Open Access Article
Open Access ArticleLinear alkylbenzene sulfonate (LAS) promotes sedimentation and lipid accumulation in Scenedesmus obliquus
Qiang Penga,
Miaomiao Zhaob,
Guangzhu Shenb,
Xinyu Ganb and
Ming Li *bcd
*bcd
aCollege of Food Science, Northwest Agriculture and Forestry University, Yangling 712100, PR China
bCollege of Resources and Environment, Northwest Agriculture and Forestry University, Yangling 712100, PR China. E-mail: lileaf@163.com
cKey Laboratory of Plant Nutrition and the Agri-environment in Northwest China, Ministry of Agriculture, PR China
dCollaborative Innovation Center of Water Security for Water Source Region of Mid-line of South-to-North Diversion Project, Henan Province, PR China
First published on 30th January 2017
Abstract
The lipids found in microalgae are promising raw materials for bio-diesel production. This study tests the effects of linear alkylbenzene sulfonate (LAS) on the growth, colony formation, sedimentation and lipid content of the green alga Scenedesmus obliquus. Growth of S. obliquus was unaffected by LAS below concentrations of 100 mg L−1. LAS treatment promoted colony formation and the number of cells per aggregate significantly increased with rising LAS concentration. Both optic and scanning electron microphotoes showed that the induced colony of S. obliquus was regular and there were clear attachment points between two neighboring cells. The sedimentation efficiency at 10 min significantly increased with the increasing LAS concentration. Under treatment of 100 mg L−1 LAS, the sedimentation efficiency was 69.0% after 40 minutes' settling, which was 30% higher than that in the control group. The sedimentation efficiency was positively related to cells per colony at all sedimentation time except for 60 min. Moreover, the lipid content was 21.2% when treated with 100 mg L−1 LAS, which was 1.18 times higher than in the control group. The main fatty acid composition of S. obliquus was α-linolenic acid (C18:3) and palmitic acid (C16:0). The degree of fatty acid unsaturation was not obviously affected by LAS addition but the proportion of fatty acid with the chain length ranging from C16 to C18 was promoted from 70.9% to 84.2% by LAS addition. This result indicated that LAS addition was beneficial to improve the quality of algal biodiesel. Our results suggested that moderate doses of LAS can effectively enhance the sedimentation efficiency of S. obliquus by inducing colony formation and improving the lipid content of the cells without inhibiting their growth.
1. Introduction
Microalgae is considered a promising raw material for bio-diesel production because of their fast growth rate and high lipid contents.1 Scenedesmus obliquus is among the most widely used lipid-producing microalgae. Although its lipid content is not very high,2 its lipid composition is suitable for biodiesel synthesis.3 Moreover, this algal species can efficiently remove nitrogen and phosphorus from wastewater, so it can be cultivated for nutrient removal from wastewater.1 Nevertheless, S. obliquus cells are too small (3–20 μm) to be harvested without energy-intensive filtration and centrifugation methods, which greatly increases the cost of microalgae diesel production.4 Consequently, it is still necessary to develop economical methods for biomass harvesting of S. obliquus.Algal biomasses are frequently harvested by adding synthetic polymer flocculants and metal coagulants, which stimulate single cells to form large aggregates.4 Generally, this method cannot be widely applied because synthetic polymer flocculants are expensive and metal coagulants are toxic to algal cells.5 Alkaline flocculation is also an alternative method for algae harvest. However, the sedimentation efficiency of alkaline flocculation was always inhibited by the extracellular polymeric substances (EPS) produced by algal cells even though addition of minim alkali is cheap.4 Therefore, a cheap and effective chemical that promotes algal biomass harvesting is imperative.
In an alternative method, Yang and Li6 stimulated colony formation of unicellular S. obliquus by adding Daphnia culture filtrates. The filtrates were considered to significantly increase the colony size of S. obliquus and hence improve the harvesting efficiency.7 Nevertheless, large Daphnia culture filtrates themselves consume much energy and demand significant financial resources. Low light intensity was also reported to induce colony formation of S. obliquus8 whereas algal biomass growth would also be inhibited due to low light intensity. Therefore, the gains of this method cannot compensate the losses.
