Open Access Article
Open Access ArticleInfrared thermochromic properties of monoclinic VO2 nanopowders using a malic acid-assisted hydrothermal method for adaptive camouflage
Haining Ji ,
Dongqing Liu*,
Haifeng Cheng,
Chaoyang Zhang,
Lixiang Yang and
Dewei Ren
,
Dongqing Liu*,
Haifeng Cheng,
Chaoyang Zhang,
Lixiang Yang and
Dewei Ren
Science and Technology on Advanced Ceramic Fibers and Composites Laboratory, National University of Defense Technology, Changsha 410073, P. R. China. E-mail: dongqingliu@ymail.com; Tel: +86 0731 84576440
First published on 17th January 2017
Abstract
Monoclinic VO2 nanopowders were synthesized using a malic acid-assisted hydrothermal method. The derived VO2 nanopowders were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, Raman spectroscopy and scanning electron microscopy. The phase transition properties of the monoclinic VO2 nanopowders were studied using differential scanning calorimetry, which displayed an obvious phase transition at 67.2 °C with a narrow thermal hysteresis width of 4.9 °C. Also, the resistance–temperature relationship and the thermal infrared images in the waveband 7.5–13 μm were analyzed. The results showed that the VO2 samples have excellent electrical properties with resistance changes as large as two orders of magnitude. The VO2 nanopowders obtained can control their infrared radiation intensity actively and lower their radiation temperature. Furthermore, the stability of the VO2 nanopowders was investigated. The results showed that the VO2 nanopowders have good thermal stability, oxidation resistance below 375 °C in an air atmosphere and humidity resistance, which has great application prospects in adaptive infrared camouflage technology.
1 Introduction
With the rapid development of modern technology, military reconnaissance and precision guided technology is widely used. Target survivability on the battlefield is also facing a great threat, so it is important to develop camouflage and stealth technology for modern warfare.1 Traditional camouflage is achieved by wearing a camouflage coat with similar colors or textures to the surroundings, which is only used to camouflage military objectives in a fixed background.2 However, as the location changes, these may be discovered by enemies because of the differences between the camouflage coat and the new background. Adaptive camouflage technologies are being developed. The target is to make military armed forces more mobile and better protected. Ideally, adaptive camouflage could exhibit active changes to the object's surroundings by varying its color and infrared characteristics, acting as perfect concealment from visual and infrared detection.3Vanadium dioxide (VO2) has intrigued researchers for almost six decades since Morin first discovered its temperature-driven metal-insulator transition (Tc ≈ 68 °C).4 Accompanied with the phase transition, VO2 shows a reversible abrupt change in resistivity and infrared emissivity.5–7 These promising properties make VO2 an ideal key material in adaptive infrared camouflage.
So far, the study on adaptive infrared camouflage of VO2 mainly focuses on thin films.8–12 Among them, Mikhail A. Kats et al.10 reported that VO2 films showed large broadband negative differential thermal emittance property and could be used in infrared camouflage. Based on this, Xiao Lin et al.11 also reported a VO2/graphene/CNT (VGC) sandwich-like structure adaptive thermal camouflage artificial system, which can blend into the surrounding background by electric heating to cheat thermal imaging cameras. Recently, our group has also prepared VO2 thin films and W doped VO2 thin films, which can control its emissivity and infrared radiation intensity actively and lower its radiation temperature.8,12 Compared with thin films, VO2 nanopowders are better suitable for the surface of substrates with a large surface area and/or complex morphology due to both technical and cost problems.13,14 And VO2 nanopowders can remarkably lessen the stress for the phase changes and have broader application.14 Therefore, it is urgent to study the infrared camouflage properties of the VO2 nanopowders.
