Open Access Article
Open Access ArticleEnhanced arsenite immobilization via ternary layered double hydroxides and application to paddy soil remediation†
Jizhi Zhou ab,
Weikang Shuab,
Yuan Gaoab,
Zhenbang Caoab,
Jia Zhangab,
Hao Houab,
Jun Zhaoab,
Xueping Chenb,
Yun Panab and
Guangren Qian*ab
ab,
Weikang Shuab,
Yuan Gaoab,
Zhenbang Caoab,
Jia Zhangab,
Hao Houab,
Jun Zhaoab,
Xueping Chenb,
Yun Panab and
Guangren Qian*ab
aCenter for Green Urban Mining & Industrial Ecology, Shanghai University, No. 99 Shangda Road, Shanghai 200444, P. R. China. E-mail: grqian@shu.edu.cn
bSchool of Environmental and Chemical Engineering, Shanghai University, No. 99 Shangda Road, Shanghai 200444, P. R. China
First published on 7th April 2017
Abstract
A ternary CaMgFe-LDH was developed for the immobilization of aqueous arsenic from capillary water in paddy soils, with an outstanding removal performance for aqueous arsenite (As(III)). In an As(III) solution, the ternary LDH achieved a removal capacity for arsenite of approximately 16 mg g−1 after 5 h, with a low equilibrium concentration of As(III) (0.048 mg L−1). The As(III) removal capacity of LDH was studied with the different Ca/Mg molar ratios in LDH, which demonstrated that ternary LDHs with a higher Ca content can remove As(III) more effectively and rapidly. Accordingly, the ternary LDH material was used in the immobilization of total arsenic from a paddy soil system, achieving a removal efficiency for As(III) of 47% and a total As concentration of 346 μg L−1 in capillary water after 40 days. Compared to that of the binary Mg–Fe LDH, the As removal performance of the ternary LDH was higher, which was attributed to the As precipitation with Ca in the first 20 days during the experiment. This, along with the efficient adsorption of As on the residual Mg–Fe-LDH framework, was responsible for the low concentration of As. Therefore, our study proposes a promising approach to the remediation of arsenic-contaminated paddy soils.
1. Introduction
Arsenic in soil poses a severe problem in several countries, such as China, Vietnam, Bangladesh, Thailand and Pakistan. Arsenic in paddy soils can be transported via capillary water of soils to rice, thus posing a high risk to human health.1,2 Thus, a lower concentration of As in capillary water would lead to a decrease in the uptake of As by rice plants. For practical applications, sequestration by adsorption has emerged as a promising technology to remove aqueous arsenic from soil as it does not yield by-products.3,4 As a result, the adsorbents such as zeolites,5 activated alumina,6 activated carbon,7 anionic clays,8 and metal oxides/hydroxides9 have been developed to remediate arsenic-contaminated soils. Particularly, Fe-containing compounds, with higher affinity for selected oxyanions, have been widely used for the removal of contaminants, particularly arsenate (As(V)).10,11 However, in paddy fields, the anaerobic medium can promote the reduction of As(V) to As(III), which is more toxic than As(V).12,13 In such a case, Fe-containing compounds such as Fe2O3 showed a lower adsorption capacity for As(III) than for As(V), which led to higher As(III) concentrations in capillary water, with further accumulation of As(III) in rice. Therefore, the efficient immobilization of arsenite is challenging.14The adsorption of anions on layered double hydroxides (LDHs) has attracted much attention. The general formula for LDHs is [M2+1−xM3+x(OH)2]x+[(An−)x/n·yH2O]x−, where M2+ and M3+ represent divalent and trivalent metal cations, respectively, and x denotes the molar ratio of M3+ to total metal, which ranges from 0.23 to 0.30 for a pure LDH structure.15 An− represents the interlayer anions, which balance the positive charge on the metal hydroxide layer. As the bonding interactions between layers and anions are relatively weak, host anions such as NO3− and Cl− in LDHs can be readily exchanged with various guest organic and inorganic anions. Based on this feature, LDHs have already been proven more efficient for the removal of various oxyanions, including phosphate, chromate, selenite and arsenate, than Fe oxides or hydroxides.16–18 Since As(III) shares a similar microstructure with the abovementioned oxyanions, LDHs could be potential candidates for As(III) immobilization.
