ and In3+ are orderly positioned in the network of Se2− lattices, in which both In3+ (In1, In2) and Se2− (Se1, Se2) occupy two inequivalent sites. The whole structure can be well described as a frame work of distorted AgSe4 and InSe4 tetrahedrons connected by common faces. As an ordered vacancy compound in chalcopyrite family, AgIn5Se8 possesses a cation deficiency. Namely, each anion is coordinated by two In atoms, one Ag atom while each cation is coordinated by four anions. Fig. 1 shows atomic positions of AgIn5Se8 projected along the b axis. As can be seen, the structure is characterized by a close packed and alternatively arranged Se12− and Se22− anion lattice, completed by the Ag, In1 and In2 cations. The atomic sites on In1 planes are totally occupied while those on the Ag and In2 planes are partially occupied, leaving voids along the 010 direction, as shown in Fig. 1.22
Fig. 2(a) displays the room temperature X-ray diffraction pattern of Ag1−xCdxIn5Se8 with nominal stoichiometric component (x = 0.0, 0.01, 0.03, 0.05) after SPS. Room temperature XRD analysis for the polycrystalline sample AgxCd1−xIn5Se8 indicate that the samples are single phase. We observe that all peaks of the samples can be indexed with the standard AgIn5Se8 card (PDF #76-1439, space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m, No. 111), and no impurity peaks were found.
2m, No. 111), and no impurity peaks were found.
Fig. 2(b) shows the photon energy dependence of the relational expression of the Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05) in terms of (αhν)2 = A(hν − Eg). In this relational expression, α stands for the absorption coefficient, h signifies the Planck constant, hν stands for the photon energy and A is a constant. By extracting the intercept on the horizontal axis of the function (αhν)2 = A(hν − Eg), we obtained the band gap of the tested AgIn5Se8 is 1.1 eV, which is consistent with the previous study.18,23
3.2 Electrical transport properties
The electrical transport properties are shown in Fig. 3. Fig. 3(a) displays the temperature dependence of the electrical conductivity of Ag1−xCdxIn5Se8 with nominal stoichiometric component (x = 0.0, 0.01, 0.03, 0.05). In this figure, the pristine AgIn5Se8 shows a relatively low electrical conductivity at room temperature and the electrical conductivity for each of sample increases with increasing temperature as a result of more carriers being activated.By substituting the Cd atoms for Ag atoms, the carrier concentration increases rapidly with respect to Cd content due to the extra valence electron provided by Cd atom, which is confirmed by the results of Hall measurement shown in Fig. 3(d). The carrier concentration of pristine AgIn5Se8 is only 1.8 × 1016 cm−3 while that of x = 0.05 sample is 2.3 × 1019 cm−3, which is more than 3 orders of magnitude enhancement. Consequently, the dramatically increased carrier concentration leads to the significantly enhanced electrical conductivity. Specifically, the electrical conductivity is raised by 78 times when x = 0.05 as compared with pristine compound. As shown in Fig. 3(d), the mobility of all the Ag1−xCdxIn5Se8 samples at room temperature drops sharply with respect to Cd content, from 498 cm2 V−1 s−1 for pristine AgIn5Se8 to 31 cm2 V−1 s−1 for Ag0.95Cd0.05In5Se8 sample. The reduction in carrier mobility can be attributed to the enhanced point defect scattering caused by the heavy doping, which largely shortens the mean free path of the carriers. This is probably the reason for the diminished electrical conductivity of doped samples at higher temperature.24
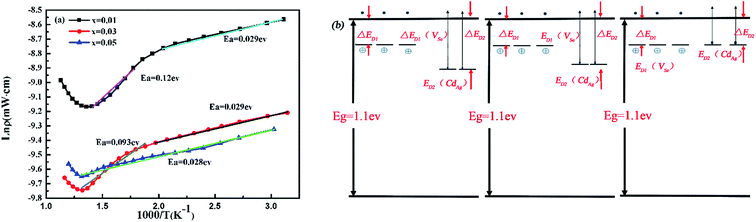
By carefully checking the curves of electrical conductivity, we observe two segments with varied slopes in the temperature dependence of electrical conductivity. In order to understand the intrinsic mechanism for the abrupt electrical conductivity enhancement of the doped samples Ag1−xCdxIn5Se8 (x = 0.01, 0.03, 0.05), the Arrhenius equation is applied to explain these different behaviors. Herein, the activation energy (Ea) for carriers is used in the Arrhenius eqn (1),25 which means the energy barrier the electrons need to overcome for conducting. Fig. 4(a) displays the Arrhenius plot with linear fit for Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05). For x = 0.01 and x = 0.03 samples, the same activation energy 0.029 eV from room temperature to 473 K is identified, which may be ascribed to the shallow donor levels created by Se vacancy (VSe).26 While