In this paper, monoclinic VO2 (VO2 (M)) nanopowders were synthesized via a novel one-step hydrothermal method with an ideal environmental friendly reducing agent malic acid. Thermal infrared images of VO2 (M) nanopowders were tested under different temperature based on thermal imaging camera working in the waveband 7.5–13 μm. And it was found that the VO2 (M) nanopowders obtained can control its infrared radiation intensity and lower its radiation temperature. Finally, the stability of VO2 (M) nanopowders was studied for further development of the application.
2 Experimental
All reagents were purchased from Aladdin chemical reagent corporation and used without further purification. VO2 (M) nanopowders were prepared by a novel one-step hydrothermal method using a vanadium source of ammonium metavanadate (NH4VO3) and a reducing agent of malic acid (C4H6O5) without additional surfactant. In a typical synthesis, 2.34 g NH4VO3 and 4.02 g C4H6O5 were dispersed in 60 mL deionized water. The mixture was stirred for 30 min and then transferred to a 100 mL Teflon-lined stainless-steel autoclave. The hydrothermal reaction was carried out at 260 °C for 24 h and then air-cooled to room temperature. The final products were collected via centrifugation, washed with deionized water and ethanol three times and dried in a vacuum drying oven at 80 °C for 12 h.The crystalline structure of VO2 (M) nanopowders was characterized by a Bruker D8 advance diffractometer equipped with monochromatic Cu Kα radiation (λ = 0.15406 nm). The composition of the nanopowders were characterized via a Bruker Vertex 70 Fourier transform infrared spectroscopy (FTIR) instrument using KBr pellet method and a Horiba JY HR Evolution Raman Spectroscopy with excitation wavelength 532 nm. The morphology was obtained using a NOVA NanoSEM 230 field-emission scanning electron microscope (FESEM). The phase transition behaviors of the nanopowders were measured by METTLER TOLEDO differential scanning calorimetry (DSC) over the temperature range from 0 to 100 °C using a liquid nitrogen cooling unit. The heating and cooling rates were set at 10 °C min−1. The thermal stability of the nanopowders was studied with thermal gravimetric analysis (TGA) by TA instruments Q600 system. The temperature was increased from room temperature to 800 °C in a ramp of 5 °C min−1 and an air flow of 60 mL min−1. The effect of humidity on the performance of the nanopowders was studied in a climate chamber which allows experiments at fixed values of temperature and humidity (RH). The treatments were performed at an ambient temperature of 25 °C and RH = 95%. The duration for the treatment was 0 h, 24 h, 48 h, 96 h, and 168 h, respectively. The samples treated were dried in a vacuum drying oven at 60 °C for 10 h in order to carry out XRD and DSC tests. The resistance measurements were performed by standard four-terminal method using a Quantum Design Physics Property Measurement System (PPMS).
In order to investigate infrared camouflage properties, thermal infrared images were tested under different temperature based on FLIR T420 thermal imaging camera operating in the waveband 7.5–13 μm. Firstly, the VO2 (M) nanopowders need to be pressed into small rectangle pellets. Vanadium pentoxide (V2O5) pellets, considered as a reference, and vanadium dioxide pellets were prepared by automatic tablet press using the same operating conditions: all rectangle pellets were realized in a rectangle die kit, involving the same uniaxial pressure, and all powder samples were prepared with the same quantity. Therefore, the thickness and the surface roughness of the samples are approximately the same.