In addition to anion exchange, the variety of cations contained in the LDH layer also improved the capture of anions. In our previous study, a Ca-based LDH was used to remove arsenic from an aqueous solution by precipitation of calcium arsenate.18 In another study, Ca was doped into a MgFe-LDH to form a ternary MgCaFe-LDH for the enhanced removal of pyrophosphate (PP) and triphosphate (TPP) in water. The results demonstrated that the release of Ca from the LDH matrix was responsible for the precipitation of anions, while anion exchange on the resultant Mg-rich LDH framework contributed to the immobilization of anions.17,19 Since the precipitation of As(III) with Ca was also verified in solution,20,21 it was suggested that As(III) may be immobilized more effectively by the Ca/Mg ternary LDH than by the binary system from both capillary water and soil. To the best of our knowledge, the application of ternary LDH systems for As(III) immobilization from paddy soils has not been reported.
Therefore, the objectives of this study were the following: (1) to prepare and characterize a series of LDHs with different Mg/Ca ratios; (2) to study the removal of As(III) by the as-prepared LDHs based on the isotherm and kinetics curves; and (3) to establish an efficient process for the removal of As by the as-synthesized LDH from a simulated paddy field system.
2. Materials & methods
2.1 Synthetic procedures
All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All reagents were of analytical grade and were used as received without further purification. To minimize contamination with carbon dioxide, deionized water was used in the experiments. The corresponding Mg3−xCaxFe–Cl-LDHs samples, where x = 0, 0.3, 1.0, 1.5 and 3.0, were denoted as LDHx. These were prepared via coprecipitation by addition of the pre-mixed metal chlorides to a basic solution under vigorous stirring. For instance, to prepare LDH3, 8.820 g of CaCl2·2H2O (0.06 mol) and 6.762 g of FeCl3·10H2O (0.02 mol) were dissolved in 50 mL of water (solution A). Solution B was prepared by dissolving 6.0 g (0.150 mol) of NaOH in 100 mL of water. Solution A was added to solution B under vigorous stirring for 4 h and subsequently, the suspension was aged for 18 h at room temperature in a N2 atmosphere. The precipitate was collected by centrifugation at 2500 rpm for 8 min and washed twice with 150 mL of deionized water. Sample was desiccated at 60 °C in a vacuum oven. Other LDHs were prepared in the similar way by varying x according to the designed value.2.2 Removal of aqueous As(III)
For the adsorption isotherms, a series of As(III) solutions with concentrations ranging from 1 to 12 mg L−1 at a pH of 7.0 were prepared by dissolving Na3AsO3. Typically, 1000 mL of the As(III) solution and 0.50 g of LDHx were mixed in a sealed conical flask. The adsorption was conducted under oscillation in a water bath at 25 °C and the remaining arsenite(III) concentration in each solution was determined accordingly within 24 h. The kinetics for the removal of As(III) by Mg3−xCaxFeCl-LDHs was investigated by monitoring the As(III) concentration in 1000 mL of a solution with an initial As(III) concentration of 10 mg L−1 to which 0.50 g of LDHx had been added. At certain intervals, 5 mL of solution were extracted, and filtered through a 0.22 μm microporous membrane prior to determining As(III) concentration. Other metal concentrations were also analyzed.2.3 Release of As(III) in a paddy field system and removal by LDH
Five duplicated samples of 450 g of paddy soil were artificially contaminated by adding 0.48 mmol of As(III). One of the duplicates was set as a control group, while LDH1 and LDH0 were added in other four duplicates. Soil samples were aged for 30 days after contamination with As(III) to ensure a better immobilization of As(III).Three circular plastic buckets (1 L, diameter 10 cm) were weighed, and the soil was added into these buckets. Rhizon sampler pipes for capillary water were placed into the soil. The water was gently added to the buckets until the height of surface water was 3 cm over soil. The buckets were maintained at a constant temperature (25 °C) and humidity (55% RH). From the flanks of the buckets, 5 mL of capillary water were sampled every day via vacuum tubes, and the pH of the water sample was measured. All samples were acidified with 0.6 mmol of HCl, and then stored at 4 °C. Then, the concentration of metal ions and As(III) was measured. The LDH dosage of 0.2 and 2.0 g was used at the equilibrium of As(III) removal.