Li et al.9 demonstrated that 100 and 1000 mg L−1 linear alkylbenzene sulfonate (LAS) induced colony formation of S. obliquus. This phenomenon revealed that LAS would promote sedimentation of S. obliquus by inducing colony formation. Meanwhile, LAS is a widely used representative surfactant.9 It is cheap, and easily manufactured and degraded. As a consequence, LAS is considered as a promising additive for promoting colony formation and sedimentation of S. obliquus. However, Li et al.9 studied the effects of LAS on colony formation of S. obliquus and found two defects limiting the development of technology to promote sedimentation of S. obliquus by LAS addition. On one hand, the concentration gradient of LAS used in their work was too big to analyze the appropriate concentration of LAS.9 On the other hand, the authors only investigated colony size of S. obliquus rather than the sedimentation efficiency.9 Hitherto, there was not a clear finding illustrating the relationship between colony size and sedimentation efficiency of S. obliquus even in the work of Zhu et al.,7 in which combined effects of nitrogen levels and Daphnia culture filtrate on colony size of S. obliquus were well studied. Therefore, it is still important to study the effects of LAS on colony size and sedimentation efficiency of S. obliquus with proper concentration gradient of LAS.
The algal fatty acids composition was another important factor affecting algal biodiesel production.10,11 The increase in the degree of fatty acid unsaturation was in favor of high-quality biodiesel production because this production was more suitable for cold weather use due to a typically gel point.12 Moreover, the chain length of fatty acids are also selective and the most optimum chain length was ranging from C16 to C18.10,11 Our knowledge about the effects of LAS on algal fatty acid composition was seriously inadequate. Therefore, the effects of LAS addition on algal fatty acid composition should also be evaluated.
This study tests the effects of LAS addition on colony formation and sedimentation efficiency of S. obliquus. As lipid productivity and fatty acid composition are two important reference standards in algal diesel production,13 the growth, lipid content and fatty acid composition of LAS-stimulated S. obliquus were also analyzed.
2. Materials and methods
2.1 Organisms and culture conditions
A unicellular strain of S. obliquus (FACHB 416) was purchased from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, Chinese Academy of Science, Wuhan, China. This strain was cultured in BG-11 medium for more than 3 months under standard conditions with a 12:12 h light:dark cycle at 50 μmol photons m−2 s−1. The temperature was set to 25 °C. In this environment, most of the algal cells were single or paired.Under the same culture conditions, the algae in logarithmic-phase were inoculated in 150 mL BG-11 medium in 250 mL conical flasks with an initial cell density of 5 × 105 cells per mL. Various amount of LAS were added to the culture medium, and their concentrations were adjusted to 0, 25, 50, 100, 200 and 500 mg L−1. LAS with a chemical formula of C18H29NaO3S was obtained from Sigma Chemical Company (USA). Six replicates of each culture were prepared. The flasks were hand-shaken two to three times daily to prevent the cells from clinging to the inner walls of the flasks. Colony formation of algae always occurred in the logarithmic growth phase14 but lipid content in the stable phase was much higher than that in the logarithmic growth phase.15 Gan et al.16 showed that the growth of S. obliquus entered the stable phase for day 16 and thus the experiment ran for 14 days in the current study as a compromise.
2.2 Cell counting
Cell density was analyzed daily. The cells were directly counted three times in a hemocytometer placed under an Olympus CX31 microscope (Olympus Corporation, Japan) at 400× magnification. If the three calculated results differed by less than 10%, they were averaged to obtain the final cell density. Otherwise, additional counting was conducted. At the end of the experiment, every two replicates were combined into a mixed sample. The number of cells per particle (where a particle denotes a single cell, a small aggregate or a colony) and the percentages of various particles were calculated by individually recording the numbers of particles and the numbers of cells per particle. At least 400 particles were counted in each sample.2.3 Optic and scanning electron microphotoes
Microphotographs of S. obliquus were taken using the microscope equipped with an Olympus C-5050 digital camera. The scanning electron microphotoes were taken by a scanning electron microscope (S-4800, Hitachi, Japan). A drop of algal sample was placed on a cover slip and air dried. Then, it was chemically fixed using 4% (w/v) glutaraldehyde for 24 h. Afterwards, the samples were rinsed in distilled water thrice and dehydrated in graded series of ethanol (30%, 50%, 70%, 80%, 90% and 100%) for 10 min each and air dried. Finally, these prepared samples were kept in desiccators until analysis of scanning electron microscope.2.4 Assessment of sedimentation rate
The OD665 of the mixed sample was measured by a spectrophotometer (UV-1780, Shimadzu, Japan) and recorded as ODi. Afterwards, six replicates of 15 mL culture of each mixed sample were injected into 15 mL centrifugal tubes respectively. All tubes were stored undisturbed at room temperature in darkness. The OD665 of the supernatant liquid in one of the replicates of each mixed sample was measured at 10 min intervals for 1 h, and recorded as ODt, where t denotes the stand-by time. The sedimentation efficiency was then calculated by eqn (1) described by Gan et al.:16| Sedimentation efficiency (%) = (1 − ODt/ODi) × 100 | (1) |
2.5 Lipid content analysis
The remaining culture medium of the mixed sample was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g rpm for 6 min. The pellet was rinsed twice with deionized water and then dried at 60 °C for lipid extraction. The photos of dried algae were taken by a camera (D60, Cannon, Japan). Algal lipid was extracted using the methanol–chloroform method proposed by Bligh and Dyer17 and 100 mg dried biomass was used to be extracted for each replicate.