3 Results and discussion
The crystalline structure of as-prepared nanopowders were characterized by XRD. Fig. 1a showed the XRD patterns of nanopowders obtained at 260 °C for 24 h. All peaks can be indexed as a single monoclinic phase VO2 (M) (JCPDS card. no. 43-1051). It can also be seen that all of the peaks were sharp and strong with relatively narrow peak widths, indicating the good crystallinity of VO2 (M). The results indicated that phase-pure and well-crystallized VO2 (M) nanopowders can be synthesized by such a novel one-step hydrothermal method. The method is very nice compared to the present ones. Firstly, for the hydrothermal synthesis, most of previously reported studies used V2O5 as a vanadium source,15–17 which exhibited a toxic property, thus limited the usage and development of this method. Secondly, the preparation of VO2 nanopowders mainly took higher-cost hydrazine as reducing agent,18–20 which increased the cost of VO2 nanopowders. Compared with the present method, the method with NH4VO3 and C4H6O5 as starting materials has some advantages such as easy obtained raw materials, lower cost and no environmental pollution.21,22 Especially malic acid, the reducing agent for the reaction, which can be directly obtained from apples, is an ideal environmental friendly reducing agent. This approach will aid in the large scale synthesis of pure vanadium dioxide nanopowders.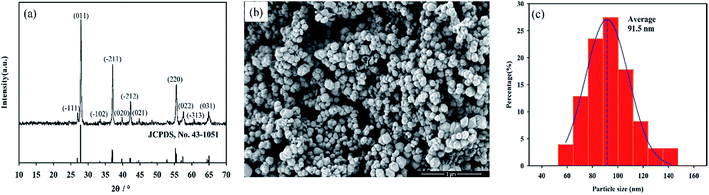 | ||
| Fig. 1 XRD patterns (a), SEM images (b) and particle size distribution (c) of as-synthesized VO2 nanopowders. | ||
The size and morphology of typical products were illustrated using SEM images. The SEM image (Fig. 1b) and particle size distributions (Fig. 1c) of nanopowders obtained from SEM image revealed that nanopowders were sphere-like with average particle size of 91.5 nm.
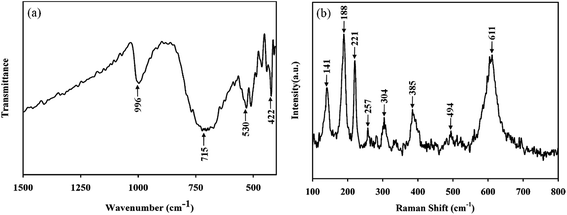
To investigate the chemical bonding between vanadium and oxygen ions and to confirm the phase purity, we performed FTIR and Raman spectrum measurement. Fig. 2a showed the FTIR spectrum of VO2 (M) samples prepared. The main vibrational bands observed from the FTIR spectrum are at 996 cm−1, 715 cm−1, 530 cm−1, 422 cm−1 and can be considered as intrinsic to vanadium dioxide, which matches well with earlier reports: the initial broad vibrational band at 530 cm−1 and 422 cm−1 are assigned to the V–O–V octahedral bending modes; the band at 996 cm−1 and 715 cm−1 is attributed to the coupled vibration of V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.23,24 These FTIR observations confirmed that the nanopowders correspond to the VO2 phase. Fig. 2b illustrated Raman spectrum of VO2 (M) samples prepared. The peaks in the Raman spectrum were all identified as 141 cm−1, 188 cm−1, 221 cm−1, 257 cm−1, 304 cm−1, 385 cm−1, 494 cm−1, and 611 cm−1 respectively, and these Raman-active modes were the clear evidence of the existing of VO2 (M), which were consistent with the previous results.25–27
O.23,24 These FTIR observations confirmed that the nanopowders correspond to the VO2 phase. Fig. 2b illustrated Raman spectrum of VO2 (M) samples prepared. The peaks in the Raman spectrum were all identified as 141 cm−1, 188 cm−1, 221 cm−1, 257 cm−1, 304 cm−1, 385 cm−1, 494 cm−1, and 611 cm−1 respectively, and these Raman-active modes were the clear evidence of the existing of VO2 (M), which were consistent with the previous results.25–27
The fully reversible phase transition of the as-synthesized VO2 (M) nanopowders are clearly revealed by DSC curves. Fig. 3(a) illustrated that the VO2 (M) nanopowders revealed a thermal hysteresis phenomenon with a width of 4.9 °C, which was narrower than the results reported in the literature.28,29 This may be caused by the interface effects.30 The heating of the VO2 (M) nanopowders was accompanied by endothermal effects at 67.2 °C in the DSC curves, corresponding to the transition temperature of VO2 from the monoclinic phase VO2 (M) to the tetragonal phase VO2 (R) (see schematic in Fig. 3b). Accordingly, the thermal analysis results provided the direct evidence for the occurrence of temperature driven first-order phase transition in VO2 (M) nanopowders.