2.4 Characterization
A portable pH-meter with a glass electrode (Aqua Cond/pH, TPS) was used to measure the pH. The concentration of heavy metals in solution was analysed by inductively coupled plasma-atomic emission spectrometry (ICP-AES, Prodigy, Leeman Co.). After centrifugation and filtration with a 0.22 μm filter, the concentration of As(III) and As(V) was determined by high performance liquid chromatography-hydride generation-atomic absorption spectroscopy (HPLC-HG-AAS). These species were separated with a Hamilton PRP-100. The total concentration of As was determined by the same method but without using a Hamilton PRP-100. The powder X-ray diffraction patterns for the solid sample were recorded on a Dmax/RB diffractometer (Rigaku Co.) with Cu Kα radiation (λ = 0.15406 nm) at 40 kV and 100 mA. Crystalline parameters were calculated by the Scherrer equation. The Powder Data File (ICDD-JCPDS) was used for the analysis of the patterns. The C content of the LDHs was determined by elemental analysis (EA3000, Leeman Co.).3. Results and discussion
3.1 Characterization of solid samples before and after As(III) removal
Fig. 1A shows the XRD patterns of the as-synthesized LDHs with various Ca contents. The XRD pattern of the Mg-based binary LDH (LDH0) displayed a series of diffraction peaks at 11.14°, 22.44°, 34.32°, 59.34° and 60.90°. The XRD pattern of LDH3 displayed diffraction peaks at 11.31°, 23.14°, 38.39°, 54.12° and 55.90°, which were indexed to the Ca-based binary LDH. In the XRD of ternary LDHx (x = 0.3, 1.0, 1.5), characteristic peaks of both Mg-LDH and Ca-LDH were observed as the Ca content increased in Mg-based LDHs. No impurity was observed in any of the samples, suggesting that pure LDH was synthesized as expected. The lattice parameters obtained from the XRD patterns are listed in Table 1. According to the Bragg's law for the crystalline, the d-spacing indicated the formation of a layered structure, and the intercalation of Ca within the layers led to a decrease of the interlayer distance. This indicated the successful establishment of a ternary LDH system. The results of the elemental analysis are also listed in Table 1. The metal composition was close to the designed value for most samples. The ratio of divalent to trivalent metal ranged from 2.26–2.9, which was within the value for pure LDH, in agreement with the XRD results. The Ca content in LDH0.3 was not as high as expected, indicating that its structure was closer to that of the binary Mg-LDH. Interestingly, despite the significant variation on the determined Mg/Ca molar ratio in LDH1 and LDH1.5 (ranged from 1.23 to 2.63), both samples demonstrated an identifiable ternary LDH structure. Due to the release of Ca from the LDHs, it was assumed that both LDH1 and LDH1.5 were able to remove As(III) by precipitation with the released Ca, as well as by adsorption on the LDH matrix. Moreover, with the increase in Ca content, the carbon content in the LDHs increased slightly, which suggested a higher number of carbonate anions in the interlayer of ternary LDHs. In addition, the LDHx (x = 0.3–1.5) formula was estimated, which indicated the proportion of Cl in ternary LDHs was higher than that in the binary Ca-LDH. Such result suggested a higher anion-exchange ability for the ternary LDHs.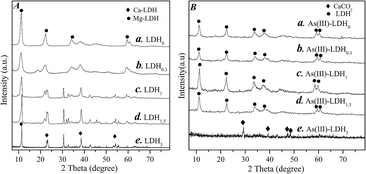 | ||
| Fig. 1 (A) XRD patterns of the synthetic LDHs, (B) XRD patterns of the LDH sample after As(III) removal; (a) LDH0, (b) LDH0.3, (c) LDH1, (d) LDH1.5 and (e) LDH3. | ||
| Samples | Carbon content (%) | d-Spacinga (nm) | Measured compositionb | Estimated LDH formulac | |
|---|---|---|---|---|---|
| Mg/Ca | M(II)/Fe(III) | ||||
| a d-Spacing was calculated by the equation d-spacing = (d003 + 2d006)/2.b M represents a divalent metal, either Mg, Ca or both.c Compositions were determined by the stoichiometric number of Fe. | |||||
| LDH3 | 3.03 | 0.763 | — | 2.87 | Ca2.9FeCl0.52(CO3)0.24·4.9H2O |
| LDH1.5 | 2.26 | 0.773 | 1.23 | 2.60 | Mg1.4Ca1.2FeCl0.70(CO3)0.15·3.2H2O |
| LDH1 | 0.99 | 0.781 | 2.63 | 2.83 | Mg2.0Ca0.8FeCl0.86(CO3)0.068·2.8H2O |
| LDH0.3 | 0.63 | 0.795 | 20.7 | 2.26 | Mg2.2Ca0.1FeCl0.93(CO3)0.037·2.1H2O |
| LDH0 | 0.37 | 0.804 | — | 2.90 | Mg2.9FeCl0.95(CO3)0.024·2H2O |
Fig. 1B shows the XRD diffraction patterns of the LDH samples after As(III) removal. On the pattern of the resultant LDH3 there were no LDH characteristic peaks, and only those attributed to CaCO3 were observed, which indicated the collapse of the LDH structure upon Ca release. On the other hand, the XRD pattern of LDH0 displayed diffraction peaks at 11.31°, 22.48°, 33.79°, 37.63°, 59.02° and 60.36°, which were indexed to Mg-LDH. This suggested the adsorption of As(III) on the binary Mg-LDH. For ternary LDH samples, the diffraction peaks of Mg-LDH could be observed while no diffraction peaks of Ca-LDH were observed regardless of Ca content before As(III) removal (such as LDH1 and LDH1.5). The d-spacing value of LDH strucutre in XRD patterns of LDH0, LDH0.3, LDH1 and LDH1.5 after As(III) adsorption was 0.7925, 0.8010, 0.7928 and 0.7929 nm, respectively, close to that of LDH0 before adsorption of As(III). These results were consistent with our previous work on phosphate removal by ternary LDHs and suggested that the Mg-LDH framework in solution was highly stable.17 Accordingly, As(III) removal can occur both via adsorption on the LDH structure and via precipitation with the Ca released from the ternary LDHs.
