Open Access Article
Open Access ArticleSolvent-free synthesis of polyhydroquinoline derivatives employing mesoporous vanadium ion doped titania nanoparticles as a robust heterogeneous catalyst via the Hantzsch reaction†
G. B. Dharma Rao *ab,
S. Nagakalyanb and
G. K. Prasad*a
*ab,
S. Nagakalyanb and
G. K. Prasad*a
aDefence Research & Development Establishment, Jhansi Road, Gwalior 474 002, M.P., India. E-mail: gbdharmarao@gmail.com; gkprasad2001@yahoo.com
bDepartment of Chemistry, Kommuri Pratap Reddy Institute of Technology, Hyderabad – 501 301, TS, India
First published on 13th January 2017
Abstract
Mesoporous vanadium ion doped titania nanoparticles (V–TiO2) were used as a reusable and robust heterogeneous catalyst for one-pot four component synthesis of polyhydroquinoline derivatives via the esteemed Hantzsch reaction using arylaldehyde, β-ketoester, dimedone and ammonium acetate at 80 °C under solvent-free conditions as a multi-component synthesis. On the other hand, the catalytic activity of V–TiO2 was compared with undoped commercial titania nanocatalyst. This protocol was successfully pertinent to a wide range of structurally diverse arylaldehydes with β-ketoester, dimedone and ammonium acetate to afford the corresponding polyhydroquinoline derivatives. Operational simplicity, short reaction time and satisfactory yields are the key features of this protocol. The catalyst could easily be recycled and reused without observable decrease in catalytic activity.
1. Introduction
1,4-Dihydropyridines (1,4-DHPs) and polyhydroquinoline (PHQ) derivatives has become a noticeable class of privileged N-heterocyclic compounds and were widely investigated in past decades due to their promising pharmacological and biological activities.1 Some of them have antitubercular properties,2 anticancer,3 neurotropic,4 neuropeptide YY1 receptor antagonists,5 neuroprotective,6 platelet anti-aggregation,7 bronchodilating8 and antidiabetic activities.9 The 1,4-DHPs and PHQ derivatives which are commercially accessible, as analogues of nicotinamide adenine dinucleotide (NADH) coenzymes are extensively used as calcium channel blockers for the treatment of cardiovascular disorder including hypertension, angina and cardiac arrhythmias.10 Besides this, the focus on the synthesis of 1,4-DHPs with respect to multidrug resistance (MDR) reversal in tumor cells provided a new aspect to their applications.11 By the evidence of the above pharmacological and biological activities, the 1,4-DHPs and PHQ core units have occupied a governing position in medicinal chemistry, which evidently expresses the extraordinary potential of new 1,4-DHP and PHQ analogues as a source of precious drug candidates.Bearing in mind the considerable applications in the fields of medicinal, bioorganic and synthetic organic chemistry, there has been incredible attention in developing proficient methods for the synthesis of PHQ. Such a medicinally significant 1,4-DHPs was initially established by Arthur Hantzsch in 1882 via the reaction of aldehydes with ethyl acetoacetate and ammonia in acetic acid or by refluxing in alcohols more than a century ago.12 Though, the yields of the desired products were modest. In addition, a notable number of citations13 for the synthesis of PHQ are currently addressed to advances the reaction conditions as maximize the product yield and minimize the reaction time along with deviation in precursors to acquire the poly-functionalized PHQ. Practically all new advanced technologies have also been contributed in synthesis of 1,4-DHPs such as, microwave-mediated synthesis,14 the support of solar thermal energy,15 ultrasound irradiation,16 infrared irradiation,17 ionic liquids,18 grinding19 and metal halides or triflates.20 Even supposing most of these processes place ahead individual advantages and some are associated with more than a few of shortcomings such as extend reaction times, expensive/toxic reagents, deadly reaction circumstances, modest-product yields and the use of volatile organic solvents.
The improvement in nanotechnology has lead to an increasing insist for multifunctional materials. Highly ordered mesoporous materials occupied as stupendous catalysts owing to their high surface area and surface functionalities. The physical and chemical properties of mesoporous V–TiO2 materials depend on their particle size as well as percentage of vanadium ion doped in TiO2 lattice. Smaller sized particles are expected to expose enhanced chemical reactivity because of their huge surface area to volume ratio, augmented number of surface defect sites. Particle size excessively plays imperative responsibility in dynamics of electron/hole recombination process and influences its catalytic properties.
Moreover, the noxious and volatile nature of many organic solvents used in organic synthesis has posed a grave menace to the environment. Accordingly, protocols that effectively minimize their use are currently the subject of substantial awareness for synthetic chemists.21 Furthermore, it is imperative to note that the amalgamation of heterogeneous catalysis with the use of solvent-free conditions stand for an appropriate way in the direction of the so-called ideal synthesis. Nanoparticle TiO2 powerfully catalyzes the conjugate 1,4-addition of indoles to α,β-unsaturated ketones and 1,2-addition of Me3SiCN to carbonyl compounds.22 They confirmed the efficiency of TiO2 NPs and attributed it to the enhanced acidic sites and surface area. In this regards and the advantage of enhanced catalytic activity of TiO2 NPs herein, we describe an expeditious, practical and one-pot four component solvent-free synthesis of polyhydroquinoline derivatives via esteemed Hantzsch reaction promoted by V–TiO2 NPs.
Above and beyond, TiO2 is more steady, copious, non hazardous and inexpensive. For this motive, we have synthesized mesoporous V–TiO2 materials of nano-size by sol–gel method and characterized them by XRD, TEM, EDX, XPS and FT-IR techniques. In prolongation of our work on new synthetic approaches23 and to determine the catalytic application of V–TiO2 (ref. 24) in this communication, we explanation the percentage of vanadium ion doped in TiO2 size dependant application of V–TiO2 nanoparticles of dissimilar sizes (6.12 nm, 6.45 nm, 5.85 nm, 6.76 nm, and 11 nm) as reusable robust heterogeneous catalysts for the synthesis of poly-functionalized hydroquinoline analogues 5a–u (Scheme 1) via Hantzsch hetero-annulation under solvent-free conditions.
2. Results and discussion
2.1. Characterization of V–TiO2 NPs
XRD patterns of the synthesized V–TiO2 catalysts are depicted in Fig. 1. Data depicts peaks at 2θ values 25.2°, 37.8°, 48.0°, 53.8°, and 62.7°. These peaks can be recognized to the presence of (1 0 1), (0 0 4), (2 0 0), (1 0 5), and (2 1 5) indices. This XRD pattern demonstrates 2θ values and relative intensities that match with (JCPDS 21-1272) data of anatase phase of TiO2. No peaks corresponding to oxides of vanadium (V2O5 or VxOy) are observed even for samples doped with 2 at% vanadium. This observation indicates the incorporation of vanadium ion into TiO2 lattice. The data also illustrated peak broadening in XRD pattern indicating the formation of crystallites with very small size. The crystallite sizes of V–TiO2 catalysts were calculated by Scherrer equation and they were found to be 6.12, 6.45, 5.85, 6.76 nm respectively for 0.1 V–TiO2, 0.25 V–TiO2, 0.55 V–TiO2 and 2 V–TiO2 respectively. The crystallite size of bare TiO2 nanoparticles (TiO2 NPs) was found to be 11 nm.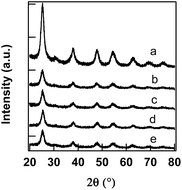 | ||
| Fig. 1 XRD patterns of (a) TiO2 (b) 0.1 V–TiO2 (c) 0.25 V–TiO2 (d) 0.55 V–TiO2 (e) 2.0 V–TiO2 nanocatalysts. | ||
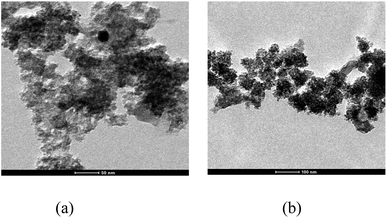
TEM was used to determine the crystallite sizes of TiO2 particles synthesized by modified sol–gel method followed by hydrothermal treatment. TEM images of both doped (0.1 V–TiO2) and bare (TiO2 NPs) samples are shown in Fig. 2(a) and (b). No noteworthy differences are observed when the images of V–TiO2 and TiO2 NPs samples are compared. TEM images visibly indicate that particles have homogeneity and quite small size. It can be seen from the images that particles are established to be embedded in the agglomerates with crystallite sizes around 6 to 11 nm which are found to be comparable to average crystallite sizes determined from XRD data.
