Open Access Article
Open Access ArticleEffect of metal on the methanol to aromatics conversion over modified ZSM-5 in the presence of carbon dioxide
Caixia Xu,
Binbo Jiang *,
Zuwei Liao,
Jingdai Wang,
Zhengliang Huang and
Yongrong Yang
*,
Zuwei Liao,
Jingdai Wang,
Zhengliang Huang and
Yongrong Yang
State Key Laboratory of Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: jiangbb@zju.edu.cn; Tel: +86-13819195891
First published on 8th February 2017
Abstract
To improve the aromatics yield of methanol to aromatics conversion (MTA) over zeolite, which has become a potential route for producing aromatics, modified ZSM-5 catalysts with equimolar metals denoted as EM-X/ZSM-5 (X = Zn, Cu, Ag, and Ni) were investigated under CO2 and N2 flow for MTA in a fixed-bed reactor. The physicochemical properties were characterized by atomic absorption spectroscopy (AAS), N2 adsorption–desorption isotherms, X-ray diffraction (XRD), and NH3 temperature-programmed desorption (NH3-TPD). Comparison with the results obtained in pure N2 flow showed that catalysts doped with Zn, Ni, and Ag could promote aromatization activity and BTX yield in the presence of CO2. Among these, EM-Zn/ZSM-5 showed an aromatics yield of 59.05%, with an increase of 8.1%, whereas EM-Cu/ZSM-5 was found to reduce the aromatization activity in the presence of CO2. Moreover, the interaction mechanism of the active sites of the catalysts with CO2 for the MTA reaction was explored on the basis of the absorbability of the catalysts for CO2, which was studied by CO2 temperature-programmed desorption (CO2-TPD); the activation ability for CO2 to combine with hydrogen was investigated by the catalytic reaction of CO2 + H2, and the verification experiments for the coupling behavior of ZSM-5 doped with different contents of Zn in the presence of CO2 were carried out.
1 Introduction
In recent years, the demand for light aromatics, such as BTX (benzene, toluene, and xylene), has sharply increased and the growth rate over the next five years is expected to be about 3.5–4.0% per year.1,2 However, aromatic production based on petrochemical processes has reduced owing to the decrease of catalytic reforming capacity, the increase of diesel demand, and the tendency towards light raw materials.3 Moreover, syngas-derived methanol production has become a serious issue with the rapid development of the coal chemical industry. Thus, the conversion of methanol to aromatics (MTA) is considered as a potential way that can not only alleviate surplus of methanol and deficiency of aromatics but also optimize the energy structure.4The low-aromatic yield and BTX selectivity are the main limiting factors of MTA that hinder the industrial application of MTA. Methanol forms aromatics via complicated reactions, including dehydration, oligomerization, and hydrogen transfer reaction, on the acid sites of zeolite and produces a considerable amount of alkanes that take up carbon atoms and reduces the aromatics yield.5 Salts of dehydro-metals, such as Ga, Cu, Zn, and Ag, have been used to modify ZSM-5 catalysts to improve the aromatics yield of MTA.6–10 The aromatics yield improved due to the fact that dehydro-metals on the ZSM-5 catalysts can speed up the hydrogen transfer of olefins and reduce the content of alkanes by replacing olefins as temporal hydrogen anion receptors.11 However, dehydrogenation and hydrogenation over dehydro-metals is a dynamic balance process, such that hydrogen produced by dehydrogenation cannot be eliminated in situ. Consequently, a large proportion of olefins will be combined with hydrogen to generate alkanes again.12 Therefore, if appropriate receptors of hydrogen are provided to cut off the hydrogenation of olefin intermediates into alkane byproducts, the aromatic yield of MTA could be greatly increased.
Oxidizing gases, such as O2, N2O, SO2, and CO2, can be utilized as hydrogen receptors.13–15 Among these, the utilization of CO2, which is a main contributor to the global warming effect, to form functional CO is very meaningful and interesting.16 It was found that carbon dioxide could significantly enhance the dehydrogenation via eliminating hydrogen produced in situ by a reverse water–gas-shift reaction in the dehydrogenation of isobutane to isobutene on VMgOx.17 The good performance of carbon dioxide has also been reported in other studies.18,19 Junhui Li et al.20 investigated the MTA reaction over NiO-ZSM-5 under CO2 atmosphere and claimed that CO2 activated by NiO species could accelerate the dehydrogenation in the conversion of olefin intermediates to aromatics by reacting with H2, produced in the MTA reaction, and could then promote aromatization. However, as is well-known, different metals loaded onto the catalyst have different dehydrogenation abilities for the aromatization reaction7,8,10 and different CO2 activation abilities for accepting the removed hydrogen;21 however, and their roles in MTA are still not clear. Therefore, the match of the loaded catalyst with CO2 and the interaction mechanism for the MTA reaction require further investigation, which could contribute a method for further improving the yield of aromatics.