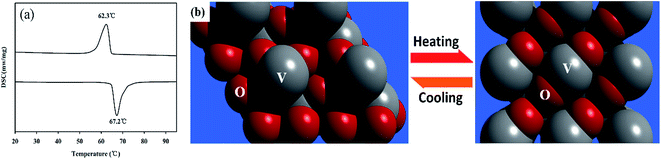 | ||
| Fig. 3 (a) DSC curves of as-synthesized VO2 nanopowder, (b) schematic of phase evolution between monoclinic VO2 (M) and tetragonal rutile VO2 (R). | ||
As mentioned above, phase transition is one of the most critical intrinsic characteristics of VO2 (M) which often reflected in the variation of resistance and infrared radiation. Then, to examine the thermochromic properties of VO2 (M) nanopowders, the electrical and infrared properties were researched.
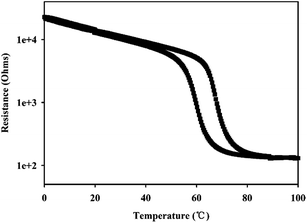
Fig. 4 showed the temperature-dependent resistance of the VO2 (M) nanopowders in temperature range of 0–100 °C, measured in the process of both cooling and heating. It can be seen that the transition temperature of VO2 (M) nanopowders was about 67 °C which was close to DSC results. At low temperature, VO2 (M) exhibited an insulating state with higher resistance; but they transited into metallic state when the temperature increased above the phase transition point characterized by abrupt jump of resistance. It should be noted that the resistance decreased by 2 order of magnitude as the temperature increased. The above results showed that VO2 (M) had excellent electrical properties. The obtained VO2 nanopowders have larger resistance jump than others' work based on thin film that resistance decreased 1.0–1.5 order of magnitude.5,31 The abrupt and large change of the resistance indicates that the VO2 nanopowders is stoichiometric and highly crystalline.
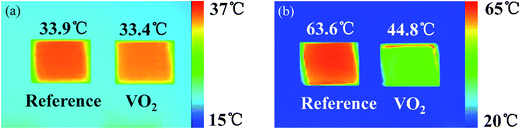
Thermal infrared images were characterized by thermal imaging camera to display the infrared radiation performance of VO2 pellets. Fig. 5 showed the thermal infrared images of reference samples (V2O5) and VO2 samples at different real temperature 40.0 °C and 75.0 °C. The emissivity of reference samples keep constant at the temperature 40.0 °C and 75.0 °C. During the measurement, we assured that reference samples and VO2 samples had the same real temperature, so the difference of the radiation temperature was due to the different emissivity.
As can be seen in Fig. 5(a), when real temperature was 40 °C, radiation temperature of reference samples and VO2 samples were 33.9 °C and 33.4 °C, respectively. There was almost no difference between the two radiation temperatures. As can be seen in Fig. 5(b), when real temperature was 75 °C, radiation temperature of reference samples and VO2 samples were 63.6 °C and 44.8 °C, respectively, radiation temperature difference reached to 18.8 °C. The results implied that at 40 °C, VO2 exhibited an insulating state with high emissivity and radiation intensity; but when the temperature increased to 75 °C, they transited into metallic state with low emissivity and radiation intensity. That is, thermal infrared radiation intensity of VO2 change adaptively with outside environment change and objects will keep consistent with the outside environment persistently. Therefore, VO2 nanopowders have great application prospects in the field of adaptive infrared camouflage technology.