above 473 K, the activation energies of these two samples are found to be 0.12 eV, 0.093 eV, resulting from the deeper donor states caused by the Cd impurities (CdAg).27 Nevertheless, only one donor level can be identified with activation energy of 0.028 eV in the whole measured temperature range for x = 0.05 sample. Here, we assume that the donor impurity level of the Cd may gradually move close to the conduction band edge as the Cd concentration increasing. When x = 0.05, the donor level of Cd may coincide with the impurity level of the selenium vacancy. The corresponding variation of donor impurity level with rising Cd concentration in Ag1−xCdxIn5Se8 samples is schematically displayed in Fig. 4(b). The additional CdAg impurity level located below the energy level of Se vacancy (VSe), provides more electrons for conducting in Ag1−xCdxIn5Se8 compounds, yielding a rapidly increased carrier concentration and thus the enhanced electrical conductivity, as compared with the pristine AgIn5Se8 the with only one VSe impurity level. The temperature for CdAg impurity level starting to function gradually shifts to room temperature with increasing Cd content, indicating the movement of CdAg impurity level towards bottom of conducting band. As a result, associated with the intrinsic Se vacancy impurity level, the two impurity levels regulation leads to the enhanced electrical performance of Ag1−xCdxIn5Se8 over the entire temperature.
 | (1) |
Fig. 3(b) displays the temperature dependence of the Seebeck coefficient of Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05). For all these samples, negative values of the Seebeck coefficients are observed from 300 to 883 K, indicating that the major carrier of these Ag1−xCdxIn5Se8 samples are electrons. At room temperature, the absolute Seebeck values decrease from 358 to 97 μV K−1 with the Cd concentration increasing, which is consistent with the general inverse relationship between electrical conductivity and Seebeck coefficient. The Seebeck coefficient of the pristine AgIn5Se8 firstly increases with lifted temperature, and then decreases as the temperature continues to raise, which is typical for non-degenerate semiconductors with low carrier concentration.
Compared with the pristine samples, the Cd doped samples shows entirely different temperature dependence behavior. Here, the eqn (2) based on a single parabolic band model is employed to provide a general understanding of the change in Seebeck coefficients of degenerate semiconductors as those Cd doped samples.28,29 The relatively lightly doped sample with x = 0.01 and x = 0.03 exhibit three segments in the curve of Seebeck coefficients with respect to temperature. Initially, the Seebeck coefficients increases with the increasing temperature since the carrier concentration in this region increases slowly and the Seebeck coefficient is mainly governed by the temperature. Then the Seebeck coefficient shows a slight decrease from 500 to 700 K due to the rapidly increased carrier concentration caused by the activation of the deep impurity donor level through lifted temperature. In this case, a large number of carriers are activated, playing a dominant role in the Seebeck coefficient change, which is given by the relationship  . After that, the Seebeck coefficients of these two samples continue to arise most of the carriers on the deep impurity level have been activated for transport and temperature dominates again for determining the absolute value of Seebeck coefficients. For the x = 0.05 sample, the Seebeck coefficient monotonically increases as a function of temperature, revealing a highly degenerate semiconductor behavior.
. After that, the Seebeck coefficients of these two samples continue to arise most of the carriers on the deep impurity level have been activated for transport and temperature dominates again for determining the absolute value of Seebeck coefficients. For the x = 0.05 sample, the Seebeck coefficient monotonically increases as a function of temperature, revealing a highly degenerate semiconductor behavior.
 | (2) |
Combining the electrical conductivity with Seebeck coefficient, the maximum power factor of 0.42 mW m−1 K−2 is obtained when the Cd content reaches x = 0.05 at 883 K, which is larger than that of the pristine AgIn5Se8 sample (0.31 mW m−1 K−2 at 883 K), as shown in Fig. 3(c). The enhanced power factor is attained primarily through the optimization of carrier concentration, regulated by the contributions from two impurity levels.