000 × g rpm for 6 min. The pellet was rinsed twice with deionized water and then dried at 60 °C for lipid extraction. The photos of dried algae were taken by a camera (D60, Cannon, Japan). Algal lipid was extracted using the methanol–chloroform method proposed by Bligh and Dyer17 and 100 mg dried biomass was used to be extracted for each replicate.
2.6 Fatty acid composition analysis
Fatty acid methyl esters were obtained following the method suggested by Lepage and Roy.18 The dried lipid obtained in above section was completely dissolved by 4 mL 14% w/v solutions of BF3–methanol and then, was refluxed by boiling water bath for 15 min. After cooling, 2 mL n-heptane and 4 mL NaCl saturated solution was added and the mixture was fully shocked. After stratification, the supernatant was filtrated using 0.2 μm polyvinylidene fluoride syringe microfilters. The fatty acid methyl esters (FAMEs) was analyzed using Shimadzu GC-2014C gas chromatography equipped with FID. A 60-m DB-5ms column was used. The injection temperature was 250 °C. The initial column temperature was 120 °C, held for 3 min. Then, the temperature increased to 220 °C at a increasing rate of 4 °C min−1, held for 5 min. The final temperature increased to 280 °C at a increasing rate of 3 °C min−1, held for 20 min.2.7 Data statistics
All data in this study are presented as their means ± standard deviations. The specific growth rate (μ) was calculated by eqn (2):| μ = ln(Dt/D0)/t | (2) |
3. Results
3.1 Growth of S. obliquus
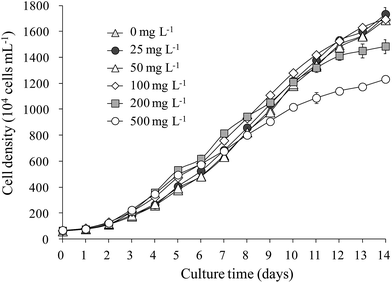
The growth of S. obliquus was not obviously influenced by LAS concentrations below 100 mg L−1. Merely, it was inhibited at higher concentrations (200 and 500 mg L−1; Fig. 1). The maximum cell density reached 1.6 × 107 cells mL−1 at LAS concentrations below 100 mg L−1, reducing to 1.4 × 107 and 1.2 × 107 cells mL−1 in the 200 and 500 mg L−1 LAS treatments, respectively. The specific growth rate was 0.25 per day when LAS concentration was below 100 mg L−1. This value slightly decreased to 0.24 and 0.23 per day while LAS concentration increased to 200 and 500 mg L−1, respectively.3.2 Colony formation of S. obliquus
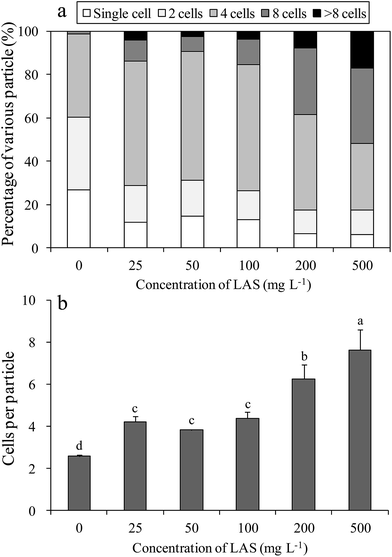