For further development of the application, the stability study of the material is also very important. To understand the stability of the VO2 (M) nanopowders, the thermal gravimetric analysis was conducted in flowing N2 atmosphere and air atmosphere respectively, as shown in Fig. 6. As can be seen from Fig. 6a, when the powder samples were heated in the N2 atmosphere from room temperature up to 800 °C, the quality was almost constant, indicating that it can be stable in the N2 atmosphere. However, when the powder samples were heated in the flowing air atmosphere from room temperature up to 800 °C, the quality of powder samples increased due to oxidation by air. Fig. 6b revealed that VO2 (M) begun to be oxidized by O2 at 375 °C and finished at 656 °C. The weight gain is ca. 9.65% in its oxidative process. The weight gain values of VO2 (M) are well corresponding to the oxidation of the bulk VO2 to V2O5 (9.64%), as represented in equation: 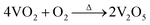 .
.
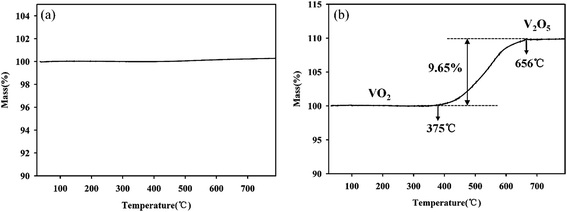 | ||
| Fig. 6 TGA analyses of the as-synthesized VO2 nanopowders in the N2 atmosphere (a) and in the air atmosphere (b). | ||
Furthermore, the effect of humidity on the performance of the nanopowders was studied. Fig. 7 showed XRD and DSC data for VO2 nanopowders after different treatment durations at an ambient temperature of 25 °C and RH = 95%. It is clear from Fig. 7a that the crystal structure of VO2 nanopowders did not change. And as can be seen from Fig. 7b, the positions of the exothermic peak and the endothermic peak and the enthalpy change almost did not change, which indicated that phase transition properties still retained after 168 h treatment. Based on the above results, VO2 (M) nanopowders have good thermal stability, oxidation resistance below 375 °C in air and humidity resistance, which can be applied to adaptive infrared camouflage in air atmosphere.
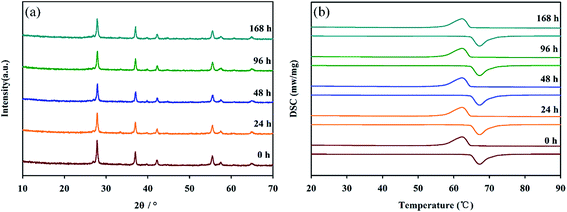 | ||
| Fig. 7 XRD spectra (a) and DSC curves (b) for VO2 nanopowders after different treatment durations at an ambient temperature of 25 °C and RH = 95%. | ||
4 Conclusion
In conclusion, VO2 (M) nanopowders were successfully synthesized by a novel one-step hydrothermal method with an ideal environmental friendly reducing agent. VO2 (M) nanopowders obtained exhibit a reversible phase transition properties with a critical temperature at 67.2 °C and a narrow hysteresis width of 4.9 °C. VO2 (M) nanopowders obtained have excellent electrical properties with resistance changes as large as two orders of magnitude. And VO2 (M) nanopowders obtained can control its infrared radiation intensity actively and lower its radiation temperature. Besides, VO2 (M) nanopowders have good thermal stability, oxidation resistance below 375 °C in air atmosphere and humidity resistance, which has great application prospects in the adaptive infrared camouflage technology.Acknowledgements
This work was supported by the Natural Natural Science Foundation of China (No. 51502344).References
- L. Li, X. L. Lü, B. Z. Li, X. P. Li and H. X. Wang, Evaluation of ground thermal infrared camouflage using pure technical and pure scale dynamic dea model, Adv. Mater. Res., 2014, 989–994, 2323–2327 CrossRef.
- J. Zhang, J. Yu, J. F. Jiao, H. Chen and P. Liu, Preparation and properties testing of intelligent color-changing camouflage coating, Adv. Mater. Res., 2013, 659, 15–18 CrossRef.