3.2 Performance of ternary LDHs for the removal of aqueous As(III)
Fig. 2 shows the kinetics and isotherm curves for the removal of aqueous As(III) by LDHs with different contents of Ca. As shown in Fig. 2A, the kinetics of As(III) removal by LDH0 over 24 h involved a relatively sharper increase in the amount removed within the first 10 h, followed by a gentle increase thereafter. The maximum removal capacity was approximately 15 mg g−1, indicating that 75% of As(III) was removed. In comparison, LDHs containing Ca exhibited a higher removal capacity for As(III). A rapid increase in the amount of As(III) removed was observed for LDHx (x = 0.3, 1, 1.5) in the first 2–7 h, with a constant removal capacity of 15 mg g−1 at equilibrium. Thus, a higher content of Ca in the LDHs appeared to favour a rapid equilibrium. For instance, LDH1.5 reached equilibrium for As(III) removal after 2 h, while LDH0.3 reached equilibrium after 7 h. Table 2 lists the results obtained after fitting the kinetics data to a Laguerre first-order equation and simplified Elovich equation, which indicated that the removal kinetics was in better accordance with a Laguerre first-order equation. It also suggested that the removal of As(III) could be described by a chemical reaction equation. Moreover, the reaction rate constant (K1) increased as the estimated removal capacity (qe) at equilibrium increased. LDH1.5 exhibited the maximum removal capacity, of 15.6 mg g−1, which was higher than that of LDH0. Thus, the improvement in the As(III) removal capacity by the incorporation of Ca suggested that the As(III) removal capacity of MgFe-LDH could be enhanced by adding Ca to the LDH structure. This was in agreement with the results obtained for the adsorption of phosphate on MgCaFe-LDH on a previous work.17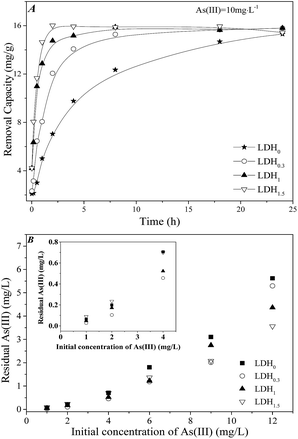 | ||
| Fig. 2 Study of the removal of As(III) by LDHs: (a) removal kinetics for a As(III) concentration of 10 mg L−1 and (b) adsorption isotherms for As(III) concentrations ranging from 1–12 mg L−1. | ||
| Samples | First order Lagergren equation qt = qe(1 − exp(−K1t)) | Simplified Elovich equation qt = A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t |
||||
|---|---|---|---|---|---|---|
| K1 | qe | R2 | A | B | R2 | |
| LDH0 | 0.0054 | 14.5140 | 0.9484 | −1.4165 | 2.0779 | 0.8299 |
| LDH0.3 | 0.0143 | 15.2834 | 0.9582 | 0.3474 | 2.2160 | 0.9087 |
| LDH1 | 0.0454 | 15.2380 | 0.8793 | 4.4352 | 1.7709 | 0.8900 |
| LDH1.5 | 0.0616 | 15.6000 | 0.8858 | 5.5665 | 1.6711 | 0.8194 |
The As(III) removal capacity of LDHx (0.3 < x < 1.5) was evaluated for various initial concentrations of As(III) ([As(III)]ini), and compared to that of LDH0. As shown in Fig. 2B, the equilibrium concentration of As(III) after its removal ([As(III)]fin) on all samples was higher for higher [As(III)]ini. For instance, for LDH1.5, the [As(III)]fin increased from 0.089 mg L−1 to 3.56 mg L−1 as [As(III)]fin increased from 1 mg L−1 to 12 mg L−1. Likewise, the [As(III)]fin for LDH0 at the highest [As(III)]ini was 5.62 mg L−1, with a [As(III)]fin of 0.065 mg L−1 at a [As(III)]ini of 1 mg L−1. The same trend was observed for LDH0.3. It should be noted that the [As(III)]fin for a high [As(III)]ini depended on the Ca content in the LDHs. As a result, the maximum removal capacity for As(III) decreased from 17.2 mg g−1 to 13.1 mg g−1 as the x value decreased from 1.5 to 0. Therefore, it was concluded that the ternary LDHs with x = 1.0 and 1.5 displayed excellent removal performances, especially for As(III) contamination at relatively high concentrations.
