Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
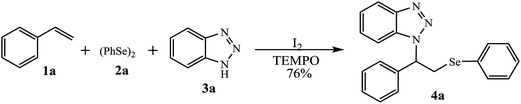
Convenient iodine-mediated aminoselenation of alkenes using benzotriazoles as nitrogen sources†
Xiaolong Wanga,
Hongjie Lia,
Min Zhub and
Jie Yan *a
*a
aCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310032, P. R. China. E-mail: jieyan87@zjut.edu.cn; Fax: +86 571 88320238
bCollege of Biological and Environmental Sciences, Zhejiang Shuren University, Hangzhou 310015, P. R. China
First published on 9th March 2017
Abstract
A new and convenient procedure mediated by I2 was developed for the preparation of aminoselenides bearing the benzotriazole structure using alkenes, diselenides, and benzotriazoles. In this protocol, molecular I2 first reacts with diselenides to form active electrophilic selenium species, following an electrophilic addition to afford the corresponding aminoselenides. This aminoselenation of alkenes requires mild reaction conditions and is a simple procedure, and it provides the products with high regioselectivity and in good yields, which extends the synthetic application of molecular iodine in organic synthesis.
Introduction
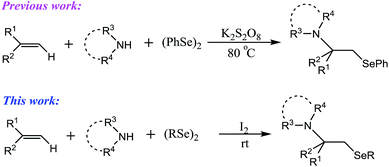
Organoselenium compounds have been increasing in importance in recent years due to their synthetic applicability,1 biological activities, such as antitumor and antibacterial activities, and other properties.2 The introduction of organoseleno groups into organic molecules has been widely studied, in which the anti-l,2-addition of an organoseleno group and a nucleophile into an alkene to form vicinal difunctional groups provides a valuable method for synthetic strategies.3 The aminoselenation of alkenes is a type of difunctionalization of alkenes, of which, both a selenium atom and amino group can be installed into the carbon–carbon double bond. Despite the vicinal aminoselenides being important in pharmaceutics and in the synthesis of various useful biologically active molecules, there are only a few studies reported on the aminoselenation of alkenes.4 Therefore, the development of simple, general, and direct methods for introducing both selenium- and nitrogen-containing groups into alkenes is highly desired.More recently, using N-(phenylseleno)phthalimide (NPSP) as both a nitrogen and selenium source and Lewis acid TiCl4 as a catalyst, Tang and co-workers reported an intermolecular aminoselenation of alkenes with high regioselectivity and diastereoselectivity.5 However, the use of metal catalyst, which is required to be removed in the purification and the pre-prepared NPSP, restricts its further application. Moreover, Sun's group demonstrated a new peroxodisulfate-mediated aminoselenation of alkenes.6 This method is very interesting because it is general, efficient, and several amino sources, including sulfamides and azoles, are effective in the reaction (Scheme 1). However, compared with various prepared aminoselenides using saccharin as nitrogen source, only one example was reported with benzotriazole as a nucleophile in the reaction.
Over the past few years, iodine-catalysed or iodine-mediated reactions have been increasingly explored because iodine is cheap, readily available, and eco-friendly, and it has metal-like behavior.7 We were interested in the oxidation and functionalization of organic compounds using hypervalent iodine reagents because they can promote the oxidative cleavage of the Se–Se bond of diphenyl diselenide, resulting in reactive electrophilic selenium species.8 In our recent research, we found that molecular iodine can replace hypervalent iodine reagents to promote some similar reactions. Based on this, to prepare more aminoselenides and research on their unknown chemical and biological properties, we investigated a novel aminoselenation of alkenes using benzotriazoles as amino sources due to the benzotriazole structure being a very useful moiety in the synthesis (Scheme 1).9 To the best of our knowledge, this convenient iodine-mediated intermolecular aminoselenation of alkenes has not been reported before.