- H. Yu, S. Shao, L. Yan, H. Meng, Y. He, C. Yao, P. Xu, X. Zhang, W. Hu and W. Huang, Side-chain engineering of green color electrochromic polymer materials: toward adaptive camouflage application, J. Mater. Chem. C, 2016, 4, 2269–2273 RSC.
- F. J. Morin, Oxides which show a metal-to-insulator transition at the neel temperature, Phys. Rev. Lett., 1959, 3, 34–36 CrossRef CAS.
- F. Guinneton, L. Sauques, J. C. Valmalette, F. Cros and J. R. Gavarri, Comparative study between nanocrystalline powder and thin film of vanadium dioxide VO2: electrical and infrared properties, J. Phys. Chem. Solids, 2001, 62, 1229–1238 CrossRef CAS.
- M. E. A. Warwick and R. Binions, Advances in thermochromic vanadium dioxide films, J. Mater. Chem. A, 2014, 2, 3275–3292 CAS.
- B. Zhu, H. Tao and X. Zhao, Effect of buffer layer on thermochromic performances of VO2 films fabricated by magnetron sputtering, Infrared Phys. Technol., 2016, 75, 22–25 CAS.
- D. Q. Liu, H. F. Cheng, W. W. Zheng and C. Y. Zhang, Infrared thermochromic properties of VO2 thin films prepared through aqueous sol–gel process, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2012, 27, 861–865 CrossRef CAS.
- Z. P. Mao, W. Wang, Y. Liu, L. P. Zhang, H. Xu and Y. Zhong, Infrared stealth property based on semiconductor (M)-to-metallic (R) phase transition characteristics of W-doped VO2 thin films coated on cotton fabrics, Thin Solid Films, 2014, 558, 208–214 CrossRef CAS.
- M. A. Kats, R. Blanchard, S. Y. Zhang, P. Genevet, C. H. Ko, S. Ramanathan and F. Capasso, Vanadium dioxide as a natural disordered metamaterial: perfect thermal emission and large broadband negative differential thermal emittance, Phys. Rev. X, 2013, 3, 041004 Search PubMed.
- L. Xiao, H. Ma, J. Liu, W. Zhao, Y. Jia, Q. Zhao, K. Liu, Y. Wu, Y. Wei and S. Fan, Fast adaptive thermal camouflage based on flexible VO2/graphene/CNT thin films, Nano Lett., 2015, 15, 8365–8370 CrossRef CAS PubMed.
- D. Liu, H. Cheng, X. Xing, C. Zhang and W. Zheng, Thermochromic properties of W-doped VO2 thin films deposited by aqueous sol–gel method for adaptive infrared stealth application, Infrared Phys. Technol., 2016, 77, 339–343 CAS.
- Z. F. Peng, W. Jiang and H. Liu, Synthesis and electrical properties of tungsten-doped vanadium dioxide nanopowders by thermolysis, J. Phys. Chem. C, 2007, 111, 1119–1122 CAS.
- J. Li, C. Y. Liu and L. J. Mao, The character of W-doped one-dimensional VO2 (M), J. Solid State Chem., 2009, 182, 2835–2839 CrossRef CAS.
- H. H. Yin, M. Luo, K. Yu, Y. F. Gao, R. Huang, Z. L. Zhang, M. Zeng, C. X. Cao and Z. Q. Zhu, Fabrication and temperature-dependent field-emission properties of bundlelike VO2 nanostructures, ACS Appl. Mater. Interfaces, 2011, 3, 2057–2062 CAS.
- W. Yu, S. Li and C. Huang, Phase evolution and crystal growth of VO2 nanostructures under hydrothermal reactions, RSC Adv., 2016, 6, 7113–7120 RSC.
- L. Zhang, F. Xia, Z. Song, N. A. S. Webster, J. C. Song, H. Luo and Y. Gao, Phase and morphology evolution during the solvothermal synthesis of VO2 polymorphs, Inorg. Chem. Front., 2015, 3, 1–8 Search PubMed.
