Open Access Article
Open Access ArticleConstruction of a well-dispersed Ag/graphene-like g-C3N4 photocatalyst and enhanced visible light photocatalytic activity†
Hao Lia,
Yue Jinga,
Xinlong Maa,
Tongyao Liua,
Linfeng Yanga,
Bin Liua,
Shu Yinb,
Yongzhi Weia and
Yuhua Wang *a
*a
aDepartment of Materials Science, School of Physical Science and Technology, Lanzhou University, Lanzhou, 730000, China. E-mail: wyh@lzu.edu.cn; Fax: +86-931-8913554; Tel: +86-931-8912772
bInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Japan
First published on 27th January 2017
Abstract
In order to overcome the intrinsic drawback of pristine g-C3N4, we demonstrate a simple thermal oxidation exfoliation-photodeposition technique to fabricate a Ag/graphene-like g-C3N4 (Ag/G-g-C3N4) photocatalyst. It was revealed that the monodispersed Ag nanoparticles were well dispersed on the surface of graphene-like g-C3N4 (G-g-C3N4). The Ag/G-g-C3N4 displayed an enhanced photocatalytic activity for methylene blue degradation and the degradation rate was 10 times higher than that of pristine g-C3N4 under visible light irradiation. The enhancement of photocatalytic activity could be attributed to the surface plasmon resonance effect of Ag and large surface area (189.9 m2 g−1) of G-g-C3N4, which improve the visible light absorption ability and provide abundant reactive sites as well as promoting photogenerated electron–hole pair separation.
Introduction
In the course of the development of economy, some issues on environmental pollution emerged, which have restricted the sustainable development of modern human society.1,2 The semiconductor photocatalytic technique, using renewable solar energy to degrade organic pollutants to non-toxic products, has been of interest to many researchers.3,4 To date, many kinds of semiconductors have been explored for photocatalytic air purification and waste water treatment. They are mainly inorganic semiconductors, such as TiO2, ZnO and Ag3PO4.5–7 Nevertheless, materials with efficient activity and adequate stability for the degradation of pollutants under visible light irradiation are still unavailable, and their development remains a significant challenge.8In recent years, a novel metal-free polymer semiconductor, g-C3N4 with a band gap of about 2.7 eV, has showed promising performance for hydrogen production and pollutant degradation under visible light irradiation.9 Compared with their inorganic semiconductor counterparts, g-C3N4 is a sustainable and environmentally friendly organic semiconductor that consists of carbon and nitrogen, which are among the most abundant elements on our planet.10 It has been clearly demonstrated that the metal-free π-conjugated g-C3N4 photocatalyst possesses interesting electronic properties as well as high thermal and chemical stability, therefore making them valuable materials for photocatalytically driven hydrogen production and pollutants degradation.11
However, there are still some key problems that restricted the application of g-C3N4 in environmental remediation,12 such as, the low surface area and high recombination rate of photogenerated electron–hole pairs.3,13 To solve these problems, many strategies such as introducing heteroatoms14 or nitrogen vacancy,15 coupling with other semiconductors or noble metal nanoparticles (Au, Ag),16–18 controlling morphology19 have been employed, and these g-C3N4-based photocatalysts have shown encouraging activity improvement.
Among various methods, construction of metal–semiconductor composites with surface plasmon resonance effect is a promising method. Moreover, the morphology control of g-C3N4 will intensively increase the photocatalytic activity.
Recently, Niu et al. developed a simple method to prepare graphene-like g-C3N4 (G-g-C3N4) by direct thermal oxidation process of bulk g-C3N4 in air.20 The nanosheets possess not only a large surface area and small sheet thickness but also a short bulk diffusion length for reducing the recombination probability of photoexcited charge carriers. Whereas, the larger bandgap of resultant nanosheets hinders its visible light absorption capacity. Furthermore, Ge et al. prepared Ag/g-C3N4 composite photocatalysts via a heating method.21 Bu et al. reported that Ag nanoparticles modified onto the g-C3N4 surface through photo-assisted reduction and studied the degradation performance for Rhodamine B.22 The photocatalytic performance of g-C3N4 can be improved with the existence of Ag nanoparticles. However, g-C3N4 in the Ag/g-C3N4 composite shows graphitic structure (low surface area), and Ag nanoparticles cannot well dispersed on the surface of g-C3N4. In a word, the previous studies did not reflect the advantage of the synergic effect between the surface plasmon resonance effect and the controlling of morphology. So the enhancement of photocatalytic activity in these works was not obvious.