Results and discussion
Initially, we utilized styrene 1a, diphenyl diselenide 2a, and benzotriazole 3a as model substrates to explore the aminoselenation of alkenes in the presence of molecular I2. It was found that on simple stirring of the mixture of 1.2 equiv. of 1a with 1.0 equiv. of 2a, 3a, and I2 in MeCN for 24 h, the expected aminoselenide, 1-[1-phenyl-2-(phenylseleno)ethyl]-1H-benzotriazole 4a was obtained in 53% yield (Table 1, entry 1). Then, the aminoselenation of 1.2 equiv. of 1a with 1.0 equiv. of 2a mediated by I2 at room temperature in air was optimized. Several solvents were first evaluated, in which 4a was obtained in the yields ranging from 31 to 53% (entries 1–6). When more 3a was added in the mixture, the yield increased (entries 7–9). When the yield was not good enough, several mixed solvents were examined to improve the reaction. Fortunately, a mixture of the solvents MeCN and EtOAc was found to be suitable for the reaction, and when the volume ratio reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield increased to 80% (entries 10–14). In the mixed solvent, the optimal amount of I2 was screened and 1.0 equiv. proved to be the best choice (entries 10, 15–17). However, in the absence of I2, 4a was formed only in 10% yield (entry 18). Other iodine-containing salts such as KI, NaI, and NH4I were also checked in place of I2, but all were ineffective in the reaction. Under the optimized conditions, the reaction smoothly progressed and was completed in 24 hours (entries 10, 19–20).
1, the yield increased to 80% (entries 10–14). In the mixed solvent, the optimal amount of I2 was screened and 1.0 equiv. proved to be the best choice (entries 10, 15–17). However, in the absence of I2, 4a was formed only in 10% yield (entry 18). Other iodine-containing salts such as KI, NaI, and NH4I were also checked in place of I2, but all were ineffective in the reaction. Under the optimized conditions, the reaction smoothly progressed and was completed in 24 hours (entries 10, 19–20).
| Entry | Benzotriazole (equiv.) | I2 (equiv.) | Solvent | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yields. | |||||
| 1 | 1.0 | 1.0 | CH3CN | 24 | 53 |
| 2 | 1.0 | 1.0 | CH2Cl2 | 24 | 52 |
| 3 | 1.0 | 1.0 | EtOAc | 24 | 40 |
| 4 | 1.0 | 1.0 | CH2ClCH2Cl | 24 | 42 |
| 5 | 1.0 | 1.0 | THF | 24 | 35 |
| 6 | 1.0 | 1.0 | DMSO | 24 | 31 |
| 7 | 1.3 | 1.0 | CH3CN | 24 | 62 |
| 8 | 1.5 | 1.0 | CH3CN | 24 | 69 |
| 9 | 2.0 | 1.0 | CH3CN | 24 | 71 |
| 10 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 80 |
| 11 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (2 EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 75 |
| 12 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (3 EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 63 |
| 13 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (5 EtOAc (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 60 |
| 14 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
24 | 49 |
| 15 | 1.5 | 1.4 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 81 |
| 16 | 1.5 | 0.6 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 53 |
| 17 | 1.5 | 0.2 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 31 |
| 18 | 1.5 | — | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 10 |
| 19 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 | 70 |
| 20 | 1.5 | 1.0 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1 EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10 | 51 |
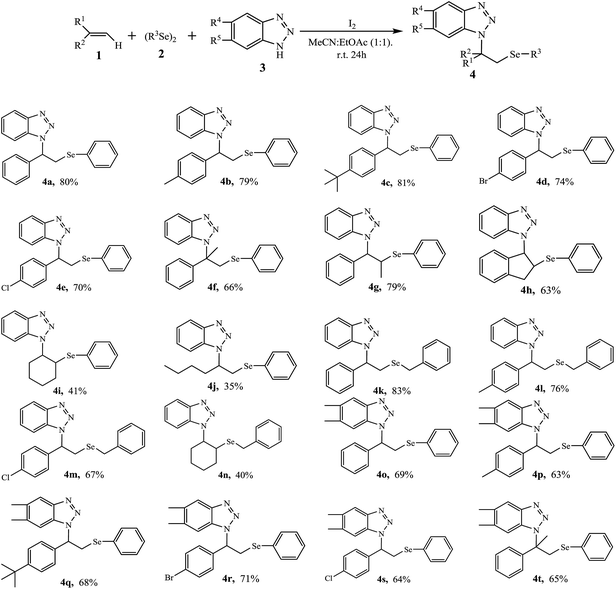
Based on the extensive screening process, we obtained the optimal reaction conditions. Next, the aminoselenation of 1.2 equiv. of alkenes 1 with 1.0 equiv. of diselenides 2 and 1.5 equiv. of benzotriazoles 3 in the presence of 1.0 equiv. of I2 in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (1
EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature for 24 hours was investigated, and as a result, a series of corresponding aminoselenides 4 were obtained. The results are summarized in Table 2.
