DOI:
10.1039/C6RA28136B
(Paper)
RSC Adv., 2017,
7, 6972-6980
A facile, simple, and inexpensive ionic liquid, 1-alkyl-3-methylimidazole chloride, as ligand for the iron(III)-mediated reverse atom transfer radical polymerization of methyl methacrylate
Received
13th December 2016
, Accepted 5th January 2017
First published on 20th January 2017
Abstract
Over the past few years, ionic liquids (ILs) have been widely reported as reaction media for reverse atom transfer radical polymerization (ATRP). In particular, almost all the ILs with the structure [Hmim][RCOO] (R represents an alkyl group) have been used as ligands for a reverse ATRP system. However, the facile, simple and low-cost ILs, 1-alkyl-3-methylimidazole chloride ([Rmim][Cl]), have rarely been employed as ligands for reverse ATRP. In this article, a [Rmim][Cl] was successfully employed as a ligand for the reverse ATRP of methyl methacrylate with Fe(III) as catalyst and 2,2′-azobisisobutyronitrile as initiator. The key to success is to adjust the FeCl3/[Rmim][Cl] molar ratio value to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results indicated that the polymerization with [Rmim][Cl] as a ligand proceeded in a controlled/“living” fashion, as evidenced by the first-order kinetic plot, low polydispersity index values, and increase in polymer molecular weight with monomer conversion. The effects of various experimental parameters, including different catalysts, reaction temperatures, solvents, and molar ratios of FeCl3/[Rmim][Cl], on the polymerization were investigated in detail. Furthermore, 1H NMR and gel permeation chromatography analyses confirmed the halogen-containing chain-end functionality of the resultant polymer.
1. The results indicated that the polymerization with [Rmim][Cl] as a ligand proceeded in a controlled/“living” fashion, as evidenced by the first-order kinetic plot, low polydispersity index values, and increase in polymer molecular weight with monomer conversion. The effects of various experimental parameters, including different catalysts, reaction temperatures, solvents, and molar ratios of FeCl3/[Rmim][Cl], on the polymerization were investigated in detail. Furthermore, 1H NMR and gel permeation chromatography analyses confirmed the halogen-containing chain-end functionality of the resultant polymer.
Introduction
In the last twenty years, atom transfer radical polymerization (ATRP) has received great attention for preparing polymers with predictable molecular weights and low polydispersity index (PDI) values.1–11 In a normal ATRP, a dynamic equilibrium is established by the oxidation–reduction reaction between activator and dormant species.12 However, the transition-metal complexes in lower oxidation states are air sensitive and easily oxidized in the presence of even traces of oxygen. Therefore, new ATRP systems, such as supplemental activator and reducing agent atom transfer radical polymerization (SARA ATRP),13,14 reverse ATRP,15–17 activators (re)generated by electron transfer (A(R)GET) ATRP,18–23 and initiators for continuous initiator regeneration (ICAR) ATRP,24–28 have been developed to overcome the above drawbacks. In the ATRP derivative systems, high-oxidation-state metals were generally converted to the corresponding lower-oxidation-state complexes with the aid of an additional reducing agent.
Ionic liquids (ILs) are organic salts in the liquid state at or near room temperature, and possess good solubility for transition-metal catalysts. Consequently, ILs have been considered to be environmentally friendly solvents, due to their nonvolatile and nonflammable properties. Furthermore, various ILs have been designed as reaction media or even ligands by varying the structures of cation and anion for ATRP, especially for reverse ATRP.29–33
Early in 2000, Carmichael et al.34 first reported the use of 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) as a solvent for the copper(I)-mediated ATRP of methyl methacrylate (MMA). Later, the application of ILs as reaction media for ATRP aroused great interest. In particular, ILs with 1-alkyl-3-methylimidazolium ([Rmim], where R represents an alkyl group) as the cation have been widely used in copper-catalyzed ATRP systems.35–38 Matyjaszewski's group investigated the normal ATRP of MMA with various ILs as ligands.39 These ILs with [Rmim] as the cations contained different anions such as Cl−, Br−, CO3−, and AlCl4−. Furthermore, due to the outstanding advantages of iron catalysts, i.e., low cost, low toxicity, and good biocompatibility, the excellent performance of iron-catalyzed ATRP ILs as reaction media has also been reported.40,41 Chen et al.42,43 reported the iron(III)/acidic ligand-catalyzed reverse ATRP of acrylonitrile (AN) and methacrylonitrile (MAN) with [Rmim][BF4] and [Rmim][PF6] as reaction media, respectively. Generally, when ILs were used as solvents, an organic ligand was usually required in the iron-catalyzed reverse ATRP systems. Meanwhile, the anions of ILs were the expensive BF4− or PF6−.44,45 Moreover, the ILs were synthesized via at least two steps (steps 1 and 2 or steps 1 and 3 in Scheme 1). The cheaper [Hmim][RCOO] (step 4 in Scheme 1) was used for the iron-catalyzed ATRP of AN46 and reverse ATRP of MAN47 without any additional ligand by Chen's group. However, the monomer conversions were relatively low. To avoid the air sensitivity of the low-oxidation-state metal in normal ATRP, other ATRP systems with ILs as reaction media have also been explored, including A(R)GET ATRP,48–52 ICAR ATRP,53 and single electron transfer-living radical polymerization (SET-LRP).54 Likewise, the ILs in these polymerization systems are generally expensive anions (PF6−, BF4−, OH−, HCO3−, CO3−, PO4−, and RCOO−) or need two or more preparation steps (Scheme 1). To the best of our knowledge, the use of the facile, simple, and cheap ILs, 1-alkyl-3-methylimidazole chloride ([Rmim][Cl], step 1 in Scheme 1) as a reaction medium or ligand for reverse ATRP has rarely been reported. We find it curious that there are few reports on [Rmim][Cl] as reaction media or ligands for reverse ATRP, especially in an Fe(III)-catalyzed system.
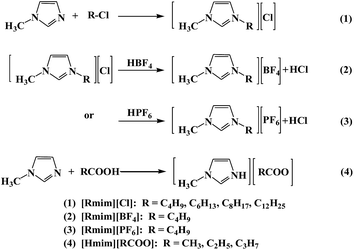 |
| | Scheme 1 Typical synthetic routes of ILs. | |
The current work is aimed to demonstrate the feasibility of using the facile, uncomplicated, and inexpensive ILs ([Rmim][Cl]) as the ligand for Fe(III)-catalyzed reverse ATRP of MMA. Kinetic studies confirmed the controlled/“living” radical polymerization characteristics. The effects of various experimental parameters on the polymerizations were investigated in detail.
Experimental
Materials
MMA (99%), ethyl methacrylate (EMA), and butyl methacrylate (BMA), were washed with 5% NaOH to remove the inhibitor (hydroquinone), washed with distilled water several times, and freshly distilled under reduced pressure after drying over Na2SO4. 2,2-Azobisisobutyronitrile (AIBN) was recrystallized twice from ethanol prior to use. 1-Butyl-3-methylimidazole chloride ([C4mim][Cl]), 1-hexyl-3-methylimidazole chloride ([C6mim][Cl]), 1-octyl-3-methylimidazole chloride ([C8mim][Cl]), and 1-dodecyl-3-methylimidazole chloride ([C12mim][Cl]) were prepared following the method in the literature.55 Acetonitrile (MeCN, 99%), N,N-dimethylformamide (DMF, 99%), dimethyl sulfoxide (DMSO, 99%), toluene (99%), and dichloromethane (CH2Cl2, 99%) were dried over Na2SO4 before use. Ferric chloride (FeCl3, 99%, Aladdin), ferric bromide (FeBr3, 99%, Aladdin), and ferric sulfate (Fe2(SO4)3, 99%) were used as received without further purification.
Polymerization
A typical polymerization was as follows. MMA and FeCl3 were added to a Schlenk tube with a magnetic stirrer bar. The tube was immersed in liquid nitrogen for one minute. Subsequently, AIBN, IL, and MeCN were rapidly added into the tube. Then the mixture was subject to three freeze–pump–thaw cycles to remove oxygen. Finally, the tube was charged with nitrogen before being sealed with a rubber septum, and placed in an oil bath at 70 °C. After the desired reaction time, the polymerization mixture was cooled by ice water, exposed to air, and the contents were then diluted with MeCN. When the mixture became a homogenous solution, the polymer was precipitated in a large excess of ethyl alcohol. The final product was separated by filtration, and dried under vacuum at 45 °C until constant weight was achieved.
Characterization
The monomer conversions were determined gravimetrically. The number-average molecular weight (Mn,GPC) and PDI values of the obtained poly(methyl methacrylate) (PMMA) samples were determined by gel permeation chromatography (GPC). The GPC system was equipped with a Waters 510 HPLC pump and a Waters 2414 RI detector and used three Waters Ultrastyragel columns (500, 103, and 105) in THF at a flow rate of 1.0 mL min−1. PMMA standards were used to calibrate the columns. 1H NMR spectra of PMMA samples were recorded on a Bruker 300 MHz spectrometer using CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard.
Results and discussion
[Rmim]Cl as ligand for the FeCl3-catalyzed reverse ATRP of MMA
As noted previously, the common, facile, and cheap ionic liquids, [Rmim][Cl] were rarely reported as reaction media or ligands for reverse ATRP.56 The ligand is very important for controlled/“living” radical polymerization, as reported by Anastasaki et al.57 Therefore, we firstly examined the feasibility of the Fe(III)/[Rmim][Cl]-catalyzed reverse ATRP of MMA by changing the amount of [Rmim][Cl], i.e., the effect of the concentration of a typical IL [C12mim][Cl] was investigated in detail under similar polymerization conditions at 70 °C. As shown in Table 1, when a large amount of IL is added into the polymerization (the ratio of [FeCl3]0/[C12mim][Cl]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, entry 1), the monomer conversion reaches only 12% within 42 h. Moreover, the measured molecular weight value (Mn,GPC) is much larger than the theoretical one (Mn,th), and the PDI value reaches 1.85. The results suggested that Fe(III) retarded or even inhibited the polymerization. By decreasing the amount of IL to a ratio of [FeCl3]0/[C12mim][Cl]0 = 2
40, entry 1), the monomer conversion reaches only 12% within 42 h. Moreover, the measured molecular weight value (Mn,GPC) is much larger than the theoretical one (Mn,th), and the PDI value reaches 1.85. The results suggested that Fe(III) retarded or even inhibited the polymerization. By decreasing the amount of IL to a ratio of [FeCl3]0/[C12mim][Cl]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, it is found, surprisingly, that the monomer conversion increases to 76% within a shorter duration (8 h), and the PDI value is lowered to 1.28 (entry 2). In addition, the measured molecular weight Mn,GPC is close to the theoretical Mn,th one. Clearly, the polymerization process proceeds in a controlled/“living” fashion. With a further decrease in [C12mim][Cl]0 to a ratio of [FeCl3]0/[C12mim][Cl]0 to 2
2, it is found, surprisingly, that the monomer conversion increases to 76% within a shorter duration (8 h), and the PDI value is lowered to 1.28 (entry 2). In addition, the measured molecular weight Mn,GPC is close to the theoretical Mn,th one. Clearly, the polymerization process proceeds in a controlled/“living” fashion. With a further decrease in [C12mim][Cl]0 to a ratio of [FeCl3]0/[C12mim][Cl]0 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the rate of polymerization is lowered again (only 24% monomer conversion within 33 h, entry 3). Therefore, by adding too much or too little IL into the polymerization system it is not possible to obtain a high rate of polymerization and control of the polymerization. Under the investigated conditions, only the reverse ATRP of MMA with [FeCl3]0/[IL]0 = 2
1, the rate of polymerization is lowered again (only 24% monomer conversion within 33 h, entry 3). Therefore, by adding too much or too little IL into the polymerization system it is not possible to obtain a high rate of polymerization and control of the polymerization. Under the investigated conditions, only the reverse ATRP of MMA with [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 shows a controlled/“living” radical polymerization (CRP) process. The controlled/“living” characteristics are confirmed by a measured molecular weight value close to the theoretical one and a low PDI value. These findings are in agreement with the results of Bai et al.58 In particular, as clearly demonstrated by Table 1, with an optimized molar ratio of 2
2 shows a controlled/“living” radical polymerization (CRP) process. The controlled/“living” characteristics are confirmed by a measured molecular weight value close to the theoretical one and a low PDI value. These findings are in agreement with the results of Bai et al.58 In particular, as clearly demonstrated by Table 1, with an optimized molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (entry 2) the IL may readily coordinate to FeCl3. With large amount of IL, with a molar ratio of 2
2 (entry 2) the IL may readily coordinate to FeCl3. With large amount of IL, with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (entry 1), the FeCl3/IL complex could be preferentially destroyed, while with a FeCl3/IL molar ratio of 2
40 (entry 1), the FeCl3/IL complex could be preferentially destroyed, while with a FeCl3/IL molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, free FeCl3 metal compound could potentially retard or inhibit the radical polymerization of MMA (entry 3).
1, free FeCl3 metal compound could potentially retard or inhibit the radical polymerization of MMA (entry 3).
Table 1 Effect of IL concentration on the reverse ATRP of MMA in bulka
| Entry |
[FeCl3]0/[IL]0 |
Time (h) |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
[MMA]0/[AIBN]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, [MMA]0 = 9.4 mol L−1, IL: [C12mim][Cl], T = 70 °C. 1, [MMA]0 = 9.4 mol L−1, IL: [C12mim][Cl], T = 70 °C. |
| 1 |
2/40 |
41 |
12 |
2000 |
9200 |
0.22 |
1.85 |
| 2 |
2/2 |
8 |
76 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.99 |
1.28 |
| 3 |
2/1 |
33 |
24 |
4000 |
5600 |
0.71 |
1.15 |
To gain further insight into the polymerization system, the effect of the IL alkyl chain length on the reverse ATRP of MMA was studied. A series of polymerization reactions with [C4mim][Cl], [C6mim][Cl], [C8mim][Cl], and [C12mim][Cl] as ligands was carried out under the same conditions, and the polymerization results are presented in Table 2. The monomer conversions reach 56%, 67%, 67%, and 76% within 8 h with [C4mim][Cl], [C6mim][Cl], [C8mim][Cl], and [C12mim][Cl] as ligands, respectively. The PDI values of the resultant polymer remain in a low range, from 1.23 to 1.28. Fig. 1 shows the dependence of monomer conversion and Mn,GPC for the reverse ATRP of MMA in bulk on the IL alkyl chain length. Obviously, the longer the alkyl chain length, the higher the monomer conversion and the PMMA molecular weight. This may originate from the fact that an IL ligand with a longer alkyl chain can more easily coordinate with the catalyst FeCl3, thus inducing a faster rate of polymerization than an IL ligand with a shorter alkyl chain. This indicates a strong dependence of polymerization on the alkyl chain length of IL. Thus, [C12mim][Cl] is an optimal ligand for the reverse ATRP of MMA in bulk, yielding the fastest rate of polymerization and the highest molecular weight.
Table 2 Effect of different ILs on the reverse ATRP of MMA in bulka
| Entry |
IL |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
[MMA]0/[AIBN]0/[FeCl3]0/[IL]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 8 h. 2, [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 8 h. |
| 1 |
[C4mim][Cl] |
56 |
9500 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.93 |
1.23 |
| 2 |
[C6mim][Cl] |
67 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.93 |
1.25 |
| 3 |
[C8mim][Cl] |
67 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.95 |
1.24 |
| 4 |
[C12mim][Cl] |
76 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.99 |
1.28 |
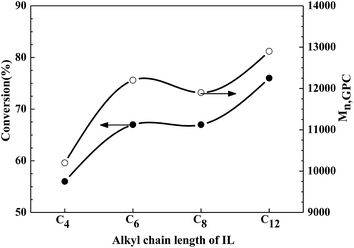 |
| | Fig. 1 Dependence of conversion and Mn,GPC on the alkyl chain length of IL for the reverse ATRP of MMA in bulk. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[IL]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. | |
To further confirm the controlled/“living” nature of the Fe(III)/[Rmim][Cl]-catalyzed reverse ATRP of MMA in bulk, kinetic studies were performed with the molar ratio of [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in bulk at 70 °C. The kinetic plot of ln([M]0/[M]) versus time is shown in Fig. 2. The plot shows an approximately straight line, proving that the concentration of propagating radicals was stable, and the side reactions can be ignored during the polymerization.59,60 In addition, an induction period of ca. 3 h is observed. The induction period probably originated from the following factors.61,62 On the one hand, Fe3+ and Fe2+ required a certain time to establish dynamic equilibrium at the beginning of the polymerization. On the other hand, AIBN decomposed relatively slowly in the initial stage of the polymerization, and the primary radical generated from AIBN reacted completely with FeCl3 to form the activator species Fe2+, leading to the result that the concentration of the reactive radical species was too low to initiate the polymerization at a noticeable rate. The Mn,th of PMMA obtained from reverse ATRP may be calculated based on the following equation:63
2 in bulk at 70 °C. The kinetic plot of ln([M]0/[M]) versus time is shown in Fig. 2. The plot shows an approximately straight line, proving that the concentration of propagating radicals was stable, and the side reactions can be ignored during the polymerization.59,60 In addition, an induction period of ca. 3 h is observed. The induction period probably originated from the following factors.61,62 On the one hand, Fe3+ and Fe2+ required a certain time to establish dynamic equilibrium at the beginning of the polymerization. On the other hand, AIBN decomposed relatively slowly in the initial stage of the polymerization, and the primary radical generated from AIBN reacted completely with FeCl3 to form the activator species Fe2+, leading to the result that the concentration of the reactive radical species was too low to initiate the polymerization at a noticeable rate. The Mn,th of PMMA obtained from reverse ATRP may be calculated based on the following equation:63
| |
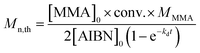 | (1) |
where [MMA]
0 and [AIBN]
0 correspond to the initial concentrations of MMA and AIBN, respectively, conv. is the monomer conversion,
MMMA is the molecular weight of MMA, the value of
kd for AIBN is 3.1 × 10
−5 at 70 °C,
64 and
t corresponds to the reaction time. The dependences of
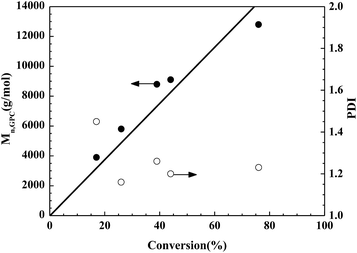
Mn and PDI of the obtained polymer on the monomer conversion are illustrated in
Fig. 3. The measured molecular weight
Mn,GPC values increase linearly from 3900 to 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
900 with monomer conversion, and are close to the theoretical
Mn,th ones. The PDI values of the obtained polymers decrease from 1.45 to 1.16 in the first stage, and then remain almost unchanged. The trend in PDI values from high to low is in accord with the CRP principle.