The LAS concentration affected the percentages of various particles in the cultures (Fig. 2a). In the control culture, most of the cells were single or double, with a considerable percentage (38.5%) of 4-cell aggregates. Under treatment with 25, 50 and 100 mg L−1 LAS, the proportion of 4-cell particles increased to approximately 60%, while the percentages of single and double cells decreased to half of the control values. When treated with 200 and 500 mg L−1 LAS, the percentage of ≥8-cell particles was 38.5% and 51.7%, respectively, versus 1.2% in the control. The number of cells per particle also increased significantly under LAS treatment (Fig. 2b). In the presence of 0 (control), 25, 50, 100, 200 and 500 mg L−1 LAS, the cells-per-particle counts were 2.6, 4.2, 3.8, 4.4, 6.3 and 7.6 respectively. The values in all the treatment were significantly higher than the control (P < 0.05).3.3 Optic and scanning electron microphotoes
Fig. 3 showed the photos of harvested biomass as well as optical and scanning electron microphotoes (SEM) of S. obliquus. There was not obvious difference in the appearance of harvested biomass among various treatments. Both optic and scanning electron microphotoes showed that the induced colony of S. obliquus was regular and there were clear attachment points between two neighboring cells. The length and width of a single cell of S. obliquus was respectively 8 and 4 μm but the value of a colony comprised of four cells was 9 and 8 μm, respectively.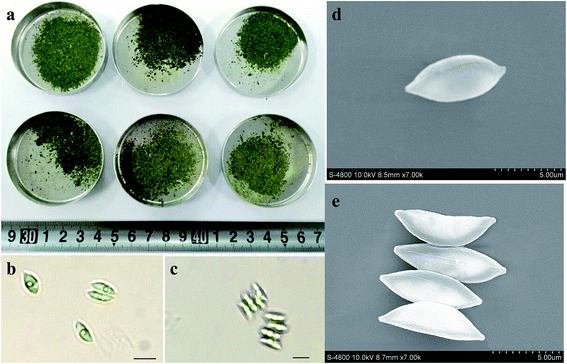 | ||
| Fig. 3 Photos of harvested biomass as well as optical and scanning electron microphotoes (SEM) of S. obliquus. | ||
3.4 Sedimentation efficiency of S. obliquus
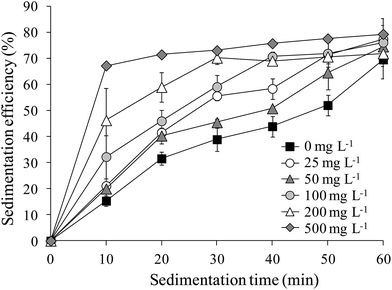
The sedimentation efficiency of S. obliquus increased with increasing LAS concentration. After 10 minutes' settling, the sedimentation efficiency was maximized at 67.2% under treatment with 500 mg L−1 LAS (Fig. 4), which was significantly higher than other treatments (P < 0.05). The values in all treatments increased at later times. At the end of the sedimentation experiment (60 minutes), the sedimentation efficiencies were 69.5%, 76.1% and 79.3% in the 0 (control), 100 and 500 mg L−1 LAS treatments, respectively. Notably, the sedimentation efficiency after 40 minutes' settling was 69.0% in the presence of 100 mg L−1 LAS, 30% higher than in the control group at that time, and approximately equal to the control value (69.5%) after 60 minutes' settling.The relationship between sedimentation efficiency at varying sedimentation time and cells per particle of S. obliquus was shown in Fig. 5. The sedimentation efficiency was positively related to cells per colony at all the sedimentation time except for 60 min. If cells per particle reached 8, sedimentation efficiency reached 60% at 10 min and the value was much higher than 70% at 20 min.