- Z. Gui, R. Fan, W. Q. Mo, X. H. Chen, L. Yang, S. Y. Zhang, Y. Hu, Z. Z. Wang and W. C. Fan, Precursor morphology controlled formation of rutile VO2 nanorods and their self-assembled structure, Chem. Mater., 2002, 14, 5053–5056 CrossRef CAS.
- Z. Zhang, Y. Gao, H. Luo, L. Kang, Z. Chen, J. Du, M. Kanehira, Y. Zhang and Z. L. Wang, Solution-based fabrication of vanadium dioxide on F: SnO2 substrates with largely enhanced thermochromism and low-emissivity for energy-saving applications, Energy Environ. Sci., 2011, 4, 4290–4297 CAS.
- Z. Gui, R. Fan, X. H. Chen and Y. C. Wu, A new metastable phase of needle-like nanocrystalline VO2·H2O and phase transformation, J. Solid State Chem., 2001, 157, 250–254 CrossRef CAS.
- J. Zou, Y. G. Peng and H. Lin, A low-temperature synthesis of monoclinic VO2 in an atmosphere of air, J. Mater. Chem. A, 2013, 1, 4250–4254 CAS.
- L. Li, J. Ge, R. Chen, F. Wu, S. Chen and X. Zhang, Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries, Waste Manag., 2010, 30, 2615–2621 CrossRef CAS PubMed.
- I. L. Botto, M. B. Vassallo, E. J. Baran and G. Minelli, IR spectra of VO2 and V2O3, Mater. Chem. Phys., 1997, 50, 267–270 CrossRef CAS.
- W. Chen, L. Mai, C. Jiang, J. Peng and Q. X. Q. Zhu, Synthesis and characterization of novel vanadium dioxide nanorods, Solid State Commun., 2003, 132, 513–516 CrossRef.
- R. Chen, L. Miao, C. Liu, J. Zhou, H. Cheng, T. Asaka, Y. Iwamoto and S. Tanemura, Shape-controlled synthesis and influence of W doping and oxygen nonstoichiometry on the phase transition of VO2, Sci. Rep., 2015, 5, 14087 CrossRef CAS PubMed.
- J. M. Wu and L. B. Liou, Room temperature photo-induced phase transitions of VO2 nanodevices, J. Mater. Chem., 2011, 21, 5499–5504 RSC.
- C. Cheng, K. Liu, B. Xiang, J. Suh and J. Q. Wu, Ultra-long, free-standing, single-crystalline vanadium dioxide micro/nanowires grown by simple thermal evaporation, Appl. Phys. Lett., 2012, 100, 103111 CrossRef.
- Z. Song, L. Zhang, F. Xia, N. A. S. Webster, J. Song, B. Liu, H. Luo and Y. Gao, Controllable synthesis of VO2 (D) and their conversion to VO2 (M) nanostructures with thermochromic phase transition properties, Inorg. Chem. Front., 2016, 3, 1035–1042 RSC.
- B. Dong, N. Shen, C. Cao, Z. Chen, H. Luo and Y. Gao, Phase and morphology evolution of VO2 nanoparticles using a novel hydrothermal system for thermochromic applications: the growth mechanism and effect of ammonium (NH4+), RSC Adv., 2016, 81559–81568 RSC.
- L. Zhong, M. Li, H. Wang, Y. Luo, J. Pan and G. Li, Star-shaped VO2 (M) nanoparticle films with high thermochromic performance, CrystEngComm, 2015, 17, 5614–5619 RSC.
- D. Zhang, K. Yang, Y. Li, Y. Liu, M. Zhu, A. Zhong, X. Cai, P. Fan and W. Lv, Employing TiO2 buffer layer to improve VO2 film phase transition performance and infrared solar energy modulation ability, J. Alloys Compd., 2016, 684, 719–725 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |