Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dioxetane formation and chemiluminescent emission upon the combination of a vinylphenol derivative with naphthalene endoperoxide†
Yui Umeharaa,
Aoi Sona,
Teruyuki Kondo*a and
Kazuhito Tanabe *b
*b
aDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: teruyuki@scl.kyoto-u.ac.jp; Fax: +81-75-383-2504; Tel: +81-75-383-7055
bDepartment of Chemistry and Biological Science, College of Science and Engineering, Aoyama Gakuin University, 5-10-1 Fuchinobe, Chuo-ku, Sagamihara, 252-5258, Japan. E-mail: tanabe.kazuhito@chem.aoyama.ac.jp; Fax: +81-42-759-6493; Tel: +81-42-759-6229
First published on 31st January 2017
Abstract
We designed a chemiluminescent system using nanoparticles. We prepared mesoporous nanoparticles bearing naphthalene endoperoxide as a singlet oxygen generator in the pore and vinyl phenol derivative as an emission unit. The combination of these functional molecules gave highly efficient and bright chemiluminescence through the formation of a 1,2-dioxetane intermediate.
Chemiluminescent technology offers considerable potential for biological imaging, and various systems have been developed to characterize biological functions and properties.1,2 In particular, the combination of luciferase and luciferin, which produces red emission through the consumption of ATP, allows biological processes to be visualized in living cells and animals.3–12 However, the practical use of conventional chemiluminescent techniques has been limited because they have several drawbacks, such as requirements for complex gene technology and cell manipulation to generate specific enzymes in the subjects, resulting in relatively low versatility. On the other hand, small-molecule-based chemiluminescent systems that are based on distorted dioxetane derivatives have been developed.13–15 However, their suitability for biological imaging is also limited because of the instability of the molecules and the difficulties involved in further modification of the core structure. Therefore, much effort has recently been made to identify simpler chemiluminescent systems.
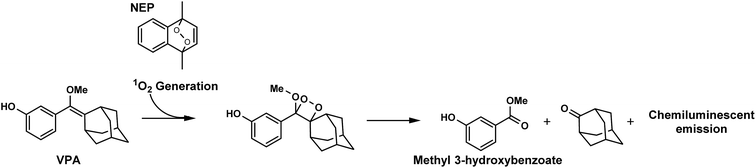
Herein, we report a novel chemiluminescent system in which dioxetane derivatives are formed in situ. We developed this system using a combination of naphthalene endoperoxide (NEP), which generates singlet oxygen (1O2) through thermolysis, and vinylphenols bearing an adamantyl group (VPA) as an emission unit. The latter is converted into dioxetane derivatives through the [2 + 2] cycloaddition of 1O2 (Fig. 1). Mixing of VPA with NEP leads to the spontaneous formation of dioxetane and the emission of chemiluminescence without any additives or other triggers. Furthermore, we prepared mesoporous nanoparticles bearing NEP within their pores (MSN-NEP) and confirmed that these particles induced the oxidation of VPA and led to chemiluminescence. The present system is a promising approach to chemiluminescence-based bio-imaging without the need for either gene or cellular manipulation.
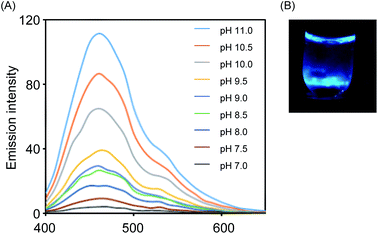
Initially, we prepared VPA and characterized its properties as an emission unit, because it is known to be a representative precursor of a chemiluminescent emission unit.13 On the other hand, NEP, as a donor of 1O2, was prepared from 1,4-dimethylnaphthalene by the photosensitization of methylene blue. To evaluate the chemiluminescence of VPA, we combined VPA and NEP and monitored the emission. As shown in Fig. 2A, we observed chemiluminescent emission at around 450 nm that was typical of oxidized VPA, indicating that VPA was spontaneously converted into the corresponding dioxetane derivative in the presence of 1O2 generated from the thermal degradation of NEP. The luminescence of VPA increased as the pH was increased, suggesting that deprotonation of the hydroxyl group on the aromatic unit is a key for the emission of VPA, which is consistent with previous reports.13 We also confirmed that the emission of VPA in the presence of NEP could be seen with the naked eye, as shown in Fig. 2B.
To evaluate this reaction in detail, we next analyzed the products obtained from [2 + 2] cycloaddition of VPA and 1O2 generated from NEP by means of GC-MS. Although the formation of dioxetane derivative of VPA was not detected, the measurement of MS spectra indicated the formation of methyl 3-hydroxybenzoate (molecular weight: 152), which was formed by the degradation of dioxetane derivative (Fig. S1†). This result strongly supports the occurrence of cycloaddition to form the dioxetane derivative.