Herein, we report a comparative study of the influence of CO2 on the performance of methanol aromatization over ZSM-5 catalysts doped by Zn, Ni, Ag, and Cu. Particularly, we mainly focused on clarifying the interaction mechanism of the active sites of the modified catalysts with CO2 for the MTA reaction on the basis of the characterization of the physicochemical properties of the prepared catalysts, the catalytic performance of the coupling of CO2 with H2, and the methanol aromatization over the catalysts modified with different contents of Zn in the presence or absence of CO2.
2 Experimental
2.1 Catalyst preparation
All ZSM-5 catalysts doped with metals were prepared using the excessive impregnation method, and all impregnating solutions had 5% excess compared with the method of incipient wetness impregnation. ZSM-5 zeolite (SiO2/Al2O3 = 38) was pressed, crushed, and sorted to obtain particles of 14–20 mesh, and then dried in an oven at 150 °C for 6 h. The metal-doped ZSM-5 catalyst was obtained by the impregnation of dried ZSM-5 zeolite particles with the nitrate solutions of precursors at room temperature for 15 h and calcination at 500 °C for 3 h after drying for 3 h at 105 °C. ZSM-5 zeolite was purchased from the Catalyst Plant of Nankai University. The metal precursors used were AgNO3, Ni(NO3)2·6H2O, Zn(NO3)2·6H2O, and Cu(NO3)2·3H2O and were purchased from Sinopharm Chemical Reagent Co., Ltd. The modified ZSM-5 with equimolar (0.229 mmol g−1) X (X = Zn, Cu, Ag, and Ni) metal was denoted as EM-X/ZSM-5. The ZSM-5 loaded with different Zn contents was denoted as nZn/ZSM-5 (where n denotes the Zn content). Note that 0.229 mmol g−1 of Zn is about 1.5 wt% of Zn.2.2 Catalyst characterization
The actual amount of metal elements in ZSM-5 zeolites was determined by atomic absorption spectroscopy (AAS, Z-2000). The samples were pretreated as follows: hydrofluoric acid was added dropwise to 100 mg catalyst powder until framework traces remained. Then, the samples were completely dissolved by concentrated nitric acid and finally diluted using deionized water.N2 adsorption–desorption experiments were performed using a physical adsorption instrument (Micromeritics, ASAP2020). The specific surface area and pore volume were calculated by the BET method and t-plot method, respectively. The crystal structure of the catalysts was characterized via X-ray diffraction analysis (XRD, X'Pert PRO X/PANalytical) using Cu Kα radiation at a scan rate of 1° min−1 in the 2θ range from 5 to 50°. NH3-TPD and CO2-TPD experiments were performed using a physical and chemical adsorption instrument (Quantachrome, ASiQ). The samples (150 mg) were outgassed and dried at 470 °C for 1 h under He flow (45 mL min−1), cooled down to 120 °C (NH3-TPD) or 100 °C (CO2-TPD), treated with NH3 or CO2 (10 wt% NH3 or CO2, He in balance) at 35 mL min−1 for 1 h, and then purged with He at the adsorption temperature for 1 h. The samples were then linearly heated at 10 °C min−1 to 600 °C (NH3-TPD) or 500 °C (CO2-TPD) in 45 mL min−1 He. NH3 or CO2 released during the heating for desorption was measured by a thermal conductivity detector (TCD).
2.3 Catalyst testing
Catalytic reactions were carried out at ambient pressure using a down-flow stainless fixed-bed reactor with an internal diameter of 13 mm. The prepared catalyst (2.4 g) was packed in a constant temperature zone and pretreated in N2 at 500 °C for 1.5 h. Reactions were carried out at 1.0 h−1 WHSV of methanol and atmospheric pressure in 30.8 mL min−1 N2 flow or the mixture of N2 and CO2 after reducing the temperature to 475 °C. C1–C6+ aliphatics were analyzed using a gas chromatograph (Agilent, GC7820A) equipped with a FID detector and a 50 m HP PLOT-Q capillary column. Benzene–C10+ was analyzed using a gas chromatograph (Agilent, GC7820A) equipped with an FID detector and a 50 m HT-1301 capillary column. H2, CO, and CO2 in tail gas were analyzed using a gas chromatograph (Agilent, GC7820A) equipped with a TCD detector and a 30 m GDX-502 molecular column (carrier gas, Ar). The yield of aromatics and BTX was calculated as follows:3 Results and discussion