65 The change in the GPC traces displays the trend visually (
Fig. 4). The curves are narrow and symmetrical. Moreover, the traces of the obtained typical polymers shift toward a shorter elution time, indicating the increase in
Mn,GPC with monomer conversion.
66 All the above observations suggest that the Fe(
III)-[Rmim][Cl]-catalyzed reverse ATRP of MMA progressed in a controlled/“living” manner.
67
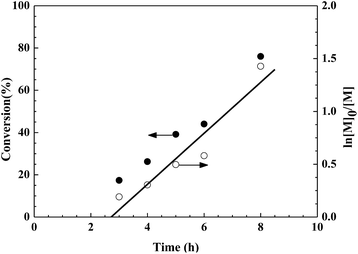 |
| | Fig. 2 Kinetic plots of ln([M]0/[M]) versus reaction time for the reverse ATRP of MMA in bulk. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. | |
 |
| | Fig. 3 Evolution of Mn (—theoretical) and PDI with monomer conversion for the reverse ATRP of MMA in bulk. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. | |
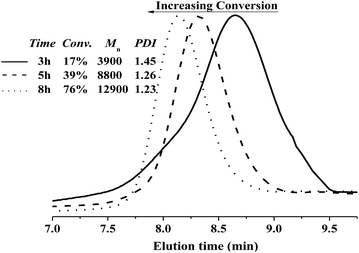 |
| | Fig. 4 The change in the GPC traces for the reverse ATRP of MMA in bulk at different monomer conversions: 17%, 39%, and 76%. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. | |
Effect of different catalysts on the reverse ATRP of MMA
The effect of different Fe(III) catalysts on the reverse ATRP of MMA in bulk was also examined, and the results are summarized in Table 3. With Fe2(SO4)3 as the catalyst to conduct the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA, the polymerization reaches only 19% monomer conversion within 24 h (entry 1). The measured molecular weight value Mn,GPC (7900) of the resultant PMMA is much larger than the theoretical one Mn,th (1900). Meanwhile, the PDI value (4.93) is the highest among the three systems. As the catalyst (Fe2(SO4)3) and initiator (AIBN) do not contain halogen elements, the polymerization cannot proceed via the ATRP mechanism. Thus, for the Fe2(SO4)3-[C12mim][Cl]-catalyzed polymerization of MMA, there is a loss of control, which is different from the result for ethyl-2-bromoisobutyrate/CuSO4·5H2O/N,N,N′,N′,N′′-pentamethyldiethylenetriamine-initiated CRP of MMA by Zhu's group.68 In contrast, with the bromine-containing catalyst FeBr3 instead of Fe2(SO4)3, the bulk polymerization yielded 70% monomer conversion after 8 h (entry 2). Obviously, the rate of polymerization increased greatly, while the PDI value (1.35) of the resultant PMMA was lowered, and the measured molecular weight value (Mn,GPC = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) is closer to the theoretical one (Mn,th = 11
500) is closer to the theoretical one (Mn,th = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800). Nevertheless, the PDI value is slightly higher than that with a chlorine-containing catalyst, FeCl3 (PDI = 1.28, entry 3). To sum up, FeCl3 is the most suitable catalyst for the investigated systems, as evidenced from the results that the corresponding polymerization yields 76% monomer conversion, and produces well-defined PMMA with a controlled Mn,GPC (12
800). Nevertheless, the PDI value is slightly higher than that with a chlorine-containing catalyst, FeCl3 (PDI = 1.28, entry 3). To sum up, FeCl3 is the most suitable catalyst for the investigated systems, as evidenced from the results that the corresponding polymerization yields 76% monomer conversion, and produces well-defined PMMA with a controlled Mn,GPC (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) close to Mn,th (12
800) close to Mn,th (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) and the lowest PDI value (1.28).
900) and the lowest PDI value (1.28).
Table 3 Effect of different catalysts on the reverse ATRP of MMA in bulka
| Entry |
Catalyst |
Time (h) |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
[MMA]0/[AIBN]0/[catalyst]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, T = 70 °C. |
| 1 |
Fe2(SO4)3 |
24 |
19 |
1900 |
7900 |
0.24 |
4.93 |
| 2 |
FeBr3 |
8 |
70 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.81 |
1.35 |
| 3 |
FeCl3 |
8 |
76 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.99 |
1.28 |
FeCl3-[C12mim][Cl]-catalyzed reverse ATRP for different monomers
According to the above results, the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA with [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is a CRP process. To investigate the generality of this approach for different monomers, EMA and BMA were selected instead of MMA as the monomer to carry out the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP under the same reaction conditions. As shown in Table 4, the monomer conversion reaches 76% after 8 h for the MMA system, and the measured molecular weight Mn,GPC value is very close to the theoretical one (entry 1). Likewise, the EMA and BMA polymerization systems achieve moderate-to-high rates of polymerization (58% and 70% monomer conversions, respectively), and the measured molecular weight Mn,GPC values (14
2 is a CRP process. To investigate the generality of this approach for different monomers, EMA and BMA were selected instead of MMA as the monomer to carry out the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP under the same reaction conditions. As shown in Table 4, the monomer conversion reaches 76% after 8 h for the MMA system, and the measured molecular weight Mn,GPC value is very close to the theoretical one (entry 1). Likewise, the EMA and BMA polymerization systems achieve moderate-to-high rates of polymerization (58% and 70% monomer conversions, respectively), and the measured molecular weight Mn,GPC values (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 18
500 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, respectively) of the resultant EMA and BMA polymers are higher than their corresponding theoretical ones (10
800, respectively) of the resultant EMA and BMA polymers are higher than their corresponding theoretical ones (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and 10
700 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, respectively). Importantly, their corresponding polymers give narrow molecular weight distribution values (PDI = 1.18 and 1.22, respectively). Therefore, the EMA and BMA polymerization systems proceed according to the CRP principle, confirming that the polymerization approach can be successfully extended to other monomers.
800, respectively). Importantly, their corresponding polymers give narrow molecular weight distribution values (PDI = 1.18 and 1.22, respectively). Therefore, the EMA and BMA polymerization systems proceed according to the CRP principle, confirming that the polymerization approach can be successfully extended to other monomers.
Table 4 Reverse ATRP for different monomers in bulka
| Entry |
Monomer |
Time (h) |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
[Monomer]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, [BMA]0 = 6.30 mol L−1, [EMA]0 = 8.04 mol L−1, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, [BMA]0 = 6.30 mol L−1, [EMA]0 = 8.04 mol L−1, T = 70 °C. |
| 1 |
MMA |
8 |
76 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.99 |
1.28 |
| 2 |
EMA |
9 |
58 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.74 |
1.18 |
| 3 |
BMA |
21 |
70 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.57 |
1.22 |
Effect of various solvents on the reverse ATRP of MMA
It is necessary to investigate the effect of various mediums including bulk, DMSO, DMF, MeCN, CH2Cl2, and toluene on the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA at 70 °C. The molar ratio of [MMA]0/[AIBN]0/[FeCl3]0/[IL]0 was set to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the original volume ratio of MMA to solvent was 2
2, and the original volume ratio of MMA to solvent was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Clearly, compared with the bulk polymerization yielding 76% monomer conversion after 8 h and the controlled PMMA with a measured Mn,GPC very close to the theoretical Mn,th and PDI = 1.28 (entry 1, Table 5), the polymerization in DMSO shows the lowest monomer conversion (23%) after 20 h and gives a less-controlled PMMA, with a Mn,GPC (7600) three times larger than the theoretical Mn,th (2300, entry 2). Similar results are found in Fe(II)-catalyzed normal ATRP in DMSO without any ligand, as reported by Matyjaszewski et al.,69 which is possibly attributable to the fact that DMSO coordinates much more strongly to the Fe catalyst and substitutes for halide ligands, thereby weakening the catalytic activity and inducing a loss of control over the polymerization. In contrast, the excellent solvent systems for PMMA, i.e., DMF, MeCN, and CH2Cl2, can reach relatively higher monomer conversions within the same reaction time of 20 h (entries 3–5): in particular, MeCN and CH2Cl2 reach up to 91% monomer conversion (entries 4 and 5). Moreover, for the less polar solvent toluene, the monomer conversion reaches 80% after 20 h (entry 6), suggesting that this less polar solvent has almost no effect on the polymerization system. On the basis of the above results that CH2Cl2 and MeCN have a significant impact on the polymerization, the non-halogen solvent MeCN is thus chosen as the optimal solvent for the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA. It is noteworthy that the measured Mn,GPC values are much larger than the theoretical Mn,th ones: that is, the initiator efficiency (f) based on f = Mn,th/Mn,GPC is lowered as the polymerization is conducted in the solvent. This may be due to the occurrence of coupling reactions of primary radicals in the initial stages of the polymerizations in various solvents. However, all investigated polymerizations consisting of bulk and solution systems produced polymers with lower PDI results (1.20–1.29), showing the high controllability of the employed Fe(III)-[C12mim][Cl] catalytic system for methacrylate monomers.
1. Clearly, compared with the bulk polymerization yielding 76% monomer conversion after 8 h and the controlled PMMA with a measured Mn,GPC very close to the theoretical Mn,th and PDI = 1.28 (entry 1, Table 5), the polymerization in DMSO shows the lowest monomer conversion (23%) after 20 h and gives a less-controlled PMMA, with a Mn,GPC (7600) three times larger than the theoretical Mn,th (2300, entry 2). Similar results are found in Fe(II)-catalyzed normal ATRP in DMSO without any ligand, as reported by Matyjaszewski et al.,69 which is possibly attributable to the fact that DMSO coordinates much more strongly to the Fe catalyst and substitutes for halide ligands, thereby weakening the catalytic activity and inducing a loss of control over the polymerization. In contrast, the excellent solvent systems for PMMA, i.e., DMF, MeCN, and CH2Cl2, can reach relatively higher monomer conversions within the same reaction time of 20 h (entries 3–5): in particular, MeCN and CH2Cl2 reach up to 91% monomer conversion (entries 4 and 5). Moreover, for the less polar solvent toluene, the monomer conversion reaches 80% after 20 h (entry 6), suggesting that this less polar solvent has almost no effect on the polymerization system. On the basis of the above results that CH2Cl2 and MeCN have a significant impact on the polymerization, the non-halogen solvent MeCN is thus chosen as the optimal solvent for the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA. It is noteworthy that the measured Mn,GPC values are much larger than the theoretical Mn,th ones: that is, the initiator efficiency (f) based on f = Mn,th/Mn,GPC is lowered as the polymerization is conducted in the solvent. This may be due to the occurrence of coupling reactions of primary radicals in the initial stages of the polymerizations in various solvents. However, all investigated polymerizations consisting of bulk and solution systems produced polymers with lower PDI results (1.20–1.29), showing the high controllability of the employed Fe(III)-[C12mim][Cl] catalytic system for methacrylate monomers.
Table 5 Effect of various solvents on the reverse ATRP of MMAa
| Entry |
Solvent |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
[MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, MMA/solvent = 2 2, MMA/solvent = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 20 h. Reaction time = 8 h. 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 20 h. Reaction time = 8 h. |
| 1b |
Bulk |
76 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.99 |
1.28 |
| 2 |
DMSO |
23 |
2500 |
7600 |
0.33 |
1.26 |
| 3 |
DMF |
90 |
9000 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.80 |
1.29 |
| 4 |
MeCN |
91 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.66 |
1.22 |
| 5 |
CH2Cl2 |
91 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.69 |
1.20 |
| 6 |
Toluene |
80 |
8400 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.67 |
1.20 |
Effect of [AIBN]0/[FeCl3]0/[IL]0 on the reverse ATRP of MMA
For an efficient reverse ATRP system, it is necessary to use the minimum amount of metal in expectation of a high rate of polymerization and for good control of the polymerization. As demonstrated in Table 6, the rate of polymerization can be changed by altering the molar ratio of [AIBN]0/[FeCl3]0/[IL]0. The polymerization reaches 99% and 92% monomer conversion within 16 h for [AIBN]0/[FeCl3]0/[IL]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (entries 1–2). For [AIBN]0/[FeCl3]0/[IL]0 = 1
1, respectively (entries 1–2). For [AIBN]0/[FeCl3]0/[IL]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the polymerization gives 91% monomer conversion within 20 h (entry 3). Decreasing [AIBN]0/[FeCl3]0/[IL]0 down to 1
2, the polymerization gives 91% monomer conversion within 20 h (entry 3). Decreasing [AIBN]0/[FeCl3]0/[IL]0 down to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the polymerizations yield only 50% conversion within 46 h (entries 4 and 5). Thus the [FeCl3]0/[IL]0 value is a crucial experimental parameter for the rate of polymerization, and adding an excess of catalyst obviously decreases monomer conversions from ca. 100% to 50% (Fig. 5). The same rule can be observed from the changes in the PDI and molecular weight values with different [FeCl3]0/[IL]0 values, as exhibited in Table 6, Fig. 5, and 6. With low [FeCl3]0/[IL]0 values of 0.5
4, the polymerizations yield only 50% conversion within 46 h (entries 4 and 5). Thus the [FeCl3]0/[IL]0 value is a crucial experimental parameter for the rate of polymerization, and adding an excess of catalyst obviously decreases monomer conversions from ca. 100% to 50% (Fig. 5). The same rule can be observed from the changes in the PDI and molecular weight values with different [FeCl3]0/[IL]0 values, as exhibited in Table 6, Fig. 5, and 6. With low [FeCl3]0/[IL]0 values of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the corresponding PDI values are relatively higher (1.91 and 1.44, respectively). With a decrease in [AIBN]0/[FeCl3]0/[IL]0 down to 1
1, the corresponding PDI values are relatively higher (1.91 and 1.44, respectively). With a decrease in [AIBN]0/[FeCl3]0/[IL]0 down to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the corresponding PDI values remain in a narrow range of 1.20–1.23. For the case of molecular weight, the final polymers obtained from five catalytic systems show a straight drop from 22
4, the corresponding PDI values remain in a narrow range of 1.20–1.23. For the case of molecular weight, the final polymers obtained from five catalytic systems show a straight drop from 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 to 8800 with the variation in [FeCl3]0/[IL]0 values. Furthermore, it is worthwhile noting the deviation between the measured molecular weight value and the corresponding theoretical one, i.e., the initiator efficiency (Fig. 6). For example, for [FeCl3]0/[IL]0 = 0.5
600 to 8800 with the variation in [FeCl3]0/[IL]0 values. Furthermore, it is worthwhile noting the deviation between the measured molecular weight value and the corresponding theoretical one, i.e., the initiator efficiency (Fig. 6). For example, for [FeCl3]0/[IL]0 = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the measured Mn,GPC values (22
1, the measured Mn,GPC values (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 and 18
600 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, respectively) of the obtained polymers are higher than their corresponding theoretical ones (11
500, respectively) of the obtained polymers are higher than their corresponding theoretical ones (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 11
900 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, respectively). Among the molar ratios investigated, the polymerization shows the highest initiator efficiency (0.66) with a molar ratio of [FeCl3]0/[IL]0 = 2
100, respectively). Among the molar ratios investigated, the polymerization shows the highest initiator efficiency (0.66) with a molar ratio of [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Therefore, all the results suggest that selecting a suitable molar ratio of [FeCl3]0/[IL]0 = 2
2. Therefore, all the results suggest that selecting a suitable molar ratio of [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is extremely important for the reverse ATRP of MMA.
2 is extremely important for the reverse ATRP of MMA.
Table 6 Effect of [AIBN]0/[FeCl3]0/[IL]0 on the reverse ATRP of MMA in MeCNa
| Entry |
[MMA]0/[AIBN]0/[FeCl3]0/[IL]0 |
Time (h) |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
MMA/MeCN = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C. 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C. |
| 1 |
200/1/0.5/0.5 |
16 |
99 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.53 |
1.91 |
| 2 |
200/1/1/1 |
16 |
92 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.60 |
1.44 |
| 3 |
200/1/2/2 |
20 |
91 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.66 |
1.22 |
| 4 |
200/1/3/3 |
46 |
50 |
5000 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.48 |
1.23 |
| 5 |
200/1/4/4 |
46 |
50 |
5000 |
8800 |
0.57 |
1.20 |
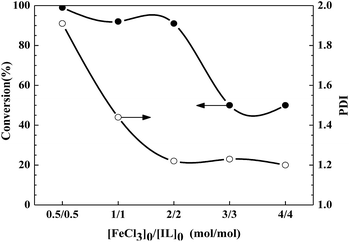 |
| | Fig. 5 Dependence of conversion and PDI on the ratio of [FeCl3]0/[IL]0 for the reverse ATRP of MMA in MeCN. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, MMA/MeCN = 2 2, MMA/MeCN = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C. 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C. | |
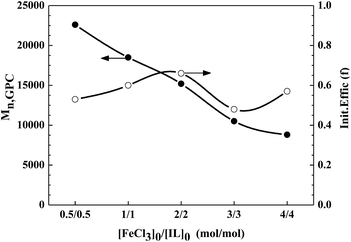 |
| | Fig. 6 Dependence of Mn,GPC and initiator efficiency (f) on the ratio of [FeCl3]0/[IL]0 for the reverse ATRP of MMA in MeCN. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, MMA/MeCN = 2 2, MMA/MeCN = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C. 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C. | |
Effect of reaction temperature on the reverse ATRP of MMA
Generally, increasing the reaction temperature can enhance the decomposition rate of radical initiators and the rate of polymerization, and thus shorten the polymerization duration.70 Three polymerization reactions were conducted and compared in MeCN at 60 °C, 70 °C, and 90 °C under the same reaction conditions and the results are summarized in Table 7. The rate of polymerization increases with increasing temperature from 60 °C to 90 °C, as evidenced by the increasing monomer conversion (from 50% to 94%) and the shortening of the polymerization time from 52 h to 11 h. When the polymerization is performed at a relatively low temperature (60 °C, entry 1), it gives only 50% monomer conversion after 52 h, and the measured molecular weight value Mn,GPC (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of the polymer is much larger than the theoretical one Mn,th (5000). In contrast, when the polymerization is performed at a relatively high temperature (90 °C, entry 3), it gives 94% monomer conversion after a shorter polymerization time (11 h). In addition, the PDI values (1.34–1.44) of the obtained polymers remain low at the three polymerization temperatures, indicating that the polymerization can be successfully conducted over a wide temperature range (60–90 °C).