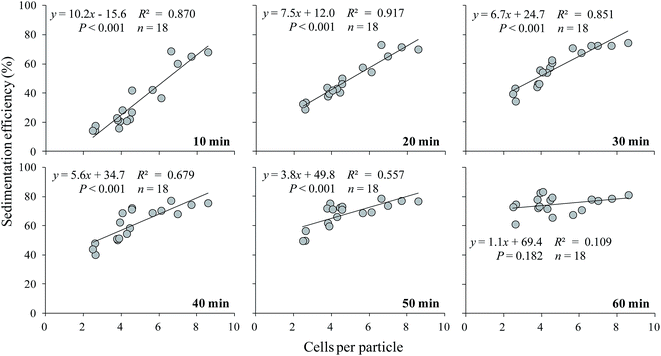 | ||
| Fig. 5 The relationship between sedimentation efficiency and cells per particle of S. obliquus at varying sedimentation time. | ||
3.5 Lipid content and composition
All treatments significantly enhanced the lipid content over the control value (18.0%; Fig. 6). The highest lipid content (24.0%) was found in the 25 mg L−1 LAS treatment. The increase in this treatment was significantly greater than in other treatments. In the 100 mg L−1 treatment, the lipid content was 21.2% (1.18 times the control value).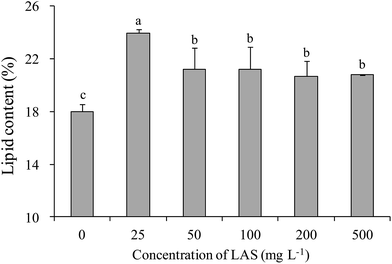 | ||
| Fig. 6 Lipid contents of S. obliquus treated with various concentrations of LAS. Different lowercase letters indicate significant differences (P < 0.05) among treatments. | ||
The main fatty acid composition of S. obliquus was α-linolenic acid (C18:3) and palmitic acid (C16:0) showing in Table 1. The proportion of α-linolenic acid (C18:3) was not affected by LAS addition except for the treatment with highest level, in which the value decreased from 45.5% to 32.2% when treated with 500 mg L−1 LAS. On the contrary, the proportion of palmitic acid (C16:0) increased from 16.3% to 23.7% with increasing LAS concentration. The degree of fatty acid unsaturation was not obviously affected by LAS addition. However, the proportion of fatty acid with the chain length ranging from C16 to C18 was promoted from 70.9% to more than 76% (highest value reached 84.2%) by LAS addition.
| Fatty acid | Concentration of LAS in the treatment (mg L−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 20 | 50 | 100 | 200 | 500 | |
| C15:0 | 0 | 3.3 | 3.8 | 0 | 0 | 0 |
| C16:0 | 16.3 | 22.6 | 18.9 | 20.0 | 22.0 | 23.7 |
| C18:0 | 0 | 1.8 | 1.6 | 0 | 0 | 0 |
| C18:1 | 0 | 8.6 | 9.1 | 8.6 | 7.7 | 22.5 |
| C18:2 | 9.1 | 3.5 | 4.6 | 0 | 4.7 | 4.9 |
| C18:3 | 45.5 | 47.7 | 46.7 | 47.4 | 46.3 | 32.2 |
| C20:5 | 16.2 | 10.4 | 13.7 | 16.2 | 13.5 | 11.7 |
| C22:0 | 12.9 | 2.1 | 1.6 | 7.8 | 5.8 | 5.0 |
| Unsaturated | 70.8 | 70.2 | 74.1 | 72.2 | 72.2 | 71.3 |
| C16–C18 | 70.9 | 84.2 | 80.9 | 76.0 | 80.7 | 83.3 |
4. Discussion
The current study clearly showed that sedimentation efficiency of S. obliquus was promoted by addition of LAS. Even though, some other factors such as culture pH, addition of FeCl3, chitosan and Al2(SO4)3 could also promote sedimentation of S. obliquus,19 the mechanism in the current study was different from previous studies. The SEM photos showed that floc was found in the previous study20 but was not found in the current study. In the present work, the sedimentation process was accompanied by colony formation of S. obliquus induced by LAS. Therefore, flocculation was the main pathway promoting sedimentation in previous studies19,20 but colony formation was the main pathway in the current study.It was also found that sedimentation efficiency at 10, 20, 30, 40 and 50 min was significantly related to cells per particle of S. obliquus. These regression analysis results could be used to predict the sedimentation performance of S. obliquus based on a large number research results about the effects of environmental factors on colony size of S. obliquus.7,8 These predictions would provide some references to choose proper agent to promote sedimentation of S. obliquus for harvesting.
Our results also illustrated that colony formation occurred in all the treatment with varying concentrations of LAS from 25 mg L−1 to 500 mg L−1. Li et al.9 reported that 100 mg L−1 LAS successfully induced colony formation of S. obliquus but 10 mg L−1 LAS did not succeed in inducing colony formation. Here, we obtained the missing information that 25 and 50 mg L−1 LAS can also induce colony formation. Colony formation in S. obliquus is thought to be triggered by increased EPS production.8,21 LAS can inhibit protein and DNA synthesis in S. obliquus, and reduce its dark respiration activity.22 Thus, the reduced polysaccharide consumption could increase the EPS content and hence induce colony formation. This mechanism has been proved previously by Li et al.9 There were clear attachment points between two neighboring cells of induced colony of S. obliquus. Frame and Sawa23 showed that single cells of S. obliquus were wrapped by a structureless slimy layer. Therefore, the pathway of colony formation in the current study was that cells of S. obliquus remain attached after binary fission due to the wrapping of slimy layer.