3.1 Methanol aromatization over the catalysts modified with different metals in the presence or absence of CO2
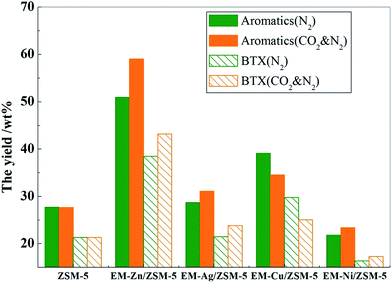
The aromatic yield and BTX yield in the MTA reaction over the catalysts-loaded different equimolar metal components in the presence and absence of CO2 are shown in Fig. 1. The detailed product distributions of the MTA reaction for the abovementioned examples are listed in Table 1. Herein, it was observed that the conversion of methanol on all the catalysts was 100% under the test conditions.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nN2 = 1
nN2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; T = 475 °C; product samples were tested at 2.25 h)
1; T = 475 °C; product samples were tested at 2.25 h)
| Catalysts atmosphere | ZSM-5 | EM-Zn/ZSM-5 | EM-Ag/ZSM-5 | EM-Cu/ZSM-5 | EM-Ni/ZSM-5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N2 | CO2 & N2 | N2 | CO2 & N2 | N2 | CO2 & N2 | N2 | CO2 & N2 | N2 | CO2 & N2 | |
| Yield/wt% | ||||||||||
| Hydrogen | 0.44 | 0.44 | 3.86 | 3.36 | 0.52 | 0.48 | 1.02 | 1.03 | 6.25 | 5.98 |
| Methane | 6.05 | 6.07 | 5.71 | 5.38 | 7.81 | 7.64 | 6.47 | 6.90 | 22.80 | 20.39 |
| Ethene | 3.91 | 3.92 | 6.74 | 6.87 | 3.03 | 3.70 | 3.72 | 3.52 | 2.60 | 3.06 |
| Ethane | 4.15 | 4.14 | 2.36 | 1.89 | 4.58 | 4.16 | 2.72 | 3.29 | 12.26 | 11.20 |
| Propene | 3.63 | 3.62 | 4.76 | 4.46 | 2.61 | 3.19 | 2.99 | 2.72 | 1.49 | 1.95 |
| Propane | 30.79 | 30.71 | 6.27 | 4.85 | 27.74 | 26.23 | 17.31 | 21.18 | 13.59 | 14.11 |
| Dimethyl ether | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Methanol | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C4 | 8.35 | 8.40 | 5.45 | 3.26 | 6.70 | 7.66 | 9.56 | 9.47 | 6.31 | 6.76 |
| C5 | 0.57 | 0.58 | 1.55 | 0.79 | 0.52 | 0.72 | 1.56 | 1.29 | 0.85 | 0.83 |
| C6 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.10 | 0.05 | 0.00 | 0.03 |
| Benzene | 2.75 | 2.72 | 2.03 | 1.64 | 3.12 | 2.85 | 2.11 | 2.10 | 2.21 | 2.05 |
| Toluene | 8.62 | 8.56 | 9.54 | 8.89 | 9.20 | 9.62 | 10.64 | 9.53 | 6.57 | 6.83 |
| C7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C8 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| m-Xylene | 0.33 | 0.31 | 0.38 | 0.43 | 0.33 | 0.34 | 0.28 | 0.23 | 0.11 | 0.12 |
| p-Xylene | 6.95 | 7.06 | 19.47 | 23.89 | 6.39 | 8.13 | 12.21 | 9.61 | 5.40 | 6.11 |
| o-Xylene | 2.72 | 2.71 | 7.04 | 8.37 | 2.47 | 2.94 | 4.56 | 3.61 | 2.11 | 2.21 |
| C9 | 1.66 | 1.67 | 1.29 | 1.24 | 2.24 | 1.72 | 2.23 | 1.77 | 2.05 | 1.95 |
| Trimethylbenzene | 6.36 | 6.38 | 12.49 | 15.84 | 7.20 | 7.21 | 9.29 | 9.48 | 5.43 | 6.12 |
| C10+ | 12.72 | 12.72 | 11.04 | 8.70 | 15.53 | 13.41 | 13.25 | 14.21 | 9.98 | 10.30 |
| Conversion of CO2/% | — | 0.00 | — | 1.06 | — | 0.11 | — | 0.00 | — | 9.75 |
In the pure N2 atmosphere, the yields of aromatics over ZSM-5 doped with Zn, Cu, and Ag were enhanced to 50.9 wt%, 39.1 wt%, and 28.72 wt%, respectively, when compared to that of ZSM-5 (27.72 wt%). As seen from Table 1, the hydrogen content in the pure N2 atmosphere over these catalysts was also increased, and the increase range was positively related to the increase of the aromatics yield. This indicates that Zn, Ag, and Cu can act as dehydro-metals, accelerate the dehydrogenation reaction, and promote the aromatization reaction, thus enhancing the aromatics yield. Other studies8,10 also showed that Zn exhibits the best catalytic performance with methanol as a feedstock in N2 atmosphere. However, Ni has an effect different from the abovementioned elements. Compared with the unmodified ZSM-5 catalyst, the hydrogen content significantly increased, whereas the yield of aromatics significantly decreased. It could be observed that a large amount of methane was generated, leading to much coking, significant reduction in the aromatization activity, and decrease in the aromatics yield.