Herein, inspired by above analyses, we designed Ag/graphene-like g-C3N4 (Ag/G-g-C3N4) photocatalyst by a simple method. The synthesized sample possesses not only the surface plasmon resonance effect of Ag but also a large surface area, which shows an enhanced photocatalytic activity.
Experimental
Synthesis of the pristine g-C3N4 and G-g-C3N4
The pristine g-C3N4 powder was synthesized by one-step polymerization of 5 g melamine according to the literature.9 In a typical procedure, the precursor melamine was thermal treated in a tube furnace at 550 °C for 4 h with a heating rate of 2 °C min−1 under air atmosphere. The yellow-colored product was collected and ground into powder for further use. The G-g-C3N4 were prepared by thermal oxidation exfoliation of the pristine g-C3N4 obtained as above in static air as follows: 400 mg of the pristine g-C3N4 was placed in an open ceramic container and was heated at 500 °C for 2 h with a ramp rate of 5 °C min−1. A light yellow powder of G-g-C3N4 was finally obtained with a yield of about 7.9%.Synthesis of Ag/G-g-C3N4 nanocomposite
Fifty milligrams of as prepared G-g-C3N4 was dispersed in water (35 mL) by mild sonication for 5 min. One milligram AgNO3 was added to this suspension and stirred in the dark for 1 h. With continuous nitrogen sparging, the resulting mixture was stirred under UV light for 1 h and washed thoroughly with distilled water and finally dried in a vacuum oven at 60 °C for further use.Characterization
The phase purity of samples was analyzed by X-ray powder diffraction (XRD) using a Bruker D2 PHASER X-ray diffractometer with graphite monochromator using Cu Kα radiation (λ = 1.54184 Å) at room temperature. Fourier transform infrared spectra (FTIR) of the samples were recorded between 400 and 2000 cm−1 on a Nicolet NEXUS 670 FTIR spectrometer. Raman spectra were obtained using a Renishaw Raman system model 2000 spectrometer. X-ray photoelectron spectroscopy (XPS, PHI-5702, Physical Electronics) was performed using a monochromated Al Kα irradiation. The morphologies of different samples were observed by field emission scanning electron microscope (FESEM, Hitachi, S-4800), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM, FEI Tecnai F30, operated at 300 kV). Diffuse reflectance ultraviolet-visible (UV-vis) absorption spectra were measured using a PerkinElmer 950 spectrometer, while BaSO4 was used as a reference. The specific surface area of the samples was measured by the dynamic Brunauer–Emmett–Teller (BET) method, in which N2 was adsorbed at 77 K using a Micromeritics ASAP 2000 system. Photoluminescence emission (PL) spectra were carried out by a FLS-920T spectrometer with Xe 900 (450 W xenon arc lamp) as the light source.Evaluation of photocatalytic activity
The photocatalytic activity of the sample was evaluated by measuring the degradation ratio of methylene blue (MB). The initial concentration of MB solution was 10 mg L−1 and the used amount of photocatalyst was 0.1 g per 300 mL of MB solution. After the sample suspension was stirred for 40 min in the dark to realize the adsorption equilibrium, the photocatalytic reaction was started using a 350 W xenon lamp (with a cutoff filter of 420 nm) for the visible-light irradiation source. A series of a certain volume of suspension were withdrawn at selected times for analysis. After recovering the catalyst by centrifugation, the concentration of MB solution was analyzed by measuring the light absorption of the clear solution using a PerkinElmer 950 spectrometer. The light energy density is 40 mW cm−2 in the photocatalytic experiments.Results and discussion