Hence keeping these particulars in observation, in this communication, we were concerned in attaining the solvent-free synthesis of polyhydroquinoline derivatives using catalytic amount of V–TiO2 nanoparticles. Our exploration started with an optimization study for the union between dimedone (1), p-methoxybenzaldehyde (2c), ethyl acetoacetate (3) and ammonium acetate (4) (Table 4, entry 3) in the presence of a catalytic amount of various size V–TiO2 NPs as well as commercial TiO2 NPs. The best result was observed when dimedone (1), p-methoxybenzaldehyde (2c), ethyl acetoacetate (3) and ammonium acetate (4) were used in the mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 employed by the catalytic amount of V–TiO2 NPs of 5.85 nm size at 80 °C under solvent-free conditions, to afford the resulting ethyl 4-(4-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (5c) (Table 4, entry 3) in 90% yield without any other bi-products. This four component union reaction proceeded efficiently at temperature (80 °C) with high yield under solvent-free reaction condition and shown in Fig. 3.
2 employed by the catalytic amount of V–TiO2 NPs of 5.85 nm size at 80 °C under solvent-free conditions, to afford the resulting ethyl 4-(4-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (5c) (Table 4, entry 3) in 90% yield without any other bi-products. This four component union reaction proceeded efficiently at temperature (80 °C) with high yield under solvent-free reaction condition and shown in Fig. 3.
From the above considerations, we examine the different catalytic concentrations for the solvent-free synthesis of polyhydroquinoline derivatives using V–TiO2 NPs with 5.85 nm particle size as heterogeneous catalyst at 80 °C. To study the feasibility of the different catalytic concentrations, the reaction of dimedone (1), p-methoxybenzaldehyde (2c), ethyl acetoacetate (3) and ammonium acetate (4) was selected as model reactants for the synthesis of corresponding polyhydroquinoline (Table 4, entry 3) and fixed the reaction time to 12 min. However, among all the catalytic concentrations, it was observed that, 2.0 mol% of V–TiO2 NPs of 5.85 nm size was found to be the most effective for the reaction of four component Hantzsch reaction with respect to yield as tabulated in Table 1.
| Entry | Catalytic conc. (mol%) | Reaction time (min) | % yield |
|---|---|---|---|
| 1 | 0.5 | 12 | 45 |
| 2 | 1.0 | 12 | 62 |
| 3 | 1.5 | 12 | 76 |
| 4 | 2.0 | 12 | 90 |
| 5 | 2.5 | 12 | 81 |
During exploratory reactions, we studied the four-component condensation reaction of model reactants dimedone (1), p-methoxybenzaldehyde (2), ethyl acetoacetate (3) and ammonium acetate (4) in the mole ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 under different addressed catalytic conditions for 12 min as reaction time. However, V–TiO2 powerfully catalyzed the reaction and afford high yield of the corresponding PHQ product (Table 2, entry 4).
2 under different addressed catalytic conditions for 12 min as reaction time. However, V–TiO2 powerfully catalyzed the reaction and afford high yield of the corresponding PHQ product (Table 2, entry 4).
The possibility of optimized reaction conditions was further extended to the synthesis of more functionalized polyhydroquinoline derivatives and experiments were performed by making use of wide range of arylaldehyde bearing variety of functional groups. As shown in the Table 2, the desired ring closure annulations of polyhydroquinoline (PHQ) ring was obtained with mono, di (Table 4, entries 6, 12 and 16) and even tri-substituted (Table 4, entries 8 and 19) arylaldehydes in good to excellent yields (70–92%). Furthermore, hetero arylaldehydes such as furfural and pyridine-3-carboxyaldehyde (Table 4, entries 14 and 15) also afforded the target products in good yields (62–78%) under the same reaction conditions. In all these cases, the reactions were clean and the products were obtained with simple work-up in good to excellent yields. Compared to reports which were addressed in the literature, this method has numerous advantages, which include operational simplicity, short reaction time and satisfactory yields, does not require chromatographic separation and wider substrate scope are the key features of this protocol. The catalyst could easily be recycled and reused without observable decrease in catalytic activity.