3.3 Thermal transport properties
Fig. 5(a) presents the temperature dependence of the total thermal conductivity κ of the Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05) samples. As temperature increases, from 300 to 883 K, the total thermal conductivity rapidly decreases, where the Umklapp process dominates. In order to investigate the intrinsic lattice thermal conductivity, the electronic contribution, which is estimated by the Wiedemann–Franz law κe = LσT, is subtracted from the total thermal conductivity. The Lorenz number L is calculated through eqn (3)–(5), based on a single parabolic model,30
 | (3) |
 | (4) |
After deriving the reduced Fermi energy ξ from the eqn (3). The Lorenz number can be calculated from the following formula (5).
 | (5) |
Fig. 5(b) plots the electronic thermal conductivity as a function of the temperature by using the calculated Lorenz number. It seems that the electronic contribution should be taken into account only for the heavily doped samples (x = 0.03 and 0.05) at high temperature. As shown in Fig. 5(c), the lattice thermal conductivity of all the samples roughly follows a T−1 law, indicating the predominant phonon scattering mechanism here is phonon–phonon scattering.30 In addition, it is important to note that the minimum lattice thermal conductivity of 0.6 W m−1 K−2 obtained in this study is among the lowest values for wide band gap diamond-like semiconductors.12,16,17
Combining the electronic transport and the total thermal conductivity data, we obtained the temperature dependence of the dimensionless thermoelectric figure of merit of the Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05) samples, shown in Fig. 5(d). Apparently, for all the samples, the zT values monotonously increase with the increasing temperature in the measured temperature range. Also, it is found that the zT value in the doped samples shows a significant increase with increasing Cd content due to the enhancement of the power factor and the slightly reduced lattice thermal conductivity in spite of the enlarged electronic thermal conductivity. The maximum zT value achieved in this study is 0.63 at 883 K for x = 0.05 sample, which is 40% enhancement compared with that of pristine sample. Similar thermoelectric performance was found in previous study,18 in which we believe the long-term annealing process (more than 30 days) would produce more Se vacancy in those compounds and give rise to similar carrier concentration to our best sample.
4. Conclusion
In summary, the n-type Ag1−xCdxIn5Se8 (x = 0.0, 0.01, 0.03, 0.05) samples were synthesized via the solid state reaction method followed by the spark plasma sintering technique. The thermoelectric performance of Cd-doped Ag1−xCdxIn5Se8 compounds has been investigated from room temperature to 883 K. With increasing Cd content, the regulation of two impurity levels arising from Cd and Se vacancy results in a rapidly increased carrier concentration, leading to a maximum power factor of 0.42 mW m−1 K−2 for x = 0.05 sample. Furthermore, the lattice thermal conductivity of Ag1−xCdxIn5Se8 samples is found to be around 0.6 W m−1 K−1, which is comparable to that of the other state of art TE materials with diamond-like structure. As a result, the maximum zT of 0.63 at 883 K is attained for Ag0.95Cd0.05In5Se8. Although the zT value obtained in this study is not as high as the mainstream TE materials, it is demonstrated here that the wide band gap semiconductors with diamond-like structure can possess low lattice thermal conductivity at high temperature. After carefully doping, those materials can also have moderate power factor, which implies the great potential in TE application. To further enhance the thermoelectric performance AgIn5Se8, more effective doping elements or method should be investigated to avoid the diminished carrier mobility.Acknowledgements
This work was financially supported in part by the National Natural Science Foundation of China (Grant No. 11344010, 11404044, 51472036), the Fundamental Research Funds for the Central Universities (106112016CDJZR308808). The work conducted at the Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences is supported by the 100 Talent Program of the Chinese Academy of Sciences (Grant No. 2013-46), the National Natural Science Foundation of China (Grant No. 51672270, 51401202), and the Project for Fundamental and Frontier Research in Chongqing (CSTC2015JCYJBX0026).References
- B. C. Sales, Science, 2002, 295, 1248–1249 CrossRef CAS PubMed.
- F. J. DiSalvo, Science, 1999, 285, 703–706 CrossRef CAS PubMed.
- L. E. Bell, Science, 2008, 321, 1457–1461 CrossRef CAS PubMed.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105–114 CrossRef CAS PubMed.
- Y. Z. Pei, A. D. LaLonde, N. A. Heinz, X. Y. Shi, S. Iwanaga, H. Wang, L. D. Chen and G. J. Snyder, Adv. Mater., 2011, 23, 5674–5678 CrossRef CAS PubMed.
- Y. Z. Pei, A. F. May and G. J. Snyder, Adv. Energy Mater., 2011, 1, 291–296 CrossRef CAS.
- X. Lu, W. Yao, G. W. Wang, X. Y. Zhou, D. Morelli, Y. S. Zhang, H. Chi, S. Hui and C. Uher, J. Mater. Chem. A, 2016, 4, 17096–17103 CAS.
- R. H. Liu, L. L. Xi, H. L. Liu, X. Shi, W. Q. Zhang and L. D. Chen, Chem. Commun., 2012, 48, 3818–3820 RSC.