On the other hand, the immobilization of As(III) at low concentrations from soil capillary water is also of much interest. As discussed above, for a [As(III)]ini of 1 mg L−1, the [As(III)]fin for LDH0 was lower than for LDH1.5. This suggested that the adsorption on the LDH led to the improvement of As(III) immobilization at low As(III) concentrations. Interestingly, the [As(III)]fin for LDH0.3 and LDH1.0 was 0.027 and 0.048 mg L−1, respectively, lower than that for LDH0. This indicated that a small portion of Ca in LDH (x = 0.3 and 1.0) was probably responsible for As(III) immobilization at low [As(III)]ini. Consequently, controlling Ca content in the LDH appears to be key for As(III) immobilization. For further investigation of the As(III) removal process, LDH1.0 was selected as a model sample due to its relatively high performance in the removal of As(III) among all the LDHx (0.3 < x < 1.5).
3.3 LDH composition after removal
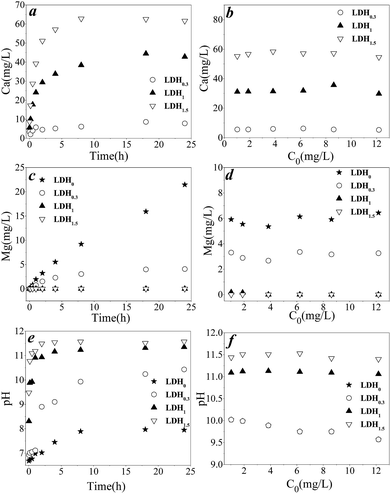
As expected, the leaching of Mg or Ca correspondingly changed the final pH when LDHs were used for anion removal.18,22 In terms of the solubility of the individual hydroxides, Ca(OH)2 (Ksp = 5.02 × 10−6) is much more soluble than Mg(OH)2 (Ksp = 5.61 × 10−12), and Fe(OH)3 is almost insoluble at pH = 8–11 (Ksp = 2.79 × 10−39).23 In the LDHs, the solubility of LDH was dependent on the dissolution of corresponding hydroxides in solution. Therefore, most Ca was dissolved in the solution, increasing water pH. In contrast, only a small fraction of Mg dissolved, lowering pH.18 In addition, the leaching of divalent metals (Mg and Ca) leaves a Fe-rich network with a higher surface area,22 which clearly improves the adsorption capacity for oxyanions.In order to gain insight on the effect of composition in LDHx, the concentration of Ca and Mg, and the pH during As(III) removal were determined, and results are shown in Fig. 3. Mg2+ was determined for LDH0, LDH0.3, LDH1 and LDH1.5 (Fig. 3c). Mg concentration remained relatively constant at 5.4–6.2 mg L−1 and 2.7–3.5 mg L−1 for LDH0.3 and LDH0, respectively, despite the fact that the amount of As(III) removed was increasing. This indicated that theoretically, a 6% and 4% of Mg had dissolved after As(III) removal for LDH0.3 and LDH0, respectively. In contrast, no Mg in solution was detected for either LDH1 or LDH1.5, which suggested that LDHs with a lower amount of Mg had a more stable structure. Fig. 3a shows the profile for the leaching of Ca during As(III) removal, which was similar to that of Mg. A lower amount of Ca within the ternary LDH clearly led to a lower equilibrium concentration of Ca. The value of dissolved Ca for LDH1.5, LDH1 and LDH0.3 was approximately 60, 40 and 5 mg L−1, suggesting that 93%, 95% and 82% of Ca had leached out, respectively. Visual MINTEQ was used to study the possibility of precipitation, and the results showed that no Ca or Mg hydroxides would precipitate in solution (the dominant species for Ca was Ca2+, and another species was Ca(OH)+; the same applied to Mg). The detailed species distribution is shown in Table S2.† As equilibrium of Ca for LDH3 was reached rapidly with a high removal amount of As(III) (Fig. S1†), the leaching of Ca in LDH1.5 and LDH1 could lead to a higher As(III) removal within a short time. Since the removal of As(III) via anion exchange was a comparatively slower process (Fig. 2), the simultaneous removal of As by precipitation, which was well exhibited on LDH1, and its practical application was further investigated in the next section.