When CO2 was added to the reaction atmosphere, it was observed that there was almost no change in the yield of aromatics and BTX over ZSM-5; however, the yield of aromatics and BTX over the modified ZSM-5 significantly changed, which could be divided into two groups with opposite trends. The aromatic yield over the ZSM-5 catalysts doped with Zn, Ag, or Ni increased when compared to those in the absence of CO2, and among these, the aromatics yield over the EM-Zn/ZSM-5 system exhibited the largest increase of 8.1%, followed by EM-Ag/ZSM-5 with an increase of 2.4%. EM-Ni/ZSM-5 exhibited the smallest increase. Moreover, the contents of hydrogen, ethane, and propane were obviously reduced over these modified ZSM-5 catalysts. This indicates that the ZSM-5 catalysts modified by Zn, Ag, and Ni can catalyze the reaction of CO2 with H, generated in the MTA process, thereby consuming hydrogen atoms and reducing the formation of alkanes, thus improving the yield of aromatics. In addition, the contents of benzene and toluene were almost unchanged, whereas the contents of p-xylene exhibited an obvious increase, which suggested that the promotion of CO2 mainly affected the formation of p-xylene. Note that the content of ethylene and propene exhibited a certain improvement over the Ag and Ni-doped catalysts. The results of Ni-doped catalysts in the CO2 and N2 atmosphere exhibit a tendency similar to that previously reported in literature.20 However, for EM-Cu/ZSM-5, addition of CO2 reduced the yield of aromatics and the contents of hydrogen, ethane, and propane were slightly increased.
From the abovementioned results, it can be seen that the Zn-modified catalyst had the best coupling effect on the aromatics yield of MTA in the presence of CO2, followed by Ag and Ni-modified catalysts, whereas the Cu-modified catalyst exhibited reduction in the aromatics yield in the presence of CO2. Therefore, it is necessary to clarify the essential reason for differences in the coupling effects of the metals over ZSM-5 in the presence of CO2 on the aromatics yield of the MTA reaction.
3.2 Physicochemical properties of the prepared catalysts
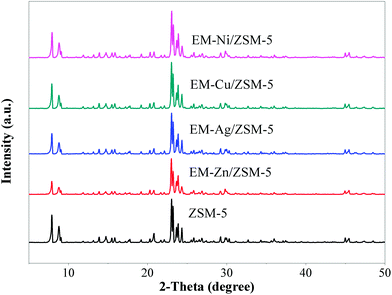
Fig. 2 shows the XRD results of ZSM-5 and modified catalysts. All loaded catalysts exhibited characteristic diffraction peaks similar to ZSM-5. However, the diffraction peaks of metals were not detected. The XRD results suggest that the metal loading did not significantly affect the structure of zeolite, forming monolayer dispersion in ZSM-5. The specific surface areas and pore volumes of the catalysts listed in Table 2 slightly decreased, which might be caused by the metal loading on the internal and external surface and orifices of zeolites. Since the molar loading of metals was almost the same and all the loadings were small (<3%), the pore volume and specific surface area of each catalyst did not change by more than 10%, which hardly affected the shape-selectivity of the channel.| ZSM-5 | EM-Zn/ZSM-5 | EM-Cu/ZSM-5 | EM-Ag/ZSM-5 | EM-Ni/ZSM-5 | ||
|---|---|---|---|---|---|---|
| Loaded molar weight, mmol g−1 (expected values) | 0.229 | 0.229 | 0.229 | 0.229 | ||
| Loaded molar weight, mmol g−1 (observed values) | 0.223 | 0.23 | 0.221 | 0.229 | ||
| Surface area, m2 g−1 | 292.3 | 278.8 | 276.9 | 268.5 | 283.4 | |
| Pore volume, mL g−1 | Mesopore | 0.064 | 0.057 | 0.057 | 0.054 | 0.059 |
| Micropore | 0.131 | 0.129 | 0.129 | 0.126 | 0.130 | |
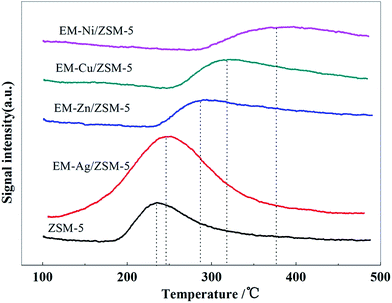
CO2 adsorption abilities for ZSM-5 and modified catalysts were studied by CO2-TPD and the results are shown in Fig. 3. There was one peak at about 240 °C for ZSM-5 that was ascribed to the desorption of weakly adsorbed CO2. However, the desorption peaks were shifted to a higher temperature upto about 375 °C after modification with Ag, Zn, Cu, and Ni. This suggested that these loaded metals could strengthen the CO2 adsorption, which could be attributed to the interaction between metals and CO2 providing basic adsorbing sites. For EM-Ag/ZSM-5, the desorption peak was slightly shifted as compared to that of ZSM-5, whereas the area of the desorption peak was significantly increased, indicating that the loading of Ag only increased the number of weak adsorption active sites, whereas it did not enhance the overall adsorption strength. The weak adsorption of CO2 over EM-Ag/ZSM-5 limited its contact with the catalyst and thus weakened the consumption of hydrogen, which could increase the aromatics yield. The desorption peak of the catalysts modified by metals, including Ni, Cu, and Zn, was shifted to a higher temperature, indicating strong interaction with CO2. Strong adsorption of CO2 contributes to its contact with the catalyst and occupation of the catalytic active sites. If the active sites can activate the adsorbed CO2 to effectively couple with hydrogen, the hydrogen in the system could be eliminated in situ, and then the yield of the aromatic hydrocarbons could be improved.