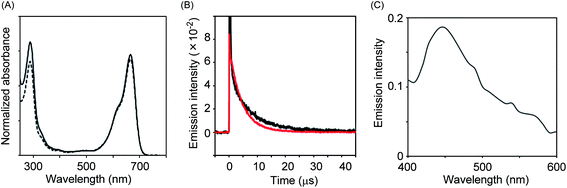
It is well known that 1O2 is a strong oxidant that can act on a huge variety of compounds including organic molecules, polymers as well as biomolecules. Thus, control of its activity is critical for the practical use of the present system in bio-imaging. It has been proposed that 1O2 species generated in the pores of nanoparticles are confined within the pores and inactivated before leaking out because of their short lifetime.16 Therefore, we designed a system in which NEP was incorporated within the pores of mesoporous silica nanoparticles (MSN-NEP). The synthesis of MSN-NEP is outlined in Scheme 1. Acid 1 was coupled with an ethylenediamine derivative to form amide 2. Removal of the Boc group in 2 under acidic conditions gave naphthalene derivative 3 possessing a primary amino group. Coupling of MSN bearing carboxylic acid groups (MSN-CO2H) with 3 formed nanoparticles possessing naphthalene units (MSN-NA). The photooxidation of MSN-NA in the presence of methylene blue as a photochemical generator of 1O2 furnished MSN-NEP. The formation of endoperoxide at the naphthalene unit was confirmed by measurement of the absorption spectrum. As shown in Fig. 3A, the absorption at around 300 nm in MSN-NA, which was assigned to absorption by the naphthalene unit, decreased as the photooxidation time increased, which indicated that NEP was formed in pores. In order to identify the thermal generation of singlet oxygen (1O2) from MSN-NEP, we measured the phosphorescence of 1O2 formed from MSN-NEP. We conducted photo-irradiation of MSN-NEP to generate heat for the formation of 1O2 and measured the emission of 1O2. We observed the emission at 1270 nm, which was identified as the phosphorescence of 1O2. In addition, we compared the lifetime of 1O2 and confirmed that 1O2 generated from MSN-NEP showed longer lifetime (9.1 μs) than that from NEP (4.8 μs) (Fig. 3B). These results and the evidence that the lifetime of 1O2 is affected by their surrounding environment such as solvents17 led the conclusion that MSN-NEP thermally generated 1O2 in their pores. We also confirmed that heating of MSN-NEP at 40 °C for 5 min resulted in an increase in absorption at around 300 nm due to the release of 1O2 (Fig. S2†). The number of naphthalene units incorporated on the surface of MSN was estimated to be 136 nmol mg−1, which was calculated from the absorption at 290 nm.
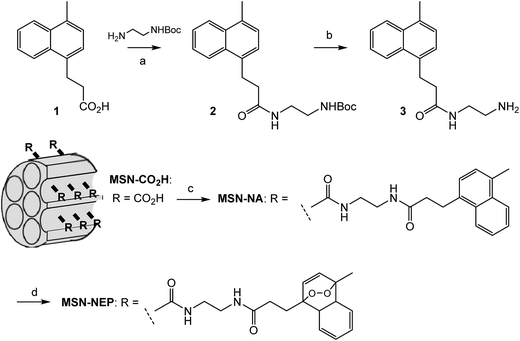 | ||
| Scheme 1 Synthesis of MSN-NEP. Reagents and conditions. (a) EDCI, HOBt, DMF, 74%; (b) HCl, 15%; (c) 3, EDCI, HOBt, DMF; (d) hν (665 nm), methylene blue. | ||
We then evaluated the reaction of VPA in the presence of MSN-NEP under basic conditions and compared the reaction efficiency between MSN-NEP and NEP upon reaction with VPA. HPLC analysis of the reaction showed that consumption amount of VPA in the presence of MSN-NEP was about half of that in the presence of NEP (Fig. S3†). Subsequently, we monitored the emission of VPA after the reaction for 1.5 h in the presence of MSN-NEP. Although the emission intensity was relatively weak, we observed chemiluminescence at around 450 nm, as shown in Fig. 3C. These results indicate that 1O2 was generated within the pores of MSN and reacted with VPA to form a dioxetane derivative, which degraded with the concomitant emission.