As a result of metal release, the pH in LDH0.3 was approximately 10, and pH was close to 11 for both LDH1 and LDH1.5. Furthermore, no evident pH changes were observed within a wide range of As(III) concentrations (Fig. 3f). It seemed that pH was only determined by the composition of LDH, as indeed a higher pH was observed for those ternary LDHs with a higher Ca content. The low leaching amount of Mg from LDH1 and LDH0.3 ensured the stability of the Mg–Fe LDH framework after leaching of Ca, which favoured the adsorption of As(III) on these two LDHs.
Accordingly, the removal of As(III) was described by the following equations:
| Mg2CaFe(OH)8Cl → Mg2Fe(OH)6Cl + Ca2+ + OH− | (1) |
| Ca2+ + 2H2AsO3− → Ca(H2AsO3)2 | (2) |
| Mg2Fe(OH)6Cl + HAsO3− → Mg2Fe(OH)6(HAsO3) + Cl− | (3) |
When LDH was added to the solutions, the majority of Mg was retained in the LDH, supporting the framework. On the other hand, Ca was leached, forming an alkaline media (eqn (1)), in which Ca(H2AsO3)2 precipitated (eqn (2)), indicating the removal of As(III) by precipitation. Meanwhile, a number of H2AsO3− entered the interlayer space of the LDH, and were restrained by the LDH structure (eqn (3)), which represented an additional amount of removed As(III). Moreover, arsenate and CO32− also reacted with Ca2+, as shown in eqn (4) and (5), respectively.
| Ca2+ + HAsO42− → CaHAsO4 | (4) |
| Ca2+ + CO32− → CaCO3 | (5) |
In summary, these ternary LDHs, with a novel composition, present an excellent stability and significant removal capacity at the same time.
3.4 Removal of released As(III) in a simulated paddy field system
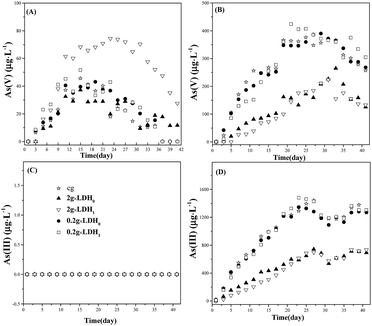
Although anion exchange also contributes to As(III) removal, the binding of As(III) to the LDH layers was driven by electrostatic forces, which may be compromised when exposed to a substrate with a complex composition, such as a paddy field. If this interaction was weakened, As(III) may be released into water again.The leaching of As(III) was monitored during a 40 day period, and results are shown in Fig. 4. LDH1 and LDH0 were selected to evaluate their removal capacity in a simulated paddy field system. A control group without LDH, denoted as cg, was also monitored. For the cg, the concentration of As(III) and As(V) in capillary water increased during the first 28 days, and then reached a stable concentration of about 1200 μg L−1 and 250 μg L−1, respectively (Fig. 4B and D). As shown in Fig. 4B and D, when 2.0 g of LDH were added, the increase in As(III) concentration was clearly less pronounced. Thus, the equilibrium As(III) concentration on adding LDH0 and LDH1 was 693.2 and 635.6 μg L−1 respectively, indicating that 45.2% and 47.0% of As(III) had been removed correspondingly. This result demonstrated that the removal of As(III) on the ternary LDH was more efficient than that on the binary LDH without Ca. It also showed that, in the first 10 days, LDH1 could maintain the concentration of As(III) and As(V) at 160.88 μg L−1 and 39.44 μg L−1, respectively, which were 50% lower than those with LDH0. It indicated that LDH1 had a better ability to limit the release of As(III) and As(V) within the short term. Furthermore, it was found that a dosage of 0.2 g of LDH failed to prevent the release of both As(III) and As(V) into capillary water, while a dosage of 2.0 g was able to reduce the concentration of As(III) and As(V) to 693.2 μg L−1 and 133 μg L−1, respectively. On the other hand, the concentration of As(III) in surface water for both samples was approximately zero (Fig. 4C), as it was probably oxidized by microorganisms and Mn(II) released from the soil24–26 (the content of Mn(II) was 0.05%, Table S1†). This led to the concentration of As(V) in surface water escalating to around 40 μg L−1 in the first 15 days (Fig. 4D). In the case of 2 g of LDH1, As(V) could be precipitated by released Ca from LDH and also being adsorbed by the LDH frame.