- Y. Dong, A. R. Khabibullin, K. Wei, Z. H. Ge, J. Martin, J. R. Salvador, L. M. Woods and G. S. Nolas, Appl. Phys. Lett., 2014, 104, 252107 CrossRef.
- K. Kurosaki, H. Matsumoto, A. Charoenphakdee, S. Yamanaka, M. Ishimaru and Y. Hirotsu, Appl. Phys. Lett., 2008, 93, 012101 CrossRef.
- T. Plirdpring, K. Kurosaki, A. Kosuga, M. Ishimaru, A. Harnwunggmoung, T. Sugahara, Y. Ohishi, H. Muta and S. Yamanaka, Appl. Phys. Lett., 2011, 98, 172104 CrossRef.
- M. L. Liu, I. W. Chen, F. Q. Huang and L. D. Chen, Adv. Mater., 2009, 21, 3808–3812 CrossRef CAS.
- X. Y. Shi, F. Q. Huang, M. L. Liu and L. D. Chen, Appl. Phys. Lett., 2009, 94, 122103 CrossRef.
- C. Sevik and T. Cagin, Appl. Phys. Lett., 2009, 95, 112105 CrossRef.
- X. Y. Shi, L. L. Xi, J. Fan, W. Q. Zhang and L. D. Chen, Chem. Mater., 2010, 22, 6029–6031 CrossRef CAS.
- A. Yusufu, K. Kurosaki, A. Kosuga, T. Sugahara, Y. Ohishi, H. Muta and S. Yamanaka, Appl. Phys. Lett., 2011, 99, 061902 CrossRef.
- T. Plirdpring, K. Kurosaki, A. Kosuga, T. Day, S. Firdosy, V. Ravi, G. J. Snyder, A. Harnwunggmoung, T. Sugahara, Y. Ohishi, H. Muta and S. Yamanaka, Adv. Mater., 2012, 24, 3622–3626 CrossRef CAS PubMed.
- J. L. Cui, Y. Y. Li, Y. Deng, Q. S. Meng, Y. L. Gao, H. Zhou and Y. P. Li, Intermetallics, 2012, 31, 217–224 CrossRef CAS.
- T. Colakoglu, M. Parlak and S. Ozder, J. Non-Cryst. Solids, 2008, 354, 3630–3636 CrossRef CAS.
- T. Colakoglu, M. Parlak, M. Kulakci and R. Turan, J. Phys. D: Appl. Phys., 2008, 41, 115308 CrossRef.
- J. L. Cui, X. J. Zhang, Y. Deng, H. Fu, Y. M. Yan, Y. L. Gao and Y. Y. Li, Scr. Mater., 2011, 64, 510–512 CrossRef CAS.
- P. Benoit, P. Charpin and C. Djegamariadassou, Mater. Res. Bull., 1983, 18, 1047–1057 CrossRef CAS.
- L. Y. Huang, H. Xu, Y. P. Li, H. M. Li, X. N. Cheng, J. X. Xia, Y. G. Xu and G. B. Cai, Dalton Trans., 2013, 42, 8606–8616 RSC.
- C. G. Fu, S. Q. Bai, Y. T. Liu, Y. S. Tang, L. D. Chen, X. B. Zhao and T. J. Zhu, Nat. Commun., 2015, 6, 8144 CrossRef PubMed.
- K. S. Weldert, W. G. Zeier, T. W. Day, M. Panthofer, G. J. Snyder and W. Tremel, J. Am. Chem. Soc., 2014, 136, 12035–12040 CrossRef CAS PubMed.
- H. Y. Ueng and H. L. Hwang, J. Phys. Chem. Solids, 1989, 50, 1297–1305 CrossRef CAS.
- H. Y. Ueng and H. L. Hwang, J. Phys. Chem. Solids, 1990, 51, 11–18 CrossRef CAS.
- C. Xiao, Z. Li, K. Li, P. C. Huang and Y. Xie, Acc. Chem. Res., 2014, 47, 1287–1295 CrossRef CAS PubMed.
- J. P. Heremans, V. Jovovic, E. S. Toberer, A. Saramat, K. Kurosaki, A. Charoenphakdee, S. Yamanaka and G. J. Snyder, Science, 2008, 321, 554–557 CrossRef CAS PubMed.
- Q. F. Chen, G. W. Wang, A. J. Zhang, D. F. Yang, W. Yao, K. L. Peng, Y. C. Yan, X. N. Sun, A. P. Liu, G. Y. Wang and X. Y. Zhou, J. Mater. Chem. C, 2015, 3, 12273–12280 RSC.
| This journal is © The Royal Society of Chemistry 2017 |