2.2. Reusability of the catalyst
In order to explore the recyclability of catalyst, it was filtered off at the end of the reaction from the reaction mixture and washed with mixture of hot ethanol and water, dried and activated at 300 °C for 2 h. Catalyst was and reused as such for subsequent experiments (up to four cycles) under the similar reaction conditions and the change in their catalytic activity was studied w.r.t time and % yield. The recyclability of catalyst was verified on the reaction of dimedone (1), p-methoxybenzaldehyde (2c), ethyl acetoacetate (3) and ammonium acetate (4) (Table 4, entry 3) was selected as model reactants and fixed the reaction time to 12 min to afford the corresponding polyhydroquinoline in 90, 86, 80 and 72% yields over four cycles (Table 3). It was noticed that yields of the product remained comparable in these experiments, and thereby pointing the reusability of the catalyst without any significant loss in catalytic activity.| Entry | R | R1 | R2 | Time (min) | Product | Yieldb (%) | Mp (°C) | Reported mp (°C) |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (1.0 mmol), 2 (1.0 mmol), 3 (1.0 mmol) and 4 (2.0 mmol) at 80 °C using V–TiO2 NPs of 5.85 nm size under solvent-free condition.b Isolated yield. | ||||||||
| 1 | H- | CH3 | OEt | 10 | 5a | 85 | 203–204 | 202–204 |
| 2 | 4-CH3- | CH3 | OEt | 12 | 5b | 90 | 261–262 | 260–261 |
| 3 | 4-CH3O- | CH3 | OEt | 12 | 5c | 90 | 256–257 | 257–259 |
| 4 | 4-Cl- | CH3 | OEt | 14 | 5d | 82 | 245–246 | 245–246 |
| 5 | 4-NO2- | CH3 | OEt | 15 | 5e | 80 | 243–244 | 242–244 |
| 6 | 2,4-Cl2- | CH3 | OEt | 12 | 5f | 74 | 242–243 | 241–243 |
| 7 | 2-Cl- | CH3 | OEt | 15 | 5g | 76 | 207–208 | 208–210 |
| 8 | 3,4,5-(OCH3)3- | CH3 | OEt | 20 | 5h | 76 | 199–200 | 198–199 |
| 9 | 2-NO2- | CH3 | OEt | 15 | 5i | 70 | 206–207 | 206–208 |
| 10 | 3-NO2- | CH3 | OEt | 14 | 5j | 82 | 178–179 | 177–178 |
| 11 | 4-F- | CH3 | OEt | 10 | 5k | 94 | 185–186 | 184–186 |
| 12 | 3,4-(OCH3)2- | CH3 | OEt | 20 | 5l | 88 | 196–198 | 198–199 |
| 13 | 4-N(CH3)2- | CH3 | OEt | 15 | 5m | 90 | 262–263 | 263–264 |
| 14 | 2-Furyl | CH3 | OEt | 12 | 5n | 65 | 247–248 | 246–248 |
| 15 | 3-Pyridyl | CH3 | OEt | 18 | 5o | 74 | 66–68 | 66–67 |
| 16 | 4-OH-3-CH3O- | CH3 | OEt | 14 | 5p | 78 | 211–212 | 210–212 |
| 17 | 4-OH- | CH3 | OEt | 18 | 5q | 82 | 230–231 | 232–234 |
| 18 | 4-Br- | CH3 | OEt | 12 | 5r | 90 | 252–253 | 253–255 |
| 19 | 3,4,5-(OCH3)3- | CH3 | OMe | 20 | 5s | 72 | 220–221 | 220–224 |
| 20 | 4-N(CH3)2- | CH3 | OMe | 12 | 5t | 78 | 257–258 | 258–260 |
| 21 | 3-Br- | CH3 | OEt | 16 | 5u | 82 | 230–232 | 234–236 |
On the basis of the above explanations and the literature precedents, the possible mechanistic path for the synthesis of polyhydroquinoline (PHQ) derivatives is given in Scheme 2. The used metal oxides species contains Lewis acid sites and Bronsted acid sites in addition to basic surface sites. Surface of V–TiO2 nanoparticles was found to be basic in nature. Further incorporation of vanadium transition metal into TiO2 lattice would increase the number of Lewis acid sites, Bronsted acid sites and other defects which play main role in increasing the reactivity of metal oxides as heterogeneous catalyst. The interaction between arylaldehyde with the acidic sites of V–TiO2 NPs catalyst surface generated the more electrophilic carbon center followed by the nucleophilic attack of β-ketoester followed by dimedone to give reactive adduct intermediate. The resulting intermediate undergoes a intramolecular cyclization in presence of NH4OAc affording the corresponding desired polyhydroquinoline followed by the elimination of water molecule.