In the current study, the proportion of fatty acid with the chain length ranging from C16 to C18 was promoted by LAS addition. That is to say LAS addition benefitted to improve the quality of algal biodiesel.10,11 The algal fatty acid composition, related to algal physiological state, was usually affected by salinity.12,24 LAS addition also increased salinity and thus affected fatty acid composition in the current study. However, the effects of increasing alkyl benzene sulfonic acid on algal fatty acid composition was still not clear and need further studies.
Compared with control, 25 mg L−1 LAS significantly stimulated lipid accumulation in S. obliquus. Merely, there was no significant change in the lipid content after addition of 50–500 mg L−1 LAS. Similar phenomenon that lipid content increased with increasing NaCl when NaCl concentration was low but decreased slightly when NaCl concentration was high was also reported by Salama et al.12 Wang et al.25 reported that low concentrations of LAS enhanced the maximal photochemical efficiency of Microcystis aeruginosa. It could also been inferred that low concentration of LAS might also enhance the maximal photochemical efficiency of S. obliquus improving its light harvesting for lipid synthesis and thus promoted the accumulation of lipid. When LAS concentration was high, photosynthesis of S. obliquus was inhibited22 and thus no significant change in the lipid content was found in the treatment of 50–500 mg L−1 LAS. As is well documented, inorganic substances such as nutrients,26 Fe,27 CO2 (ref. 2) and salt12 can promote lipid accumulation in S. obliquus. Glucose addition also reportedly increases the lipid content,26 because some S. obliquus strains are facultative heterotrophs.28 However, the lipid content of S. obliquus did not monotonically increase with LAS concentration, indicating that LAS is not an organic carbon source for S. obliquus in the current study. The effects of Na+ can also be excluded, because the maximum Na+ concentration in the present treatments was 39.9 mg L−1, too low to exert any significant effect. Additionally, the levels of triacylglycerol (TAG) and other small molecules (such as glycerol) increase under changes in osmotic pressure,29 which could be induced by surfactant addition. These molecules promote lipid accumulation in the algal cells.12
Although the 25 mg L−1 LAS treatment significantly stimulated lipid accumulation in S. obliquus (Fig. 6), its effect on sedimentation efficiency was not superior. In contrast, 100 mg L−1 LAS maximized the sedimentation efficiency at 40 minutes (to >30% above the control group), but yielded moderate lipid content (1.18 times the control lipid content). The filtrates can be recycled to reduce costs. Lürling30 reported that surfactant (FFD-6) induces colony formation of S. obliquus within 24 h. Therefore, the LAS concentration in the culture medium could be adjusted to 25 mg L−1 using recycled filtrate, then adjusted to 100 mg L−1 by LAS addition one day before harvesting. The first step would boost the cell yield and the second would enhance the lipid content.
5. Conclusions
Our results indicated that LAS concentrations below 100 mg L−1 did not affect the growth of a potential biofuel producer, S. obliquus. The number of cells per particle significantly increased with increasing LAS concentration from 2.6 to 7.6. The sedimentation efficiency at 10 min significantly increased along with increasing LAS concentration. In the 100 mg L−1 LAS treatment, the sedimentation efficiency was 69.0% after 40 minutes' settling (30% higher than in the control treatment). Meanwhile, the lipid content was 1.18 times higher than in the control. The proportion of fatty acid with the chain length ranging from C16 to C18 was promoted from 70.9% to 84.2% by LAS addition. Synthesis consideration of sedimentation efficiency and lipid production, the LAS concentration in the culture medium could be adjusted to 25 mg L−1 using recycled filtrate, then adjusted to 100 mg L−1 by LAS addition one day before harvesting. The first step would boost the cell yield and the second would enhance the lipid content.Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (Grant 51409216) and the Tenth Special Scientific Research Project of Water Pollution Control of Lake Taihu (TH2016302).References
- S. Mandal and N. Mallick, Appl. Environ. Microbiol., 2011, 77, 374–377 CrossRef CAS PubMed.