000) of the polymer is much larger than the theoretical one Mn,th (5000). In contrast, when the polymerization is performed at a relatively high temperature (90 °C, entry 3), it gives 94% monomer conversion after a shorter polymerization time (11 h). In addition, the PDI values (1.34–1.44) of the obtained polymers remain low at the three polymerization temperatures, indicating that the polymerization can be successfully conducted over a wide temperature range (60–90 °C).
Table 7 Effect of different temperature on the reverse ATRP of MMAa
| Entry |
Temperature (°C) |
Time (h) |
Conv. (%) |
Mn,th (Da) |
Mn,GPC (Da) |
Initiat. effic. |
PDI |
[MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, MMA/MeCN = 2 1, MMA/MeCN = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1. 1 (v/v), [MMA]0 = 9.4 mol L−1. |
| 1 |
60 |
52 |
50 |
5000 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.45 |
1.38 |
| 2 |
70 |
16 |
92 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.60 |
1.44 |
| 3 |
90 |
11 |
94 |
9400 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.70 |
1.34 |
End-group analysis of the resultant PMMA
The chemical structure of the obtained PMMA was characterized by 1H NMR spectroscopy. Fig. 7 illustrates the representative 1H NMR spectrum of the well-defined PMMA with Mn = 7600 and PDI = 1.16. The signals at 0.66–1.30, 1.32–2.18, and 3.46–3.72 ppm are attributed to the protons of the methyl groups (CH3, peak b), methylene groups (CH2, peak a), and methoxy groups (OCH3, peak c), respectively. Moreover, the absorption at 3.74–3.81 is attributed to the protons of OCH3 next to the halogen chain end (peak d), proving the presence of the halogen-containing end group, CH2CCl(CH3)(COOCH3), which agrees with the work reported by Zhu's group.71 The Mn,NMR value of PMMA can be estimated from the ratio of the methylene protons (peak a) to the terminal ones (peak d). The estimated Mn,NMR value (4900) is slightly lower than the theoretical molecular weight value Mn,th (7300) and the measured molecular weight value Mn,GPC (7600). Overall, the results confirmed the distinct controlled/“living” characteristics of the polymerization.
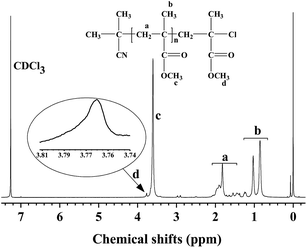 |
| | Fig. 7 1H NMR spectrum of PMMA obtained from the reverse ATRP of MMA in bulk. Reaction conditions: [MMA]0/[AIBN]0/[FeCl3]0/[C12mim][Cl]0 = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, reaction time = 4 h, monomer conversion = 26%, T = 70 °C. 2, [MMA]0 = 9.4 mol L−1, reaction time = 4 h, monomer conversion = 26%, T = 70 °C. | |
Conclusions
A small amount of facile, simple, and inexpensive IL ([Rmim][Cl]) was successfully used as a ligand for the Fe(III)-catalyzed reverse ATRP of MMA. Polymerization kinetic studies confirmed the living/controlled radical polymerization characteristics, as evidenced by the first-order kinetics of polymerization, linear increase in Mn with monomer conversion, controlled Mn close to Mn,th values, and low PDI values. The polymerization was studied in detail in terms of different alkyl chain lengths of IL (from C4 to C12), concentration of IL, catalyst, solvent, molar ratio of FeCl3/[Rmim][Cl], and reaction temperature. Longer alkyl chain lengths of IL and a higher polymerization temperature can accelerate the rate of polymerization. With a suitable molar ratio of [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the bulk polymerization achieved a controlled/“living” radical polymerization process with a high molecular weight (Mn,th = 12
2, the bulk polymerization achieved a controlled/“living” radical polymerization process with a high molecular weight (Mn,th = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 and Mn,GPC = 12
800 and Mn,GPC = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) and initiator efficiency (f = 0.99), and low PDI value (PDI = 1.28). In addition, the polymerization system can be successfully extended to other vinyl monomers. The polymerizations in different solvents, including DMSO, DMF, MeCN, CH2Cl2, and toluene, gave varied control of molecular weight and PDI values, and showed that MeCN can be the best solvent for the polymerization system. Moreover, the halogen-containing chain-end functionality of the resultant PMMA was confirmed by GPC and 1H NMR analyses.
900) and initiator efficiency (f = 0.99), and low PDI value (PDI = 1.28). In addition, the polymerization system can be successfully extended to other vinyl monomers. The polymerizations in different solvents, including DMSO, DMF, MeCN, CH2Cl2, and toluene, gave varied control of molecular weight and PDI values, and showed that MeCN can be the best solvent for the polymerization system. Moreover, the halogen-containing chain-end functionality of the resultant PMMA was confirmed by GPC and 1H NMR analyses.
Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21074127) for financial support.
Notes and references
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- J. Ran, L. Wu, Z. Zhang and T. Xu, Prog. Polym. Sci., 2013, 39, 124–144 CrossRef.
- A. Pal and S. Pal, RSC Adv., 2016, 6, 2958–2965 RSC.
- H. Zhou, W. Jiang, N. An, Q. P. Zhang, S. D. Xiang, L. P. Wang and J. Tang, RSC Adv., 2015, 5, 42728–42735 RSC.
- C. Zhou, S. S. Qian, X. J. Li, F. Yao, J. Forsythe and G. D. Fu, RSC Adv., 2014, 4, 54631–54640 RSC.
- M. Zhang, Q. Xiong, W. Shen and Q. Zhang, RSC Adv., 2014, 4, 30566–30572 RSC.
- Q. Zhang, X. D. Tang, T. S. Wang, F. Q. Yu, W. J. Guo and M. S. Pei, RSC Adv., 2014, 4, 24240–24247 RSC.
- P. Mandal and N. Singha, RSC Adv., 2014, 4, 213–215 Search PubMed.
- J. C. Theriot, C. H. Lim, H. Yang, M. D. Ryan, C. B. Musgrave and G. M. Miyake, Science, 2016, 352, 1082–1086 CrossRef CAS PubMed.
- C. Boyer, N. A. Corrigan, K. Jung, D. Nguyen, T.-K. Nguyen, N. N. M. Adnan, S. Oliver, S. Shanmugam and J. Yeow, Chem. Rev., 2016, 116, 1803–1949 CrossRef CAS PubMed.
- C. Boyer, A. H. Soeriyadi, P. B. Zetterlund and M. R. Whittaker, Macromolecules, 2011, 44, 8028–8033 CrossRef CAS.
- J. S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117, 5614–5615 CrossRef CAS.
- A. Anastasaki, V. Nikolaou and D. M. Haddleton, Polym. Chem., 2016, 7, 1002–1026 RSC.
- A. Anastasaki, V. Nikolaou, G. Nurumbetov, P. Wilson, K. Kempe, J. F. Quinn, T. P. Davis, M. R. Whittaker and D. M. Haddleton, Chem. Rev., 2015, 116, 835–877 CrossRef PubMed.
- Y. P. Jiao, J. S. Jiang, H. T. Zhang, K. Y. Shi and H. Q. Zhang, Eur. Polym. J., 2014, 54, 95–108 CrossRef CAS.
- O. S. Taskin, G. Yilmaz, M. A. Tasdelen and Y. Yagci, Polym. Int., 2014, 63, 902–907 CrossRef CAS.
- Y. J. Chen, B. J. Wu, F. S. Wang, M. H. Chi, J. T. Chen and C. H. Peng, Macromolecules, 2015, 48, 6832–6838 CrossRef CAS.
- L. F. Zhang, Z. P. Cheng, S. P. Shi, Q. H. Li and X. L. Zhu, Polymer, 2008, 49, 3054–3059 CrossRef CAS.
- J. Miao, H. J. Jiang, L. F. Zhang, Z. Q. Wu, Z. P. Cheng and X. L. Zhu, RSC Adv., 2011, 2, 840–847 RSC.
- Y. H. Yu, X. H. Liu, D. Jia, B. W. Cheng, Y. L. Ren, F. J. Zhang, H. N. Li, P. Chen and S. Xie, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1690–1694 CrossRef CAS.
- Y. H. Yu, X. H. Liu, D. Jia, B. W. Cheng, F. J. Zhang, P. Chen and S. Xie, Polymer, 2013, 54, 148–154 CrossRef CAS.
- J. Y. Peng, M. Q. Ding, Z. P. Cheng, L. F. Zhang and X. L. Zhu, RSC Adv., 2015, 5, 104733–104739 RSC.
- L. J. Bai, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2012, 3, 2685–2697 RSC.
- X. H. Liu, J. Wang, F. J. Zhang, S. L. An, Y. L. Ren, Y. H. Yu, P. Chen and S. Xie, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4358–4364 CrossRef CAS.
- X. H. Liu, J. Wang, J. S. Yang, S. L. An, Y. L. Ren, Y. H. Yu and P. Chen, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1933–1940 CrossRef CAS.
- X. W. Jiang, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2016, 37, 1337–1343 CrossRef CAS PubMed.