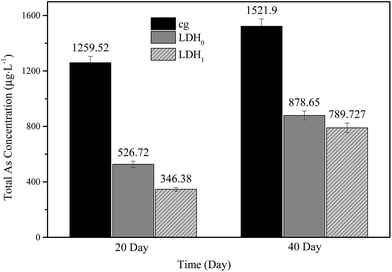
Mg and Ca concentrations in surface water and capillary water were monitored when 2.0 g of LDH were added to the soil samples, and results are shown in Fig. 5 (the composition of soil is shown in Table S1†). The initial Mg concentration was as low as to be negligible in both surface and capillary water in soil system (Fig. 5a and b). When the LDH was added, the Mg concentration in both surface water was increased, which was attributed to the Mg leaching from LDH. The similar situation was observed in the capillary water. The equilibrium concentration of Mg in surface water and capillary water were approximately 30 and 120 mg L−1, respectively, suggesting that the majority of Mg was still retained within the LDH matrix. Meanwhile, the Ca concentration in surface water increased during the first 10 days and remained constant thereafter, at about 100 mg L−1, irrespective of whether LDH was added or not (Fig. 5c). However, the Ca concentration in capillary water when adding LDH0 and LDH1 was clearly lower than in the cg (Fig. 5d), indicating the participation of Ca in As(III) removal. However, the removal amount of As(III) was not as high as the theoretical value in the hypothesis that all As(III) was precipitated with Ca in capillary water. This was attributed to the consuming of Ca in the precipitation of As(V) as the decrease in the total concentration of As. Furthermore, as shown in Fig. 6, for the control sample, the concentration of total As was 1295 μg L−1 after remediation for 20 days and increased to 1521 μg L−1 after 40 days. By contrast, both LDH0 and LDH1 inhibited the release of As such that a total As concentration of 526 and 346 μg L−1 was found after 20 days, respectively. Thus, at lower As concentrations, LDH1 showed a better performance for As immobilization than LDH0. However, after 40 days, an increase in the As concentration was also observed for the soil samples treated with LDH addition. The lower concentration of As in capillary water in the cg indicated the immobilization by LDH. In addition, a relatively low increase in pH was observed when LDH was added to the soil (Fig. S2†). Therefore, it was concluded that the ternary LDH could be a promising material for As immobilization from paddy soils.
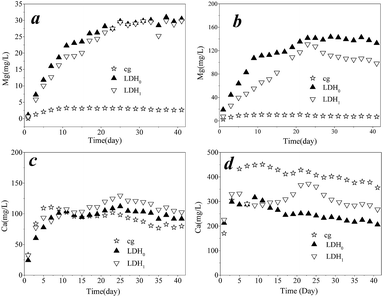 | ||
| Fig. 5 Mg concentration in (a) surface water and (b) capillary water; Ca concentration in (c) surface water and (d) capillary water. | ||
4. Conclusions
A ternary LDH with enhanced removal capacity for As(III) has been successfully applied in a paddy field system. This environmentally friendly material has demonstrated an outstanding chemical stability within aqueous systems owing to the Mg-dominant matrix, and an efficient removal via precipitation with Ca2+ ions released from the LDH matrix and interlayer anion exchange. The efficiency of the synthesized LDHs in paddy field remediation was also verified, and approximately a 47% of As(III) was removed, with a low concentration of total As of 346 μg L−1 in capillary water. Additionally, total As was immobilized more efficiently on ternary LDHs than on the binary Mg–Fe LDH, indicating that the ternary MgCaFe LDH is a promising material for remediation of As(III)-polluted sites.Acknowledgements
This project was supported by the National Nature Science Foundation of China (no. 51174132, no. 21207086, no. 41402311 and no. 20877053), and the Shanghai Municipal Education Commission Innovation project (15ZZ051). We also appreciate the technical support from Instrumental Analysis & Research Center of Shanghai University.References
- M. Gil-Díaz, S. Diez-Pascual, A. González, J. Alonso, E. Rodríguez-Valdés, J. R. Gallego and M. C. Lobo, Chemosphere, 2016, 149, 137–145 CrossRef PubMed.