1) at room temperature for 24 hours was investigated, and as a result, a series of corresponding aminoselenides 4 were obtained. The results are summarized in Table 2.
As shown in Table 2, the I2-mediated aminoselenation was compatible with the studied alkenes 1a–1h, affording the corresponding aminoselenides 4a–4h and 4o–4t in good yields. It is obvious that the substituents on benzene ring for these aromatic alkenes, whether they were electron-donating (methyl and t-butyl) or electron-withdrawing (bromo and chloro) groups, usually had no significant influence on their reactivity. However, when cyclohexene 1i and 1-hexene 1j were treated as representative aliphatic alkenes, low yields of 41% and 35% were obtained for 4i and 4j, respectively, indicating our protocol is more suitable for aromatic alkenes. Dibenzyl diselenide 2b, similar to 2a, an aliphatic diselenide, was also effective in the reaction, resulting in the corresponding products 4k–4m from aromatic alkenes in good yields and a yield of 40% for 4n.
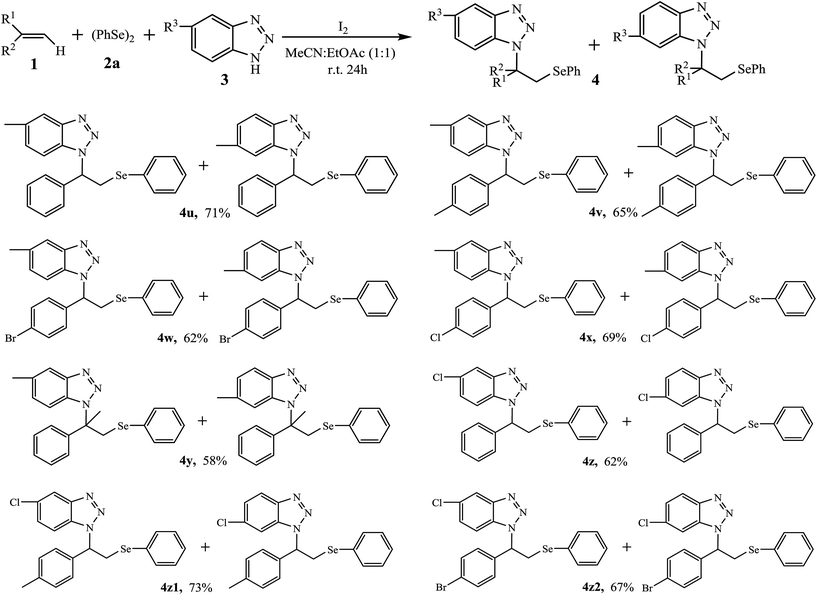
After investigated the aminoselenation of alkenes using benzotriazole 3a and 5,6-dimethylbenzotriazole 3b as amino sources, to prepare more aminoselenides that bear the benzotriazole structure, we then explored the activities of 5-methylbenzotriazole 3c and 5-chlorobenzotriazole 3d in the reaction. Due to low yields that were usually obtained with aliphatic alkenes, the following aminoselenation was mainly focused on aromatic alkenes (Table 3). Under the optimal reaction conditions, both monosubstituted benzotriazoles typically gave 5-substituted and 6-substituted isomer mixtures 4u–4z2 with yields ranging from 58 to 73%. It is important to isolate the mixed products; however, after several attempts, we failed to achieve the goal. Finally, with 1H-NMR analysis, the ratio was nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for most 5-substituted and 6-substituted isomers.
1 for most 5-substituted and 6-substituted isomers.