3.3 The activation of CO2 to react with H2 over the prepared catalysts
Ogonowski et al.17 reported that CO2 could react with H2 removed from alkenes and alkanes to form water and CO by a reversed water–gas-shift reaction. A new product of CO did appear over these modified ZSM-5 catalysts in the MTA reaction. CO2 conversion over these catalysts was calculated using CO produced through the reversed water–gas-shift reaction and the results are listed in Table 1. Note that the trend of CO2 conversion was different from the trend of aromatics yield on different metal-modified catalysts.The reactions between CO2 and H2 (as raw materials) over these catalysts were investigated to explore their ability to activate CO2 to react with H2. Results demonstrated that CO2 could be activated to react with H2 and the conversion of CO2 remained stable during the course of the reaction over all the doped catalysts. It could be seen from Table 3 that not only CO but also a small amount of CH4 was produced, wherein CO made up the largest part of the products over the catalysts including EM-Zn/ZSM-5, EM-Cu/ZSM-5, and EM-Ag/ZSM-5, whereas CH4 was a relatively larger product over EM-Ni/ZSM-5. The results suggested that the reaction of generated CO with H2 might further undergo eqn (2) when CO2 is reacted with H2 via the reversed water–gas-shift reaction, as shown in eqn (1). For example, when CO2 was added to the MTA reaction mixture, both reactions (not just the reversed water–gas-shift reaction) occurred to rapidly consume hydrogen from the intermediates, as reported by Li et al.20 Moreover, hydrogen was mainly consumed via eqn (2) over EM-Ni/ZSM-5, whereas it was mostly consumed via eqn (1) over the other three catalysts.
| CO2 + H2 → CO + H2O | (1) |
| CO + 3H2 → H2O + CH4 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nN2 = 1
nN2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; T = 475 °C; ratio of H2/N2 is approximately equal to 2.0; product samples were tested at 2.25 h)
1; T = 475 °C; ratio of H2/N2 is approximately equal to 2.0; product samples were tested at 2.25 h)
| Composition & volume content/% | EM-Zn/ZSM-5 | EM-Cu/ZSM-5 | EM-Ag/ZSM-5 | EM-Ni/ZSM-5 |
|---|---|---|---|---|
| H2 | 49.94 | 50.67 | 49.91 | 45.24 |
| N2 | 23.76 | 21.99 | 22.27 | 25.87 |
| CO | 4.59 | 1.67 | 3.58 | 2.79 |
| CO2 | 21.57 | 25.61 | 24.10 | 22.76 |
| CH4 | 0.14 | 0.06 | 0.14 | 3.34 |
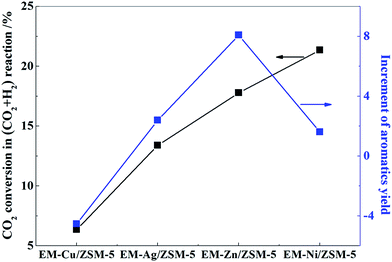
Fig. 4 shows the CO2 conversion for the reaction of CO2 with H2 and the increase of aromatics yield in the MTA reaction in the presence of CO2 over these modified ZSM-5 catalysts at the same TOS (TOS = 2.25 h). It can be seen that CO2 conversion for the reaction of CO2 with H2 gradually increased over the catalysts in the order of Cu > Ag > Zn > Ni-modified catalysts, indicating that the catalytic abilities for activating CO2 to react with H2 increase in turn. The aromatics yield increased in the same order except for the Ni-modified catalyst, and the value increased from −4.5% over EM-Cu/ZSM-5 to 8.1% over EM-Zn/ZSM-5. The conversion of CO2 over EM-Ni/ZSM-5 was the highest, whereas the increase in aromatics yield was only 1.61%.
Based on the abovementioned results, it can be concluded that the increase of aromatics yield of MTA over the modified catalysts in CO2 atmosphere is determined by the interaction of three essential catalytic factors: the ability of the catalyst to adsorb CO2, the ability of the metal over the catalyst to catalyze the reaction of CO2 with H2, and the dehydroaromatization ability of the catalyst. If the metal over the catalyst is capable of efficiently activating CO2 and catalyzing the reaction of CO2 with H2 to form CO and CH4 via eqn (1) and (2), the metal can effectively displace the olefin as the temporary hydrogen receptors to rapidly remove the hydrogen produced during the MTA, reducing the formation of alkanes and thereby increasing the yield of aromatics. If the catalyst cannot effectively activate CO2 to react with H2, whereas has a strong adsorption capacity for CO2, CO2 cannot be timely consumed and will occupy the active sites of the catalyst to inhibit the MTA reaction. When the dehydroaromatization ability of the catalyst itself is insufficient, adding CO2 hardly promotes the aromatization reaction of methanol even if the ability of the catalyst to adsorb CO2 and to catalyze the reaction of CO2 with H2 is excellent.