3. Experimental details
3.1. Materials
Vanadyl acetylacetonate, titanium tetraisopropoxide (TTIP) were procured from Acros organics, UK. Dichloromethane, ethanol and acetonitrile were obtained from E. Merck India Ltd. Arylaldehydes, methyl/ethyl β-ketoester, dimedone and ammonium acetate were purchased from Sigma-Aldrich, India. Chemicals purchased from Across, Sigma-Aldrich and Merck (India) were used without further purification.3.2. Preparation of vanadium ion doped TiO2 nanoparticles (V–TiO2 NPs)
V–TiO2 NPs of various dimensions were synthesized in our laboratory by modified sol–gel hydrolysis of titanium(IV) isopropoxide (TTIP) subsequently hydrothermal treatment procedure as follows: initially, the mixture of deionized water and ethanol were placed at room temperature with constant vigorous stirring. To this mixture add TTIP drop wise which is dissolved in anhydrous ethanol. As the observed gel was relocated into Teflon lined autoclave and heated at 80 °C for 24 h. The accomplished solid was dried at room temperature and finally tagged as bare TiO2 nanoparticles (TiO2 NPs). At last, the obtained TiO2 NPs were washed with excess of ethanol to eradicate organic moieties.25 In the same way, metal ion dopants were incorporated through adding appropriate amounts of vanadyl acetylacetonate into distilled water foregoing to the hydrolysis of TTIP. By varying concentration of vanadyl acetylacetonate, diverse nanoparticles were synthesized. The nanoparticles were tagged as 0.1 V–TiO2 (0.1 at% V in TiO2), 0.25 V–TiO2 (0.25 at% V in TiO2), 0.55 V–TiO2 (0.55 at% V in TiO2) and 2.0 V–TiO2 (2.0 at% V in TiO2).3.3. Catalyst characterization techniques
Powder X-ray diffraction (XRD) patterns of the synthesized samples were recorded on X'Pert Pro Diffractometer of M/s Panalytical, Netherlands make using Cu Kα radiation. Transmission electron microscopy (TEM) measurements were done on Tecnai transmission electron microscope of FEI make. Samples were suspended in 30 mL of acetone, and the suspension was sonicated for 30 min. After that, suspension was placed on carbon coated copper grids of 3 mm dia and dried at room temperature prior to the analysis. Nitrogen adsorption measurements were done on ASAP 2020 surface area analyzer of Micrometrics, USA. FT-IR measurements were done as KBr pellets on Perkin Elmer, USA instrument. X-ray photoelectron spectroscopy (XPS) data was recorded on KRATOS AXIS 165 instrument. TiO2, V–TiO2 nanoparticles are confirmed by Energy-Dispersive X-ray spectroscopy (EDX) spectrum that reveals the presence of Ti, V and O elements. Au and C signals could be due to gold coating and carbon film supporting the specimen during SEM observation.3.4. Catalytic activity: synthesis of polyhydroquinoline derivatives via Hantzsch reaction