- X. W. Jiang, J. Wu, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2014, 35, 1879–1885 CrossRef CAS PubMed.
- T. Guo, L. F. Zhang, X. Q. Pan, X. H. Li, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2013, 4, 3725–3734 RSC.
- B. Tadeusz and K. Przemysław, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3454–3459 CrossRef.
- S. M. Gong, H. Y. Ma and X. H. Wan, Polym. Int., 2006, 55, 1420–1425 CrossRef CAS.
- E. J. Tang, K. D. Du, X. Y. Feng, M. Yuan, S. J. Liu and D. S. Zhao, Eur. Polym. J., 2015, 66, 228–235 CrossRef CAS.
- P. Kubisa, Prog. Polym. Sci., 2004, 29, 3–12 CrossRef CAS.
- A. Anastasaki, V. Nikolaou, G. Nurumbetov, N. P. Truong, G. S. Pappas, N. G. Engelis, J. F. Quinn, M. R. Whittaker, T. P. Davis and D. M. Haddleton, Macromolecules, 2015, 48, 5140–5147 CrossRef CAS.
- A. J. Carmichael, D. M. Haddleton, S. A. F. Bon and K. R. Seddon, Chem. Commun., 2000, 1237–1238 RSC.
- T. Biedroń and P. Kubisa, Polym. Int., 2003, 52, 1584–1588 CrossRef.
- H. Y. Ma, X. H. Wan, X. F. Chen and Q. F. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2005, 41, 143–151 CrossRef.
- H. Y. Ma, X. H. Wan, X. F. Chen and Q. F. Zhou, Polymer, 2003, 44, 5311–5316 CrossRef CAS.
- S. J. Ding, M. Radosz and Y. Shen, Macromolecules, 2005, 38, 5921–5928 CrossRef CAS.
- T. Sarbu and K. Matyjaszewski, Macromol. Chem. Phys., 2001, 202, 3379–3391 CrossRef CAS.
- Z. J. Deng, L. H. Qiu, L. J. Bai, Y. X. Zhou, B. C. Lin, J. Zhao, Z. P. Cheng, X. L. Zhu and F. Yan, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1605–1610 CrossRef CAS.
- L. J. Bai, S. Q. Huang, W. X. Wang, H. Xu, H. Chen, Y. Z. Niu and M. H. Wang, Polym. Int., 2015, 64, 1754–1761 CrossRef CAS.
- H. Chen, R. J. Qu, C. N. Ji, C. M. Sun and C. H. Wang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2701–2707 CrossRef.
- Y. Liang, H. Chen and W. Y. Zhou, J. Macromol. Sci., Part A: Pure Appl.Chem., 2009, 46, 759–764 CrossRef CAS.
- H. Chen, Y. Liang, M. L. Wang, P. L. Lv and Y. H. Xuan, Chem. Eng. J., 2009, 147, 297–301 CrossRef CAS.
- H. Chen, C. H. Wang, D. L. Liu, M. Wang and C. N. Ji, J. Appl. Polym. Sci., 2011, 122, 3298–3302 CrossRef CAS.
- H. Chen, R. J. Qu, C. M. Sun, C. N. Ji, C. H. Wang, L. Ying, N. Jiang, F. Xiu and L. F. Chen, Polymer, 2008, 49, 3424–3427 CrossRef.
- H. Chen, Y. F. Meng, Y. Liang, Z. X. Lu and P. L. Lv, J. Mater. Res., 2009, 24, 1880–1885 CrossRef CAS.
- H. Chen, D. L. Liu, Y. T. Song, R. J. Qu and C. H. Wang, Polym. Adv. Technol., 2011, 22, 1513–1517 CrossRef CAS.
- H. Chen, L. F. Chen, C. H. Wang and R. J. Qu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1046–1049 CrossRef CAS.
- G. X. Wang, M. Lu, L. C. Liu, H. Wu and M. Zhong, J. Appl. Polym. Sci., 2013, 128, 3077–3083 CrossRef CAS.
- Z. Deng, J. Guo, L. Qiu, Y. Zhou, L. Xia and F. Yan, Polym. Chem., 2012, 3, 2436–2443 RSC.
- X. Y. Du, J. L. Pan, M. T. Chen, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Chem. Commun., 2014, 50, 9266–9269 RSC.
- G. X. Wang, M. Lu and H. Wu, Polymer, 2012, 53, 1093–1097 CrossRef CAS.
- J. Ma, H. Chen, M. Zhang and M. M. Yu, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 609–613 CrossRef CAS.
- J. G. Huddleston and R. D. Rogers, Chem. Commun., 1998, 1765–1766 RSC.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- A. Anastasaki, C. Waldron, P. Wilson, R. Mchale and D. M. Haddleton, Polym. Chem., 2013, 4, 2672–2675 RSC.
- L. J. Bai, W. X. Wang, H. Chen, L. F. Zhang, Z. P. Cheng and X. L. Zhu, RSC Adv., 2015, 5, 62577–62584 RSC.
- L. F. Zhang, Z. P. Cheng, Y. T. Lü and X. L. Zhu, Macromol. Rapid Commun., 2009, 30, 543–547 CrossRef CAS PubMed.
- B. J. Zhang, X. Y. Jiang, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2015, 6, 6616–6622 RSC.
- G. H. Zhu, L. F. Zhang, Z. B. Zhang, J. Zhu, Y. F. Tu, Z. P. Cheng and X. L. Zhu, Macromolecules, 2011, 44, 3233–3239 CrossRef CAS.
- L. F. Zhang, J. Miao, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2010, 31, 275–280 CrossRef CAS PubMed.
- J. S. Wang and K. Matyjaszewski, Macromolecules, 1995, 28, 7572–7573 CrossRef CAS.
- Z. G. Xue, D. He and X. L. Xie, Polym. Chem., 2015, 6, 1660–1687 RSC.
- X. H. Liu, H. N. Li, F. J. Zhang, S. Xie, Z. J. Liu and Y. G. Li, Chin. J. Polym. Sci., 2015, 33, 362–370 CrossRef CAS.
- V. S. Vo, S. Mahouchechergui, J. Babinot, V. H. Nguyen, S. Naili and B. Carbonnier, RSC Adv., 2016, 6, 89322–89327 RSC.
- X. H. Liu, H. N. Li, F. J. Zhang, Q. Zhu, X. H. Hu, W. L. Di, S. Zhao and Y. G. Li, Polymer, 2016, 107, 170–176 CrossRef CAS.
- J. F. Zhao, W. X. Wang, L. J. Bai, L. L. Zhou, Z. P. Cheng, Z. B. Zhang and X. L. Zhu, Polym. Chem., 2012, 3, 3220–3223 RSC.
- Y. Wang and K. Matyjaszewski, Macromolecules, 2010, 43, 4003–4005 CrossRef CAS.
- Z. J. Deng, L. H. Qiu, L. J. Bai, Y. X. Zhou, B. C. Lin, J. Zhao, Z. P. Cheng, X. L. Zhu and F. Yan, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1605–1610 CrossRef CAS.
- G. Wang, X. L. Zhu, Z. P. Cheng and J. Zhu, Eur. Polym. J., 2003, 39, 2161–2165 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *,
Qian Zhu,
Qiuyan Zhang,
Yanguang Zhang and
Chen Ding
*,
Qian Zhu,
Qiuyan Zhang,
Yanguang Zhang and
Chen Ding
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results indicated that the polymerization with [Rmim][Cl] as a ligand proceeded in a controlled/“living” fashion, as evidenced by the first-order kinetic plot, low polydispersity index values, and increase in polymer molecular weight with monomer conversion. The effects of various experimental parameters, including different catalysts, reaction temperatures, solvents, and molar ratios of FeCl3/[Rmim][Cl], on the polymerization were investigated in detail. Furthermore, 1H NMR and gel permeation chromatography analyses confirmed the halogen-containing chain-end functionality of the resultant polymer.