- H. Ho, C. Y. Chen and J. S. Chang, Bioresour. Technol., 2012, 113, 244–252 CrossRef PubMed.
- S. H. Ho, W. M. Chen and J. S. Chang, Bioresour. Technol., 2010, 101, 8725–8730 CrossRef CAS PubMed.
- D. Vandamme, A. Beuckels, E. Vadelius, O. Depraetere, W. Noppe, A. Dutta, I. Foubert, L. Laurens and K. Muylaert, Water Res., 2016, 88, 301–307 CrossRef CAS PubMed.
- C. González-Fernández and M. Ballesteros, J. Appl. Phycol., 2013, 25, 991–999 CrossRef.
- Z. Yang and J. J. Li, J. Freshwater Ecol., 2007, 22, 249–253 CrossRef.
- X. Zhu, J. Yang, N. Xu, G. Chen and Z. Yang, Algal Res., 2015, 9, 94–98 CrossRef.
- M. Li, L. Gao and L. Lin, Annales de Limnologie/International Journal of Limnology, 2015, 51, 329–334 CrossRef.
- M. Li, W. Zhu, X. Dai and X. Li, Fresenius Environ. Bull., 2013, 22, 1189–1194 CAS.
- X. Meng, J. Yang, X. Xu, L. Zhang, Q. Nie and M. Xian, Renewable Energy, 2009, 34, 1–5 CrossRef CAS.
- D. Tang, W. Han, P. Li, X. Miao and J. Zhong, Bioresour. Technol., 2011, 102, 3071–3076 CrossRef CAS PubMed.
- S. Salama, H. C. Kim, R. A. Abou-Shanab, M. K. Ji, Y. K. Oh, S. H. Kim and B. H. Jeon, Bioprocess Biosyst. Eng., 2013, 36, 827–833 CrossRef PubMed.
- J. Griffiths and S. T. Harrison, J. Appl. Phycol., 2009, 21, 493–507 CrossRef.
- W. Wang, Y. Liu and Z. Yang, J. Limnol., 2010, 69, 193–198 CrossRef.
- X. Li, H. Hu and J. Yang, New Biotechnol., 2010, 27, 59–63 CrossRef CAS PubMed.
- X. Gan, G. Shen, B. Xin and M. Li, Desalination, 2016, 400, 1–6 CrossRef CAS.
- G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef PubMed.
- G. Lepage and C. C. Roy, J. Lipid Res., 1984, 25, 1391–1396 CAS.
- L. Chen, C. Wang, W. Wang and J. Wei, Bioresour. Technol., 2013, 133, 9–15 CrossRef CAS PubMed.
- S. Guo, X. Zhao, C. Wan, Z. Huang, Y. Yang, M. A. Alam, S. Ho, F. Bai and J. Chang, Bioresour. Technol., 2013, 145, 285–289 CrossRef CAS PubMed.
- Z. Yang, F. Kong, X. Shi, P. Xing and M. Zhang, Int. Rev. Hydrobiol., 2007, 92, 618 CrossRef.
- G. Chawla, P. N. Viswanathan and S. Devi, Environ. Exp. Bot., 1987, 27, 311321–319323 CrossRef.
- P. Frame and T. Sawa, Comparative anatomy of Charophyta: II. The axial nodal complex—an approach to the taxonomy of Lamprothamnium, J. Phycol., 1975, 11(2), 202–205 Search PubMed.
- R. Rao, C. Dayananda, R. Sarada, T. R. Shamala and G. A. Ravishankar, Bioresour. Technol., 2007, 98, 560–564 CrossRef PubMed.
- Z. Wang, J. Zhang, L. Song, E. Li, X. Wang and B. Xiao, Environ. Sci. Pollut. Res., 2015, 22, 5491–5499 CrossRef CAS PubMed.
- S. Mandal and N. Mallick, Appl. Microbiol. Biotechnol., 2009, 84, 281–291 CrossRef CAS PubMed.
- A. El Baky, G. S. El-Baroty, A. Bouaid, M. Martinez and J. Aracil, Bioresour. Technol., 2012, 119, 429–432 CrossRef PubMed.
- P. Devi, G. V. Subhash and S. V. Mohan, Renewable Energy, 2012, 43, 276–283 CrossRef.
- Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert and A. Darzins, Plant J., 2008, 54, 621–639 CrossRef CAS PubMed.
- M. Lürling, Chemosphere, 2006, 62, 1351–1358 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2017 |