For the EM-Cu/ZSM-5 catalyst, it is difficult to promote the dehydroaromatization due to its poor ability to catalyze the reaction of CO2 with H2 (Fig. 4); however, there is a strong absorption for CO2 (Fig. 3). On adding CO2 to the reaction atmosphere, it is easy for CO2 to occupy a large number of active sites, which not only hardly accelerates the elimination of hydrogen from the intermediate, but also weakens the aromatization reaction, and then decreases the yield of aromatics.
For EM-Ag/ZSM-5 and EM-Zn/ZSM-5 catalysts, the latter has a better ability to adsorb CO2 (Fig. 3) and to catalyze the reaction of CO2 with H2 (Fig. 4), as well as better dehydroaromatization ability (Fig. 1 and Table 1). Consequently, the increase in aromatics yields over EM-Zn/ZSM-5 was larger than that over EM-Ag/ZSM-5 when adding CO2 into the MTA reaction atmosphere. In addition, due to the weaker dehydroaromatization ability of EM-Ag/ZSM-5, the olefin intermediates obtained from the dehydrogenation cannot fully undergo the aromatization reaction, resulting in the higher content of ethane and propane and lower aromatics yield over EM-Ag/ZSM-5 than those over EM-Zn/ZSM-5 in the presence of CO2. The EM-Ni/ZSM-5 catalyst has a strong ability to adsorb and activate CO2 (Fig. 3); however, its coking deactivation suppresses its ability to catalyze fundamental carbocation reactions, especially the aromatization reaction of the intermediates. As a consequence, although the EM-Ni/ZSM-5 catalyst can catalyze the reaction between CO2 and H2 to reduce the content of alkanes and increase the content of olefins, it cannot greatly improve the aromatics yield when CO2 is added into the MTA atmosphere.
3.4 Methanol aromatization over catalysts modified with different contents of Zn in the presence or absence of CO2
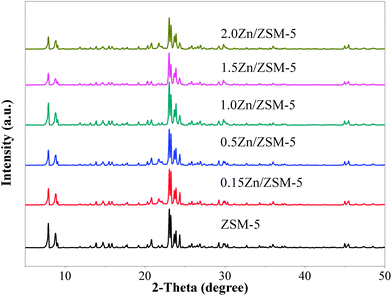
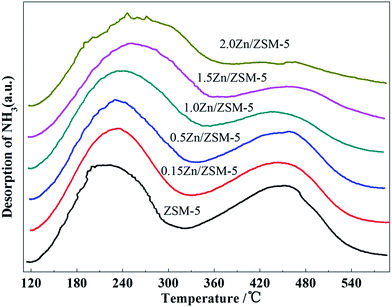
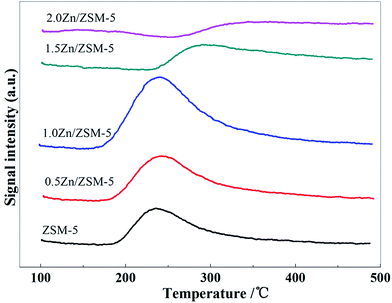
The XRD results (Fig. 5) indicate that the structure of ZSM-5 did not change when ZSM-5 was impregnated with different contents of Zn, even up to 2.0 wt%. As shown in Fig. 6, the introduction of zinc species on ZSM-5 had a certain influence on the distribution of acid sites and acidity. With the increase of Zn content, the amount of weak acid sites increased and the amount of strong acid sites decreased, which may be due to the fact that some acid sites were covered and Zn-L weak acid sites were generated.22 Fig. 7 shows the CO2-TPD results of the ZSM-5 modified with different contents of Zn. When Zn content was increased to 1.0 wt%, desorption peak temperature remained at about 240 °C, whereas the area of the desorption peak significantly increased. The desorption peak was shifted to a higher temperature when the Zn content continued to increase. These changes indicate that the ability of the catalyst to adsorb CO2 increased with the increasing Zn content.Fig. 8 shows the conversion of CO2 in the reaction of CO2 with H2 catalyzed by the ZSM-5 modified with different contents of Zn. It was observed that the conversion of CO2 remained very low up to a Zn content of 0.5 wt%, and then rapidly increased with the increase in the Zn content upto 1.5 wt%. The conversion of CO2 slightly increased when the content of Zn increased from 1.5 wt% to 2.0 wt%. This suggests that the ability of the metal over the catalyst to catalyze the reaction of CO2 with H2 increased with the increasing Zn content.