1. The results indicated that the polymerization with [Rmim][Cl] as a ligand proceeded in a controlled/“living” fashion, as evidenced by the first-order kinetic plot, low polydispersity index values, and increase in polymer molecular weight with monomer conversion. The effects of various experimental parameters, including different catalysts, reaction temperatures, solvents, and molar ratios of FeCl3/[Rmim][Cl], on the polymerization were investigated in detail. Furthermore, 1H NMR and gel permeation chromatography analyses confirmed the halogen-containing chain-end functionality of the resultant polymer.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, entry 1), the monomer conversion reaches only 12% within 42 h. Moreover, the measured molecular weight value (Mn,GPC) is much larger than the theoretical one (Mn,th), and the PDI value reaches 1.85. The results suggested that Fe(III) retarded or even inhibited the polymerization. By decreasing the amount of IL to a ratio of [FeCl3]0/[C12mim][Cl]0 = 2
40, entry 1), the monomer conversion reaches only 12% within 42 h. Moreover, the measured molecular weight value (Mn,GPC) is much larger than the theoretical one (Mn,th), and the PDI value reaches 1.85. The results suggested that Fe(III) retarded or even inhibited the polymerization. By decreasing the amount of IL to a ratio of [FeCl3]0/[C12mim][Cl]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, it is found, surprisingly, that the monomer conversion increases to 76% within a shorter duration (8 h), and the PDI value is lowered to 1.28 (entry 2). In addition, the measured molecular weight Mn,GPC is close to the theoretical Mn,th one. Clearly, the polymerization process proceeds in a controlled/“living” fashion. With a further decrease in [C12mim][Cl]0 to a ratio of [FeCl3]0/[C12mim][Cl]0 to 2
2, it is found, surprisingly, that the monomer conversion increases to 76% within a shorter duration (8 h), and the PDI value is lowered to 1.28 (entry 2). In addition, the measured molecular weight Mn,GPC is close to the theoretical Mn,th one. Clearly, the polymerization process proceeds in a controlled/“living” fashion. With a further decrease in [C12mim][Cl]0 to a ratio of [FeCl3]0/[C12mim][Cl]0 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the rate of polymerization is lowered again (only 24% monomer conversion within 33 h, entry 3). Therefore, by adding too much or too little IL into the polymerization system it is not possible to obtain a high rate of polymerization and control of the polymerization. Under the investigated conditions, only the reverse ATRP of MMA with [FeCl3]0/[IL]0 = 2
1, the rate of polymerization is lowered again (only 24% monomer conversion within 33 h, entry 3). Therefore, by adding too much or too little IL into the polymerization system it is not possible to obtain a high rate of polymerization and control of the polymerization. Under the investigated conditions, only the reverse ATRP of MMA with [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 shows a controlled/“living” radical polymerization (CRP) process. The controlled/“living” characteristics are confirmed by a measured molecular weight value close to the theoretical one and a low PDI value. These findings are in agreement with the results of Bai et al.58 In particular, as clearly demonstrated by Table 1, with an optimized molar ratio of 2
2 shows a controlled/“living” radical polymerization (CRP) process. The controlled/“living” characteristics are confirmed by a measured molecular weight value close to the theoretical one and a low PDI value. These findings are in agreement with the results of Bai et al.58 In particular, as clearly demonstrated by Table 1, with an optimized molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (entry 2) the IL may readily coordinate to FeCl3. With large amount of IL, with a molar ratio of 2
2 (entry 2) the IL may readily coordinate to FeCl3. With large amount of IL, with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (entry 1), the FeCl3/IL complex could be preferentially destroyed, while with a FeCl3/IL molar ratio of 2
40 (entry 1), the FeCl3/IL complex could be preferentially destroyed, while with a FeCl3/IL molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, free FeCl3 metal compound could potentially retard or inhibit the radical polymerization of MMA (entry 3).
1, free FeCl3 metal compound could potentially retard or inhibit the radical polymerization of MMA (entry 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, [MMA]0 = 9.4 mol L−1, IL: [C12mim][Cl], T = 70 °C.
1, [MMA]0 = 9.4 mol L−1, IL: [C12mim][Cl], T = 70 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 8 h.
2, [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 8 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in bulk at 70 °C. The kinetic plot of ln([M]0/[M]) versus time is shown in Fig. 2. The plot shows an approximately straight line, proving that the concentration of propagating radicals was stable, and the side reactions can be ignored during the polymerization.59,60 In addition, an induction period of ca. 3 h is observed. The induction period probably originated from the following factors.61,62 On the one hand, Fe3+ and Fe2+ required a certain time to establish dynamic equilibrium at the beginning of the polymerization. On the other hand, AIBN decomposed relatively slowly in the initial stage of the polymerization, and the primary radical generated from AIBN reacted completely with FeCl3 to form the activator species Fe2+, leading to the result that the concentration of the reactive radical species was too low to initiate the polymerization at a noticeable rate. The Mn,th of PMMA obtained from reverse ATRP may be calculated based on the following equation:63
2 in bulk at 70 °C. The kinetic plot of ln([M]0/[M]) versus time is shown in Fig. 2. The plot shows an approximately straight line, proving that the concentration of propagating radicals was stable, and the side reactions can be ignored during the polymerization.59,60 In addition, an induction period of ca. 3 h is observed. The induction period probably originated from the following factors.61,62 On the one hand, Fe3+ and Fe2+ required a certain time to establish dynamic equilibrium at the beginning of the polymerization. On the other hand, AIBN decomposed relatively slowly in the initial stage of the polymerization, and the primary radical generated from AIBN reacted completely with FeCl3 to form the activator species Fe2+, leading to the result that the concentration of the reactive radical species was too low to initiate the polymerization at a noticeable rate. The Mn,th of PMMA obtained from reverse ATRP may be calculated based on the following equation:63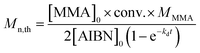
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 with monomer conversion, and are close to the theoretical Mn,th ones. The PDI values of the obtained polymers decrease from 1.45 to 1.16 in the first stage, and then remain almost unchanged. The trend in PDI values from high to low is in accord with the CRP principle.65 The change in the GPC traces displays the trend visually (Fig. 4). The curves are narrow and symmetrical. Moreover, the traces of the obtained typical polymers shift toward a shorter elution time, indicating the increase in Mn,GPC with monomer conversion.66 All the above observations suggest that the Fe(III)-[Rmim][Cl]-catalyzed reverse ATRP of MMA progressed in a controlled/“living” manner.67
900 with monomer conversion, and are close to the theoretical Mn,th ones. The PDI values of the obtained polymers decrease from 1.45 to 1.16 in the first stage, and then remain almost unchanged. The trend in PDI values from high to low is in accord with the CRP principle.65 The change in the GPC traces displays the trend visually (Fig. 4). The curves are narrow and symmetrical. Moreover, the traces of the obtained typical polymers shift toward a shorter elution time, indicating the increase in Mn,GPC with monomer conversion.66 All the above observations suggest that the Fe(III)-[Rmim][Cl]-catalyzed reverse ATRP of MMA progressed in a controlled/“living” manner.67

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C.
2, [MMA]0 = 9.4 mol L−1, T = 70 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) is closer to the theoretical one (Mn,th = 11
500) is closer to the theoretical one (Mn,th = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800). Nevertheless, the PDI value is slightly higher than that with a chlorine-containing catalyst, FeCl3 (PDI = 1.28, entry 3). To sum up, FeCl3 is the most suitable catalyst for the investigated systems, as evidenced from the results that the corresponding polymerization yields 76% monomer conversion, and produces well-defined PMMA with a controlled Mn,GPC (12
800). Nevertheless, the PDI value is slightly higher than that with a chlorine-containing catalyst, FeCl3 (PDI = 1.28, entry 3). To sum up, FeCl3 is the most suitable catalyst for the investigated systems, as evidenced from the results that the corresponding polymerization yields 76% monomer conversion, and produces well-defined PMMA with a controlled Mn,GPC (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) close to Mn,th (12
800) close to Mn,th (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) and the lowest PDI value (1.28).
900) and the lowest PDI value (1.28).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, T = 70 °C.
2, [MMA]0 = 9.4 mol L−1, T = 70 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is a CRP process. To investigate the generality of this approach for different monomers, EMA and BMA were selected instead of MMA as the monomer to carry out the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP under the same reaction conditions. As shown in Table 4, the monomer conversion reaches 76% after 8 h for the MMA system, and the measured molecular weight Mn,GPC value is very close to the theoretical one (entry 1). Likewise, the EMA and BMA polymerization systems achieve moderate-to-high rates of polymerization (58% and 70% monomer conversions, respectively), and the measured molecular weight Mn,GPC values (14
2 is a CRP process. To investigate the generality of this approach for different monomers, EMA and BMA were selected instead of MMA as the monomer to carry out the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP under the same reaction conditions. As shown in Table 4, the monomer conversion reaches 76% after 8 h for the MMA system, and the measured molecular weight Mn,GPC value is very close to the theoretical one (entry 1). Likewise, the EMA and BMA polymerization systems achieve moderate-to-high rates of polymerization (58% and 70% monomer conversions, respectively), and the measured molecular weight Mn,GPC values (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 18
500 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, respectively) of the resultant EMA and BMA polymers are higher than their corresponding theoretical ones (10
800, respectively) of the resultant EMA and BMA polymers are higher than their corresponding theoretical ones (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and 10
700 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, respectively). Importantly, their corresponding polymers give narrow molecular weight distribution values (PDI = 1.18 and 1.22, respectively). Therefore, the EMA and BMA polymerization systems proceed according to the CRP principle, confirming that the polymerization approach can be successfully extended to other monomers.
800, respectively). Importantly, their corresponding polymers give narrow molecular weight distribution values (PDI = 1.18 and 1.22, respectively). Therefore, the EMA and BMA polymerization systems proceed according to the CRP principle, confirming that the polymerization approach can be successfully extended to other monomers.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [MMA]0 = 9.4 mol L−1, [BMA]0 = 6.30 mol L−1, [EMA]0 = 8.04 mol L−1, T = 70 °C.