To a mixture of methyl/ethyl acetoacetate (1.0 mmol), dimedone (1.0 mmol), arylaldehyde (1.0 mmol) and ammonium acetate (2.0 mmol), catalytic amount (2.0 mol%) of V–TiO2 nanoparticle was added at room temperature under stirring. The reaction mixture was heated on oil bath at 80 °C. The reaction advancement was monitored by TLC. After the completion of reaction, it was cooled to room temperature and the resultant reaction mixture was washed with brine and extracted with ethyl acetate. The catalyst was separated out by filtration from the extraction mixture. Organic layer was separated, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was then purified by re-crystallization from hot ethanol and water to afford 1,4-dihydroquinoline derivatives in high yield. The structures of the products were confirmed from physical and spectroscopic data (IR and 1H NMR) in comparison with the literature data.4. Conclusion
In conclusion, a simple, efficient and environmentally benign procedure has been developed using mesoporous vanadium ion doped titania nanoparticles (V–TiO2) as robust heterogeneous nanocatalyst for the synthesis of polyhydroquinoline derivatives via esteemed Hantzsch reaction of arylaldehyde, β-ketoester, dimedone and ammonium acetate at 80 °C under solvent-free conditions as multi-component synthesis. This protocol was successfully pertinent to a wide range of structurally diverse arylaldehydes with β-ketoester, dimedone and ammonium acetate to afford the corresponding polyhydroquinoline derivatives. The advantages of performing the Hantzsch reaction in the presence of V–TiO2 NPs as catalyst can be summarized as follows: (1) use of a safe, non-volatile, non-corrosive and easily handled V–TiO2 NPs; (2) recovery of V–TiO2 NPs at the end of the reactions by simple filtration and washing; (3) desired products are obtained in admirable yields under mild reaction conditions; and (4) the reactions are carried out under solvent-free conditions with economic benefits. Excellent reusability of the catalyst and ease of isolation of product are the other added advantages that make this approach an attractive alternative for the synthesis of these polyhydroquinoline derivatives.Acknowledgements
Authors express deep sense of gratitude to Ravi K. Gujjula, Principal, KPRIT, Hyderabad and also special thanks to IISER, Bhopal.Notes and references
- R. Shan, C. Velazquez and E. E. Knaus, J. Med. Chem., 2004, 47, 254–261 CrossRef CAS PubMed.
- P. S. Eharkar, B. Desai, H. Gaveria, B. Varu, R. Loriya, Y. Naliapara, A. Shah and V. M. Kulkarni, J. Med. Chem., 2002, 45, 4858–4867 CrossRef.
- T. Tsuruo, H. Iida, M. Nojiri, S. Tsukagoshi and Y. Sakurai, Cancer Res., 1983, 43, 2905–2910 CAS.
- A. Krauze, S. Germane, O. Eberlins, I. Sturms, V. Klusa and G. Duburs, Eur. J. Med. Chem., 1999, 34, 301–310 CrossRef CAS.
- G. S. Poindexter, M. A. Bruce, J. G. Breitenbucher, M. A. Higgins, S. Y. Sit, J. L. Romine, S. W. Martin, S. A. Ward, R. T. McGovern, W. Clarke, J. Russell and I. Antal-Zimanyi, Bioorg. Med. Chem., 2004, 12, 507–521 CrossRef CAS PubMed.
- V. Klusa, Drugs Future, 1995, 20, 135–138 CrossRef.
- R. G. Bretzel, C. C. Bollen, E. Maeser and K. F. Federlin, Am. J. Kidney Dis., 1993, 21, 53–64 CrossRef CAS PubMed.
- R. W. Chapman, G. Danko and M. I. Siegels, Pharmacology, 1984, 29, 282–291 CrossRef CAS PubMed.
- A. K. Ogawa, C. A. Willoughby, R. Bergeron, K. P. Ellsworth, W. M. Geissler, R. W. Myer, J. Yao, G. Harris and K. T. Chapman, Bioorg. Med. Chem. Lett., 2003, 13, 3405–3408 CrossRef CAS PubMed.
- Comprehensive Medicinal Chemistry, Ed. J. C. Emmet, Pergamon Press, Oxford, 1990, ch. 14.1, vol. 3 Search PubMed.