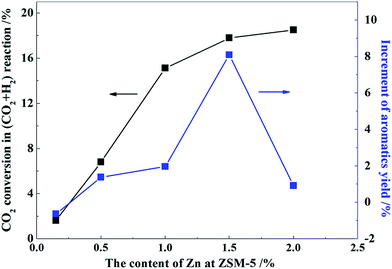
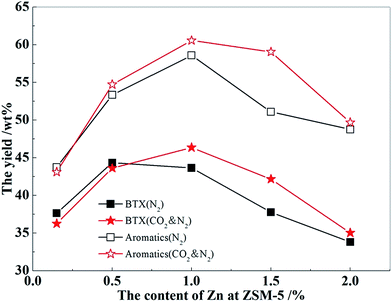
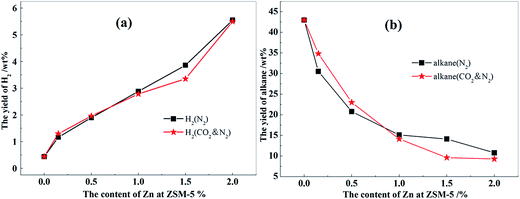
The results of methanol aromatization over the catalysts loaded with different contents of Zn in the presence and absence of CO2 are shown in Fig. 9. The changes in the aromatics yield for the MTA reaction in the presence of CO2 is shown in Fig. 8. As can be seen, aromatics yield increased with the increasing Zn content in the N2 atmosphere upto a maximum Zn content of 1.0 wt%, and then gradually decreased. After the addition of CO2, the aromatics yield slightly reduced than that in a N2 atmosphere when the content of Zn was not greater than 0.5 wt%. Then, the increase of the aromatics yield in the MTA reaction in the presence of CO2 was positive with the increasing Zn content and reached a maximum of 8.1% at a Zn content of 1.5 wt%. When Zn content was low, a large number of strong acid sites of the catalysts (Fig. 6) promoted the hydrogen transfer reaction of biomolecules, resulting in a large amount of alkanes, a poor aromatics yield, and little hydrogen generation. Moreover, the small number of metal centers over the catalysts had an insufficient ability to catalyze the reaction of CO2 with H2 (Fig. 8). Therefore, adding CO2 did not increase the yield of aromatic hydrocarbons, whereas weakened the dehydroaromatization ability of the catalyst due to the competitive adsorption of CO2, accompanied by the increase of alkanes and hydrogen (Fig. 10). As the Zn content increased from 1.0 to 1.5 wt%, the adsorption and activation capability of the catalysts for CO2 were significantly boosted (Fig. 7 and 8), such that the elimination of hydrogen was accelerated and the balance of dehydrogenation and hydrogenation was further broken. Hence, the change in aromatics yield was positive and was significantly improved (Fig. 8). At the same time, the amount of alkanes and hydrogen was decreased (Fig. 10) on adding CO2 into the MTA atmosphere.
When the content of Zn was increased to 2.0 wt%, a large number of strong acid sites of ZSM-5 were destroyed, which affected the reaction of the carbocation at the acid site, and the aromatics yield rapidly decreased. Thus, the catalyst could not effectively catalyze the aromatization reaction of olefin intermediates in spite of the strong ability of the catalyst to adsorb CO2 and catalyze the reaction of CO2 with H2. Therefore, in the presence of CO2, the yield of aromatics could not be significantly improved compared to the nitrogen atmosphere, and the content of alkanes and hydrogen could not decrease further either. Thus, there was a flat trend, as shown in Fig. 10.
4 Conclusions
ZSM-5 catalysts modified by Zn, Ni, Ag, and Cu were investigated in the presence or absence of CO2 for methanol to aromatics in a fixed-bed reactor. It was demonstrated that catalysts doped with Zn, Ni, and Ag were capable of increasing the total aromatics yield via promoting the rapid elimination of hydrogen in the system. This was achieved by catalyzing the reaction of CO2 with hydrogen to generate CO and CH4 when adding CO2 into the MTA reaction atmosphere. The EM-Zn/ZSM-5 showed 59.05 wt% aromatics yield, which was an increase of 8.1% compared to that in N2 atmosphere. However, EM-Cu/ZSM-5 was found to reduce the aromatics yield as a result of the fact that the active sites were occupied by a large number of CO2 molecules.Moreover, methanol aromatization over the catalysts modified with different contents of Zn in the presence or absence of CO2 was investigated. It was found that the aromatics yield increased with the increasing Zn content in the N2 atmosphere, up to a maximum Zn content of 1.0 wt%, and then gradually decreased. With the increase of the Zn content, the change of the aromatics yield in the MTA reaction in the presence of CO2 was negative, then turned positive, and finally reached a maximum of 8.1% at a Zn content of 1.5 wt%.