In conclusion, we prepared a novel chemiluminescent system using a vinyl phenol derivative (VPA) and naphthalene endoperoxide (NEP). In this system, VPA and NEP act as an emission unit and thermal 1O2 generator, respectively. The combination of VPA and NEP resulted in the formation of a dioxetane derivative via the [2 + 2] cycloaddition of VPA and 1O2; subsequent decomposition of a dioxetane derivative led to bright chemiluminescence. In addition, we prepared mesoporous silica nanoparticles bearing NEP within the pores (MSN-NEP) and confirmed that selective oxidation of VPA occurred within the pores to give a chemiluminescence. Although both the efficiency of the reaction and the emission intensity need to be improved, this approach may lead to a chemiluminescent emission system for biological imaging that does not require complex techniques such as gene or cellular manipulation. Further biological experiments are in progress.
Acknowledgements
We sincerely thank Professor Tadashi Suzuki (Aoyama Gakuin University) for the measurement of emission and lifetime of singlet oxygen. This work is partly supported by the Innovative Techno-Hub for Integrated Medical Bio-imaging Project of the Special Coordination Funds for Promoting Science and Technology, and by Grant-in-Aid for Scientific Research (for K. T. Grant number 23113508, for A. S. Grant number 16K12876), from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.References
- A. Roda and M. Guardigli, Anal. Bioanal. Chem., 2012, 402, 69 CrossRef CAS PubMed.
- A. Roda, M. Guardigli, P. Pasini, M. Mirasoli, E. Michelini and M. Musiani, Anal. Chim. Acta, 2005, 541, 25 CrossRef CAS.
- R. Geiger, E. Shneider, K. Wallenfels and W. Miska, Biol. Chem. Hoppe-Seyler, 1992, 373, 1187 CrossRef CAS PubMed.
- W. Zhou, M. P. Valley, J. Shultz, E. M. Hawkins, L. Bernad, T. Good, D. Good, T. L. Riss, D. H. Klaubert and K. V. J. Wood, J. Am. Chem. Soc., 2006, 128, 3122 CrossRef CAS PubMed.
- M. P. Valley, W. Zhou, E. M. Hawkins, J. Shultz, J. J. Cali, T. Worzella, L. Bernad, T. Good, D. Good, T. L. Riss, D. H. Klaubert and K. V. Wood, Anal. Biochem., 2006, 359, 238 CrossRef CAS PubMed.
- H. Yao, M.-K. So and J. Rao, Angew. Chem., Int. Ed., 2007, 46, 7031 CrossRef CAS PubMed.
- W. Zhou, C. Andrews, J. Liu, J. W. Shultz, M. P. Valley, J. J. Cali, E. M. Hawkins, D. H. Klaubert, R. F. Bulleit and K. V. Wood, ChemBioChem, 2008, 9, 714 CrossRef CAS PubMed.
- A. Dragulescu-Andrasi, G. Liang and J. Rao, Bioconjugate Chem., 2009, 20, 1660 CrossRef CAS PubMed.
- M. Kindermann, H. Roschitzki-Voser, D. Caglic, U. Repnik, C. Miniejew, P. R. E. Mittl, G. Kosec, M. G. Grütter, B. Turk and K. U. Wendt, Chem. Biol., 2010, 17, 999 CrossRef CAS PubMed.
- G. C. Van de Bittner, E. A. Dubikovskaya, C. R. Bertozzi and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21316 CrossRef CAS PubMed.
- A. S. Cohen, E. A. Dubikovskaya and C. R. Bertozzi, J. Am. Chem. Soc., 2015, 1966, 88 Search PubMed.
- G. C. Van de Bittner, C. R. Bertozzi and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 1783 CrossRef CAS PubMed.
- M. Matsumoto, J. Photochem. Photobiol., C, 2004, 5, 27 CrossRef CAS.
- A. P. Schaap, R. S. Handley and B. P. Giri, Tetrahedron Lett., 1987, 28, 935 CrossRef CAS.
- N. Hananya, A. E. Boock, C. R. Bauer, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2016, 138, 13438 CrossRef CAS PubMed.
- T. Nakamura, A. Son, Y. Umehara, T. Ito, R. Kurihara, Y. Ikemura and K. Tanabe, Bioconjugate Chem., 2016, 27, 1058 CrossRef CAS PubMed.
- B. Rånby, J. F. Rabek, Singlet Oxygen: Reactions with organic compounds and polymers, John Wiley & Sons, Chichester, 1978 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detail of the experimental procedure and MS spectra of VPA, absorption spectra of MSN-NEP and HPLC profile of VPA before and after the reaction. See DOI: 10.1039/c6ra28079j |
| This journal is © The Royal Society of Chemistry 2017 |