- D. Ociński, I. Jacukowicz-Sobala, P. Mazur, J. Raczyk and E. Kociołek-Balawejder, Chem. Eng. J., 2016, 294, 210–221 CrossRef.
- C. M. Babu, R. Vinodh, B. Sundaravel, A. Abidov, M. P. Mei, S. C. Wang and H. T. Jang, J. Taiwan Inst. Chem. Eng., 2016, 62, 199–208 CrossRef CAS.
- B. Lan, Y. Wang, X. Wang, X. Zhou, Y. Kang and L. Li, Chem. Eng. J., 2016, 292, 389–397 CrossRef CAS.
- M. T. Olguín and S. Deng, J. Hazard. Mater., 2015, 302, 341–350 CrossRef PubMed.
- D. Ghosh and A. Gupta, Resour., Conserv. Recycl., 2012, 61, 118–124 CrossRef.
- X. J. Gong, W. G. Li, D. Y. Zhang, W. B. Fan and X. R. Zhang, Int. Biodeterior. Biodegrad., 2015, 102, 256–264 CrossRef CAS.
- S. Paikaray and M. J. Hendry, Appl. Clay Sci., 2013, 77–78, 33–39 CrossRef CAS.
- M. Martínez-Cabanas, M. López-García, J. L. Barriada, R. Herrero and M. E. S. D. Vicente, Chem. Eng. J., 2016, 301, 83–91 CrossRef.
- A. Sigdel, J. Park, H. Kwak and P. K. Park, J. Ind. Eng. Chem., 2016, 35, 277–286 CrossRef CAS.
- S. Matsumoto, J. Kasuga, T. Makino and T. Arao, Environ. Exp. Bot., 2016, 125, 42–51 CrossRef CAS.
- M. B. Baskan and A. Pala, J. Hazard. Mater., 2009, 166, 796–801 CrossRef CAS PubMed.
- K. V. Snyder, T. M. Webster, G. Upadhyaya, K. F. Hayes and L. Raskin, J. Environ. Manage., 2016, 171, 21–28 CrossRef CAS PubMed.
- E. M. Farrow, J. Wang, J. G. Burken, H. Shi, W. Yan, J. Yang, B. Hua and B. Deng, Ecotoxicol. Environ. Saf., 2015, 118, 55–61 CrossRef CAS PubMed.
- P. S. Braterman, Z. P. Xu and F. Yarberry, Layered double hydroxides (LDHs), in Handbook of Layered Materials, ed. S. M. Auerbach, K. A. Carrado and P. K. Dutta, Marcel Dekker, New York, 1st edn, 2004, ch. 8, p. 373 Search PubMed.
- Z. Gu, J. J. Atherton and Z. P. Xu, ChemInform, 2015, 51, 3024–3036 CAS.
- J. Zhou, Z. P. Xu, S. Qiao, Q. Liu, Y. Xu and G. Qian, J. Hazard. Mater., 2011, 189, 586–594 CrossRef CAS PubMed.
- Y. Xu, Y. Dai, J. Zhou, Z. P. Xu, G. Qian and G. Q. M. Lu, J. Mater. Chem., 2010, 20, 4684–4691 RSC.
- G. Carja, S. Ratoi, G. Ciobanu and I. Balasanian, Desalination, 2008, 223, 243–248 CrossRef CAS.
- M. Stachowicz, T. Hiemstra and W. H. V. Riemsdijk, J. Colloid Interface Sci., 2008, 320, 395–396 CrossRef PubMed.
- K. Grover, S. Komarneni and H. Katsuki, Water Res., 2009, 43, 3884–3890 CrossRef CAS PubMed.
- J. Zhou, Z. P. Xu, S. Qiao, Q. Liu, Y. Xu and G. Qian, J. Hazard. Mater., 2011, 189, 586–594 CrossRef CAS PubMed.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, Boca Raton, FL, 2007 Search PubMed.
- R. Seith and M. Jekel, Vom Wasser, 1997, 89, 283–296 CAS.
- W. Driehaus, R. Seith and M. Jekel, Water Res., 1995, 29, 297–305 CrossRef CAS.
- B. Hambsch, B. Raue and H. Brauch, Acta Hydrochim. Hydrobiol., 1995, 23, 166–172 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28116h |
| This journal is © The Royal Society of Chemistry 2017 |