2, [MMA]0 = 9.4 mol L−1, [BMA]0 = 6.30 mol L−1, [EMA]0 = 8.04 mol L−1, T = 70 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the original volume ratio of MMA to solvent was 2
2, and the original volume ratio of MMA to solvent was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Clearly, compared with the bulk polymerization yielding 76% monomer conversion after 8 h and the controlled PMMA with a measured Mn,GPC very close to the theoretical Mn,th and PDI = 1.28 (entry 1, Table 5), the polymerization in DMSO shows the lowest monomer conversion (23%) after 20 h and gives a less-controlled PMMA, with a Mn,GPC (7600) three times larger than the theoretical Mn,th (2300, entry 2). Similar results are found in Fe(II)-catalyzed normal ATRP in DMSO without any ligand, as reported by Matyjaszewski et al.,69 which is possibly attributable to the fact that DMSO coordinates much more strongly to the Fe catalyst and substitutes for halide ligands, thereby weakening the catalytic activity and inducing a loss of control over the polymerization. In contrast, the excellent solvent systems for PMMA, i.e., DMF, MeCN, and CH2Cl2, can reach relatively higher monomer conversions within the same reaction time of 20 h (entries 3–5): in particular, MeCN and CH2Cl2 reach up to 91% monomer conversion (entries 4 and 5). Moreover, for the less polar solvent toluene, the monomer conversion reaches 80% after 20 h (entry 6), suggesting that this less polar solvent has almost no effect on the polymerization system. On the basis of the above results that CH2Cl2 and MeCN have a significant impact on the polymerization, the non-halogen solvent MeCN is thus chosen as the optimal solvent for the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA. It is noteworthy that the measured Mn,GPC values are much larger than the theoretical Mn,th ones: that is, the initiator efficiency (f) based on f = Mn,th/Mn,GPC is lowered as the polymerization is conducted in the solvent. This may be due to the occurrence of coupling reactions of primary radicals in the initial stages of the polymerizations in various solvents. However, all investigated polymerizations consisting of bulk and solution systems produced polymers with lower PDI results (1.20–1.29), showing the high controllability of the employed Fe(III)-[C12mim][Cl] catalytic system for methacrylate monomers.
1. Clearly, compared with the bulk polymerization yielding 76% monomer conversion after 8 h and the controlled PMMA with a measured Mn,GPC very close to the theoretical Mn,th and PDI = 1.28 (entry 1, Table 5), the polymerization in DMSO shows the lowest monomer conversion (23%) after 20 h and gives a less-controlled PMMA, with a Mn,GPC (7600) three times larger than the theoretical Mn,th (2300, entry 2). Similar results are found in Fe(II)-catalyzed normal ATRP in DMSO without any ligand, as reported by Matyjaszewski et al.,69 which is possibly attributable to the fact that DMSO coordinates much more strongly to the Fe catalyst and substitutes for halide ligands, thereby weakening the catalytic activity and inducing a loss of control over the polymerization. In contrast, the excellent solvent systems for PMMA, i.e., DMF, MeCN, and CH2Cl2, can reach relatively higher monomer conversions within the same reaction time of 20 h (entries 3–5): in particular, MeCN and CH2Cl2 reach up to 91% monomer conversion (entries 4 and 5). Moreover, for the less polar solvent toluene, the monomer conversion reaches 80% after 20 h (entry 6), suggesting that this less polar solvent has almost no effect on the polymerization system. On the basis of the above results that CH2Cl2 and MeCN have a significant impact on the polymerization, the non-halogen solvent MeCN is thus chosen as the optimal solvent for the Fe(III)-[C12mim][Cl]-catalyzed reverse ATRP of MMA. It is noteworthy that the measured Mn,GPC values are much larger than the theoretical Mn,th ones: that is, the initiator efficiency (f) based on f = Mn,th/Mn,GPC is lowered as the polymerization is conducted in the solvent. This may be due to the occurrence of coupling reactions of primary radicals in the initial stages of the polymerizations in various solvents. However, all investigated polymerizations consisting of bulk and solution systems produced polymers with lower PDI results (1.20–1.29), showing the high controllability of the employed Fe(III)-[C12mim][Cl] catalytic system for methacrylate monomers.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, MMA/solvent = 2
2, MMA/solvent = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 20 h.b Reaction time = 8 h.
1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C, reaction time = 20 h.b Reaction time = 8 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (entries 1–2). For [AIBN]0/[FeCl3]0/[IL]0 = 1
1, respectively (entries 1–2). For [AIBN]0/[FeCl3]0/[IL]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the polymerization gives 91% monomer conversion within 20 h (entry 3). Decreasing [AIBN]0/[FeCl3]0/[IL]0 down to 1
2, the polymerization gives 91% monomer conversion within 20 h (entry 3). Decreasing [AIBN]0/[FeCl3]0/[IL]0 down to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the polymerizations yield only 50% conversion within 46 h (entries 4 and 5). Thus the [FeCl3]0/[IL]0 value is a crucial experimental parameter for the rate of polymerization, and adding an excess of catalyst obviously decreases monomer conversions from ca. 100% to 50% (Fig. 5). The same rule can be observed from the changes in the PDI and molecular weight values with different [FeCl3]0/[IL]0 values, as exhibited in Table 6, Fig. 5, and 6. With low [FeCl3]0/[IL]0 values of 0.5
4, the polymerizations yield only 50% conversion within 46 h (entries 4 and 5). Thus the [FeCl3]0/[IL]0 value is a crucial experimental parameter for the rate of polymerization, and adding an excess of catalyst obviously decreases monomer conversions from ca. 100% to 50% (Fig. 5). The same rule can be observed from the changes in the PDI and molecular weight values with different [FeCl3]0/[IL]0 values, as exhibited in Table 6, Fig. 5, and 6. With low [FeCl3]0/[IL]0 values of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the corresponding PDI values are relatively higher (1.91 and 1.44, respectively). With a decrease in [AIBN]0/[FeCl3]0/[IL]0 down to 1
1, the corresponding PDI values are relatively higher (1.91 and 1.44, respectively). With a decrease in [AIBN]0/[FeCl3]0/[IL]0 down to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the corresponding PDI values remain in a narrow range of 1.20–1.23. For the case of molecular weight, the final polymers obtained from five catalytic systems show a straight drop from 22
4, the corresponding PDI values remain in a narrow range of 1.20–1.23. For the case of molecular weight, the final polymers obtained from five catalytic systems show a straight drop from 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 to 8800 with the variation in [FeCl3]0/[IL]0 values. Furthermore, it is worthwhile noting the deviation between the measured molecular weight value and the corresponding theoretical one, i.e., the initiator efficiency (Fig. 6). For example, for [FeCl3]0/[IL]0 = 0.5
600 to 8800 with the variation in [FeCl3]0/[IL]0 values. Furthermore, it is worthwhile noting the deviation between the measured molecular weight value and the corresponding theoretical one, i.e., the initiator efficiency (Fig. 6). For example, for [FeCl3]0/[IL]0 = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the measured Mn,GPC values (22
1, the measured Mn,GPC values (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 and 18
600 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, respectively) of the obtained polymers are higher than their corresponding theoretical ones (11
500, respectively) of the obtained polymers are higher than their corresponding theoretical ones (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 11
900 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, respectively). Among the molar ratios investigated, the polymerization shows the highest initiator efficiency (0.66) with a molar ratio of [FeCl3]0/[IL]0 = 2
100, respectively). Among the molar ratios investigated, the polymerization shows the highest initiator efficiency (0.66) with a molar ratio of [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Therefore, all the results suggest that selecting a suitable molar ratio of [FeCl3]0/[IL]0 = 2
2. Therefore, all the results suggest that selecting a suitable molar ratio of [FeCl3]0/[IL]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is extremely important for the reverse ATRP of MMA.
2 is extremely important for the reverse ATRP of MMA.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C.
1 (v/v), [MMA]0 = 9.4 mol L−1, T = 70 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of the polymer is much larger than the theoretical one Mn,th (5000). In contrast, when the polymerization is performed at a relatively high temperature (90 °C, entry 3), it gives 94% monomer conversion after a shorter polymerization time (11 h). In addition, the PDI values (1.34–1.44) of the obtained polymers remain low at the three polymerization temperatures, indicating that the polymerization can be successfully conducted over a wide temperature range (60–90 °C).
000) of the polymer is much larger than the theoretical one Mn,th (5000). In contrast, when the polymerization is performed at a relatively high temperature (90 °C, entry 3), it gives 94% monomer conversion after a shorter polymerization time (11 h). In addition, the PDI values (1.34–1.44) of the obtained polymers remain low at the three polymerization temperatures, indicating that the polymerization can be successfully conducted over a wide temperature range (60–90 °C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, MMA/MeCN = 2
1, MMA/MeCN = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), [MMA]0 = 9.4 mol L−1.
1 (v/v), [MMA]0 = 9.4 mol L−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the bulk polymerization achieved a controlled/“living” radical polymerization process with a high molecular weight (Mn,th = 12
2, the bulk polymerization achieved a controlled/“living” radical polymerization process with a high molecular weight (Mn,th = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 and Mn,GPC = 12
800 and Mn,GPC = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) and initiator efficiency (f = 0.99), and low PDI value (PDI = 1.28). In addition, the polymerization system can be successfully extended to other vinyl monomers. The polymerizations in different solvents, including DMSO, DMF, MeCN, CH2Cl2, and toluene, gave varied control of molecular weight and PDI values, and showed that MeCN can be the best solvent for the polymerization system. Moreover, the halogen-containing chain-end functionality of the resultant PMMA was confirmed by GPC and 1H NMR analyses.
900) and initiator efficiency (f = 0.99), and low PDI value (PDI = 1.28). In addition, the polymerization system can be successfully extended to other vinyl monomers. The polymerizations in different solvents, including DMSO, DMF, MeCN, CH2Cl2, and toluene, gave varied control of molecular weight and PDI values, and showed that MeCN can be the best solvent for the polymerization system. Moreover, the halogen-containing chain-end functionality of the resultant PMMA was confirmed by GPC and 1H NMR analyses.