It was further found that the increase of the aromatics yield of the MTA reaction over the modified catalysts in a CO2 atmosphere is determined by the interaction of three essential catalytic factors: the ability of the catalyst to adsorb CO2, the ability of the metal over the catalyst to catalyze the reaction of CO2 with H2, and the dehydroaromatization ability of the catalyst. If the metal over the catalyst is capable of efficiently activating CO2 and catalyzing the reaction of CO2 with H2, the metal can effectively displace the olefin as a temporary hydrogen receptor to rapidly remove hydrogen produced during the MTA, reducing the formation of alkanes, thereby increasing the yield of aromatics. If the catalyst cannot effectively activate CO2 to react with H2, whereas has a strong adsorption capacity for CO2, CO2 cannot be timely consumed and will occupy the active sites of the catalyst to inhibit the MTA reaction. When the dehydroaromatization ability of the catalyst itself is insufficient, addition of CO2 hardly promotes the aromatization reaction of methanol even if the ability of the catalyst to adsorb CO2 and catalyze the reaction of CO2 with H2 is excellent.
It has also been demonstrated that there are two reactions that will generate CO and CH4, not just the reversed water–gas-shift reaction, when CO2 is added into the MTA reaction atmosphere.
Acknowledgements
The authors gratefully acknowledge the support and encouragement of the National Natural Science Foundation of China (21176208), National Basic Research Program of China (2012CB720500), and Fundamental Research Funds for the Central Universities (2011QNA4032).References
- Y. Q. Zhang, Technology & Economics in Petrochemicals, 2013, vol. 29, pp. 3–6 Search PubMed.
- Y. C. Zhang, H. X. Wang, X. W. Zhang and K. J. Li, Chem. Ind. Eng. Prog., 2016, 35, 801–805 Search PubMed.
- BP Statistical Review of World Energy, 2015 Search PubMed.
- T. Wang, X. P. Tang, X. F. Huang, W. Z. Qian, Y. Cui, X. Y. Hui, W. Yang and F. Wei, Catal. Today, 2014, 233, 8–13 CrossRef CAS.
- Z. H. Zeng, Shape selective catalysis, China Petrochemical Press, Beijing, 1994 Search PubMed.
- D. L. Zeng, J. Yang, J. Q. Wang, J. Xu, Y. X. Yang, C. H. Ye and F. Deng, Microporous Mesoporous Mater., 2007, 98, 214–219 CrossRef CAS.
- T. Tian, Z. W. Qian, X. P. Tang, H. Song and F. Wei, Acta Phys.-Chim. Sin., 2010, 26, 3305–3309 CAS.
- J. A. Lopez-Sanchez, M. Conte, P. Landon, J. K. Bartley, S. H. Taylor and A. F. Carley, Catal. Lett., 2012, 142, 1049–1056 CrossRef CAS.
- Y. M. Ni, A. M. Sun, X. L. Wu, G. L. Hai, J. L. Hu, T. Li and G. X. Li, Microporous Mesoporous Mater., 2011, 143, 435–442 CrossRef CAS.
- M. Conte, J. A. Lopez-Sanchez, Q. He, D. J. Morgan, Y. Ryabenkova, J. K. Bartley, A. F. Carley, S. H. Taylor, C. J. Kiely, K. Khalid and G. J. Hutchings, Catal. Sci. Technol., 2012, 2, 105–112 CAS.
- J. G. Zhang, W. Z. Qian, C. Y. Kong and F. Wei, ACS Catal., 2015, 5, 2982–2988 CrossRef CAS.
- Y. Bi, Y. L. Wang, X. Chen, Z. X. Yu and L. Xu, Chin. J. Catal., 2014, 35, 1740–1751 CrossRef CAS.
- J. S. Chang, V. P. Vislovskiy, M. S. Park, D. Y. Hong, J. S. Yoo and S. E. Park, Green Chem., 2003, 5, 587–590 RSC.
- N. R. Shiju, M. Anilkumar, S. P. Mirajkar and C. V. Satyanarayana, J. Catal., 2005, 230, 484–492 CrossRef CAS.
- C. R. Adams and T. J. Jennings, J. Catal., 1970, 17, 157–177 CrossRef CAS.
- G. S. Sun, Q. Z. Huang, H. Q. Li, H. T. Liu, Z. Zhang, X. R. Wang, Q. P. Wang and J. S. Wang, Chin. J. Catal., 2011, 32, 1424–1429 CAS.
- J. Ogonowski and E. Skrzynska, Catal. Commun., 2009, 11, 132–136 CrossRef CAS.
- G. S. Sun, Q. Z. Huang, H. Q. Li, H. T. Liu, Z. Zhang, X. R. Wang, Q. P. Wang and J. S. Wang, Chin. J. Catal., 2011, 32, 1424–1429 CAS.
- J. F. Ding, Z. F. Qin, X. K. Li, G. F. Wang and J. G. Wang, J. Mol. Catal. A: Chem., 2010, 315, 221–225 CrossRef CAS.
- J. H. Li, C. Hu, K. Tong, H. Xiang, Z. R. Zhu and Z. H. Hu, RSC Adv., 2014, 4, 44377–44385 RSC.
- P. Michorczyk and J. Ogonowski, React. Kinet. Catal. Lett., 2003, 78, 41–47 CrossRef CAS.
- J. Y. Wang, W. H. Li and J. X. Hu, J. Fuel Chem. Technol., 2009, 37, 607–612 CAS.
| This journal is © The Royal Society of Chemistry 2017 |