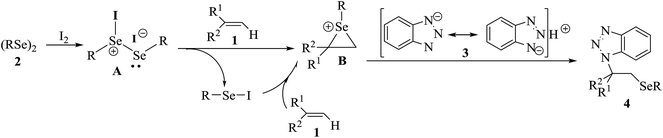
To explore the mechanism, a stoichiometric radical scavenger, 2,2,6,6-tetramethylpiperidine1-oxyl (TEMPO), was added in the reaction of 1a with 2a, 3a, and I2 under the optimized conditions. It was found that the aminoselenation still carried out well, affording the product 4a in 76% yield (Scheme 2). This result suggests that the reaction may not undergo a radical pathway. According to the above mentioned results and control experiments, a plausible electrophilic addition mechanism mediated by I2 is shown in Scheme 3. Thus, molecular I2 first smoothly reacts with diselenide 2 to form the active intermediate A, followed by a rapid Se–Se bond cleavage.10 The in situ generated active electrophilic selenium species then reacts with alkene 1 to produce the unstable cyclic seleniranium intermediate B. Finally, intermediate B is attacked by nucleophile benzotriazole 3 to provide the desired product amonoselenide 4 as a single isomer via an SN1 mechanism. Accompanying the reaction, another active intermediate ArSeI11 can further transfer a second equivalent of electrophilic selenium to alkene 1. As for monosubstituted benzotriazoles 3c and 3d resulting in 5-substituted and 6-substituted isomer mixtures, it is mainly that 1H-benzotriazole forms a conjugated structure N− ion when it acts as a nucleophile in the reaction.
Conclusions
In summary, we developed a new and convenient procedure for the preparation of aminoselenides from alkenes, diselenides, and benzotriazoles mediated by I2 at room temperature. This aminoselenation of alkenes has some advantages such as mild reaction conditions and simple procedure, which provides a series of aminoselenides containing benzotriazole structure with high regioselectivity and in good yields. Furthermore, this reaction will extend the application scope of molecular iodine in organic synthesis.References
- (a) T. Wirth, Tetrahedron, 1999, 55, 1 CrossRef CAS; (b) T. Wirth, Angew. Chem., Int. Ed., 2000, 39, 3740 CrossRef CAS; (c) D. M. Freudendahl, S. A. Shahzad and T. Wirth, Eur. J. Org. Chem., 2009, 1649 CrossRef CAS; (d) A. J. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Chem. Rev., 2010, 110, 4357 CrossRef CAS PubMed; (e) D. N. Jones, D. Mundy and R. D. Whitehouse, J. Chem. Soc., 1970, 86 CAS; (f) R. Walter and J. Roy, J. Org. Chem., 1971, 36, 2561 CrossRef CAS PubMed; (g) G. Zeni, M. P. Stracke, C. W. Nogueira, A. L. Braga, P. H. Menezes and H. A. Stefani, Org. Lett., 2004, 6, 1135 CrossRef CAS PubMed.
- (a) L. Letavayova, V. Vlckova and J. Brozmanova, Toxicology, 2006, 227, 1 CrossRef CAS PubMed; (b) P. Erkekoglu, W. Rachidi, O. G. Yuzugullu, B. Giray, A. Favier, M. Ozturk and F. Hincal, Toxicol. Appl. Pharmacol., 2010, 248, 52 CrossRef CAS PubMed; (c) H. E. Ganther, Carcinogenesis, 1999, 20, 1657 CrossRef CAS PubMed; (d) A. Hartwig, H. Blessing, T. Schwerdtle and I. Walter, Toxicology, 2003, 193, 161 CrossRef CAS PubMed; (e) P. Erkekoglu, B. Giray, W. Rachidi, I. Hininger-Favier, A.-M. Roussel, A. Favier and F. Hincal, Environ. Toxicol., 2014, 29, 98 CrossRef CAS PubMed; (f) K. Wallace, K. T. Kelsey, A. Schned, J. S. Morris, A. S. Andrew and M. R. Karagas, Cancer Prev. Res., 2009, 2, 70 CrossRef CAS PubMed; (g) K. El-Bayoumy, P. Upadhyaya, V. Date, O. S. Sohn, E. S. Fiala and B. Reddy, Chem. Res. Toxicol., 1991, 4, 560 CrossRef CAS PubMed; (h) M. P. Rayman, Proc. Nutr. Soc., 2005, 64, 527 CrossRef CAS PubMed; (i) P. Erkekoglu, M.-W. Chao, W. Ye, J. Ge, L. J. Trudel, P. L. Skipper, B. Kocer-Gumusel, B. P. Engelward, G. N. Wogan and S. R. Tannenbaum, Food Chem. Toxicol., 2014, 72, 98 CrossRef CAS PubMed.
- (a) A. A. Vieira, J. B. Azeredo, M. Godoi, C. Santi, E. N. da Silva Júnior and A. L. Braga, J. Org. Chem., 2015, 80, 2120 CrossRef CAS PubMed; (b) T. G. Back, The Chemistry of Organic Selenium and Tellurium Compounds, ed. S. Patai, Wiley, Chichester, 1987, vol. 2, pp. 115–134 Search PubMed; (c) M. Tiecco, L. Testaferri, M. Tingoli, D. Bartoli and R. Balducci, J. Org. Chem., 1990, 55, 429 CrossRef CAS; (d) M. Tiecco, L. Testaferri, M. Tingoli, D. Chianelli and D. Bartoli, Tetrahedron Lett., 1989, 30, 1417 CrossRef CAS; (e) C. Bosman, A. D'Annibale, S. Resta and C. Trogolo, Tetrahedron Lett., 1994, 35, 6525 CrossRef CAS; (f) N. Taniguchi, J. Org. Chem., 2006, 71, 7874 CrossRef CAS PubMed; (g) T. Wirth, Angew. Chem., Int. Ed., 2000, 39, 3740 CrossRef CAS; (h) T. Wirth, Organoselenium Chemistry: Synthesis and Reactions, Wiley-VCH, Weinheim, 2012 Search PubMed; (i) E. S. Conner, K. E. Crocker, R. G. Fernando, F. R. Fronczek, G. G. Stanley and J. R. Ragains, Org. Lett., 2013, 15, 5558 CrossRef CAS PubMed.
- (a) A. Toshimitsu, K. Nakano, T. Mukai and K. Tamao, J. Am. Chem. Soc., 1996, 118, 2756 CrossRef CAS; (b) M. Tiecco, L. Testaferri, C. Santi, C. Tomassini, F. Marini, L. Bagnoli and A. Temperini, Tetrahedron: Asymmetry, 2002, 13, 429 CrossRef CAS; (c) A. Toshimitsu, T. Aoai, S. Uemura and M. Okano, J. Chem. Soc., Chem. Commun., 1980, 1041 RSC; (d) A. Toshimitsu, T. Aoai, H. Owada, S. Uemura and M. Okano, J. Org. Chem., 1981, 46, 4727 CrossRef CAS; (e) A. Toshimitsu, G. Hayashi, K. Terao and S. Uemura, J. Chem. Soc., Perkin Trans. 1, 1986, 343 RSC; (f) M. Tiecco, L. Testaferri, C. Santi, C. Tomassini, F. Marini, L. Bagnoli and A. Temperini, Angew. Chem., Int. Ed., 2003, 42, 3131 CrossRef CAS PubMed.
- E. Tang, W.-L. Wang, Y.-J. Zhao, M. Zhang and X. Dai, Org. Lett., 2016, 18, 176 CrossRef CAS PubMed.
- K. Sun, X. Wang, Y.-H. Lv, G. Li, H.-Z. Jiao, C.-W. Dai, Y.-Y. Li, C. Zhang and L. Liu, Chem. Commun., 2016, 52, 8471 RSC.
- (a) R. D. Richardson and T. Wirth, Angew. Chem., Int. Ed., 2006, 45, 4402 CrossRef CAS PubMed; (b) T. Dohi and Y. Kita, Chem. Commun., 2009, 2073 RSC; (c) M. Uyanik, D. Suzuki, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2011, 50, 5331 CrossRef CAS PubMed; (d) J. Feng, S. Liang, S.-Y. Chen, J. Zhang, S.-S. Fu and X.-Q. Yu, Adv. Synth. Catal., 2012, 354, 1287 CrossRef CAS; (e) M. Uyanik, H. Okamoto, T. Yasui and K. Ishihara, Science, 2010, 328, 1376 CrossRef CAS PubMed; (f) E. Shi, Y. Shao, S. Chen, H. Hu, Z. Liu, J. Zhang and X. Wan, Org. Lett., 2012, 14, 3384 CrossRef CAS PubMed; (g) S. Tang, Y. Wu, W. Q. Liao, R. P. Bai, C. Liu and A. W. Lei, Chem. Commun., 2014, 50, 4496 RSC; (h) A. Sagar, S. Vidyacharan and D. S. Sharada, RSC Adv., 2014, 4, 37047 RSC; (i) K. Yang, M. Ke and Y. G. Lin, Green Chem., 2015, 17, 1395 RSC; (j) S.-Ke. Wang, M.-T. Chen, D.-Y. Zhao, X. You and Q.-L. Luo, Adv. Synth. Catal., 2016, 358, 4093 CrossRef CAS; (k) Y.-N. Duan, Z. Zhang and C. Zhang, Org. Lett., 2016, 18, 6176 CrossRef CAS PubMed; (l) Y. Siddaraju and K. R. Prabhu, Org. Lett., 2016, 18, 6090 CrossRef CAS PubMed; (m) S. Ambethkar, M. Vellimalai, V. Padmini and N. Bhuvanesh, New J. Chem., 2017, 41, 75 RSC; (n) D.-D. Xu, W.-W. Sun, Y.-L. Xie, J.-K. Liu, B. Liu, Y.-B. Zhou and B. Wu, J. Org. Chem., 2016, 81, 11081 CrossRef CAS PubMed.
- (a) M. Tingoli, M. Tiecco, D. Chianelli, R. Balducci and A. Temperini, J. Org. Chem., 1991, 56, 6809 CrossRef CAS; (b) M. Tingoli, M. Tiecco, L. Testaferri and R. Balducci, Synlett, 1993, 211 CrossRef CAS; (c) M. Tingoli, M. Tiecco, L. Testaferri and A. Temperini, Chem. Commun., 1994, 1883 RSC; (d) M. Tingoli, M. Tiecco, L. Testaferri and A. Temperini, Synth. Commun., 1998, 28, 1769 CrossRef CAS; (e) J. P. Das, U. K. Roy and S. Roy, Organometallics, 2005, 24, 6136 CrossRef CAS; (f) L. Yu, B. Chen and X. Huang, Tetrahedron Lett., 2007, 48, 925 CrossRef CAS; (g) Y. V. Mironov, A. A. Sherman and N. E. Nifantiev, Tetrahedron Lett., 2004, 45, 9107 CrossRef CAS; (h) M. Shi, B.-Y. Wang and J. Li, Eur. J. Org. Chem., 2005, 759 CrossRef CAS.
- (a) P. P. Dixit, P. S. Nair, V. J. Patil, S. Jain, S. K. Arora and N. Sinha, Bioorg. Med. Chem. Lett., 2005, 15, 3002 CrossRef CAS PubMed; (b) N. Mishra, P. Arora, B. Kumar, L. C. Mishra, A. Bhattacharya, S. K. Awasthi and V. K. Bhasin, Eur. J. Med. Chem., 2008, 43, 1530 CrossRef CAS PubMed; (c) Z. Rezaei, S. Khabnadideh, K. Pakshir, Z. Hossaini, F. Amiri and E. Assadpour, Eur. J. Med. Chem., 2009, 44, 3064 CrossRef CAS PubMed; (d) S. Demirayak, K. Benkli and G. Given, Pharm. Acta Helv., 1998, 72, 285 CrossRef CAS PubMed; (e) M. R. Barbachyn and C. W. Ford, Angew. Chem., Int. Ed., 2003, 42, 2010 CrossRef CAS PubMed; (f) V. Arora, M. M. Salunkhe, N. Sinha, R. K. Sinha and S. Jain, Bioorg. Med. Chem. Lett., 2004, 14, 4647 CrossRef CAS PubMed.
- (a) H.-W. Shi, C. Yu, M. Zhu and J. Yan, J. Organomet. Chem., 2015, 776, 117 CrossRef CAS; (b) C. Muangkaew, P. Katrun, P. Kanchanarugee, M. Pohmakotr, V. Reutrakul, D. Soorukram, T. Jaipetch and C. Kuhakarn, Tetrahedron, 2013, 69, 8847 CrossRef CAS.
- (a) Z.-Z. Huang, X. Huang and Y.-Z. Huang, J. Chem. Soc., Perkin Trans. 1, 1995, 95 RSC; (b) A. Toshimitsu, S. Uemura and M. Okano, J. Chem. Soc., Chem. Commun., 1982, 87 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra27202a |
| This journal is © The Royal Society of Chemistry 2017 |