- S. Tasaka, H. Ohmori, N. Gomi, M. Iino, T. Machida, A. Kiue, S. Naito and M. Kuwano, Bioorg. Med. Chem. Lett., 2001, 11, 275–277 CrossRef CAS PubMed.
- A. Hantzsch, Chem. Ber., 1881, 14, 1637–1638 CrossRef.
- (a) M. Tajbakhsh, E. Alaee, H. Alinezhad, M. Khanian, F. Jahani, S. Khaksar, P. Rezaee and M. Tajbakhsh, Chin. J. Catal., 2012, 33, 1517–1522 CrossRef CAS , references there in; (b) M. Nasr-Esfahani, M. Montazerozohori and R. Raeatikia, Maejo Int. J. Sci. Technol., 2014, 8, 32–40 Search PubMed; (c) J. D. Akbari, S. D. Tala, M. F. Dhaduk and H. S. Joshi, ARKIVOC, 2008, 126–135 CAS.
- S. J. Tu, J. F. Zhou, X. Deng, P. J. Cai, H. Wang and J. C. Feng, Chin. J. Org. Chem., 2001, 21, 313 CAS.
- R. A. Mekheimer, A. A. Hameed and K. U. Sadek, Green Chem., 2008, 10, 592 RSC.
- J.-Y. He, H.-Z. Jia, Q.-G. Yao, S.-J. Liu, H.-K. Yue, H.-W. Yu and R.-S. Hu, Ultrason. Sonochem., 2015, 22, 144–148 CrossRef CAS PubMed.
- R. Gómez-Pliego, R. Osnaya, I. Zamora, B. Velasco-Bejarano, G. Arroyo, E. Ramírez-San Juan, J. Trujillo, F. Delgado and R. Miranda, J. Mex. Chem. Soc., 2007, 51, 181–184 Search PubMed.
- X. Y. Zhang, Y. Z. Li, X. S. Fan, G. R. Qu, X. Y. Hu and J. J. Wang, Chin. Chem. Lett., 2006, 17, 150 CAS.
- S. Kumar, P. Sharma, K. K. Kapoor and M. S. Hundal, Tetrahedron, 2008, 64, 536 CrossRef CAS.
- L. M. Wang, J. Sheng, L. Zhang, J. W. Han, Z. Y. Fan, H. Tian and C. T. Qian, Tetrahedron, 2005, 61, 1539 CrossRef CAS.
- J. H. Clark and C. N. Rhodes, Clean Synthesis using Porous Inorganic Solid Catalysts and Supported Reagents, Royal Society of Chemistry, UK, 1st edn, 2000 Search PubMed.
- B. M. Khadikar, V. G. Gaikar and A. A. Chitnavis, Tetrahedron Lett., 1995, 36, 8083 CrossRef.
- (a) G. B. Dharma Rao, B. N. Acharya, S. K. Verma and M. P. Kaushik, Tetrahedron Lett., 2011, 52, 809–812 CrossRef; (b) G. B. Dharma Rao, B. Anjaneyulu and M. P. Kaushik, Tetrahedron Lett., 2014, 55, 19–22 CrossRef CAS; (c) G. B. Dharma Rao, B. N. Acharya and M. P. Kaushik, Tetrahedron Lett., 2013, 54, 6644–6647 CrossRef CAS.
- (a) X. X. Yang, C. Cao, K. Hohn and L. Erickson, J. Catal., 2007, 252, 296 CrossRef CAS; (b) P. V. R. K. Ramacharyulu, J. Praveen Kumar, G. K. Prasad, G. Pranav Kumar and K. Dwivedi, Adv. Porous Mater., 2013, 1, 1 CrossRef; (c) S. T. Martin, C. L. Morrison and M. R. Hoffmann, J. Phys. Chem., 1994, 98, 13695 CrossRef CAS.
- Z. Zhang, C. C. Wang, R. Zakaria and J. Y. Wing, J. Phys. Chem. B, 1998, 102, 10871 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental information, spectral characterization data and copies of ESI-MS and GC-MS spectra of the products. See DOI: 10.1039/c6ra26664a |
| This journal is © The Royal Society of Chemistry 2017 |