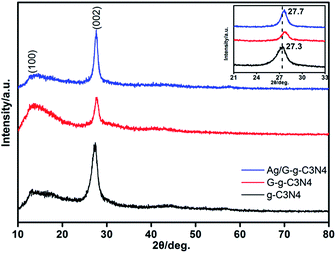
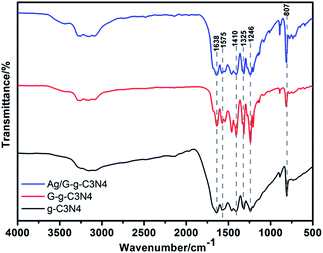
The crystal structure of as-prepared g-C3N4, G-g-C3N4 and Ag/G-g-C3N4 were analyzed by XRD patterns. As presented in Fig. 1, all of the peaks in the XRD patterns of the samples could be easily indexed to the hexagonal phase of g-C3N4 (JCPDS 87-1526). The peak at about 27° is due to the stacking of the conjugated aromatic system, which is indexed for graphitic materials as the (002) peak of g-C3N4. Another minor diffraction peak around 13° is assigned to the (100) plane associated with the in-plane repeated unites of g-C3N4.21 With respect to the pristine g-C3N4, the peaks originated from the periodic stacking of layers in the G-g-C3N4 and Ag/G-g-C3N4 are shifted from 27.3° to 27.7°, indicating a decreased gallery distance between the basic sheets in the G-g-C3N4 and Ag/G-g-C3N4. The reason is that the single layer in bulk g-C3N4 is potentially undulated, but it could be planarized by further heating, resulting in a denser stacking.23 No significant diffraction peaks of any other phases or impurities can be detected in the composite sample, which indicate that the introducing of Ag species does not affect the crystal structure of g-C3N4. What is more, no diffraction peaks of Ag species are detected, which may be explained by the small amounts of Ag species introducing (2 wt%) and high dispersion in the Ag/G-g-C3N4.The crystal structure of the g-C3N4, G-g-C3N4 and Ag/G-g-C3N4 can be further confirmed by FTIR spectroscopy. As shown in Fig. 2, for pristine g-C3N4 sample, the broad peak at the range of 3000–3500 cm−1 corresponds to N–H stretching vibration of uncondensed amino groups and O–H vibration from adsorbed water on the surface.24 The peak at 1638 cm−1 could be ascribed to C–N stretching and the four peaks at 1575 cm−1, 1410 cm−1, 1325 cm−1 and 1246 cm−1 can attribute to aromatic C–N stretching vibration.25 The absorption peak at 807 cm−1 accords with characteristic breathing mode of triazine units.26 All main characteristic vibration FTIR peaks related to g-C3N4 can be distinctly found in G-g-C3N4 and Ag/G-g-C3N4, suggesting that the overall structure of g-C3N4 keeps intact after thermal oxidation exfoliation and depositing the Ag nanoparticles, which is in accordance with XRD results.
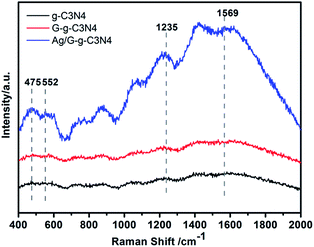
Raman spectroscopy is usually used to search the vibrational properties of carbon materials. Fig. 3 shows Raman spectra of g-C3N4, G-g-C3N4 and Ag/G-g-C3N4 samples. There are no significant Raman signals observed on pristine g-C3N4 and G-g-C3N4, but a clear Raman spectrum is detected on Ag/G-g-C3N4. The band at 1569 cm−1 corresponds to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of g-C3N4, which is also defined as graphitic G band, indicating the formation of graphite like structure, while the 552 cm−1 belongs to in-plane symmetrical stretching vibration of heptazine heterocycles.27 The peaks located at 475 cm−1, and 1235 cm−1 stem from the vibration modes of CN heterocycles in g-C3N4.28 In general, Raman intensity can be greatly enhanced by the electromagnetic interaction or coupling Ag nanoparticles with proper shape and size. Ling et al. reported that graphite-like materials also have the potential enhancing the Raman effects and found that the Raman enhancement factors can be distinguished through three classes that correspond to the symmetry of vibrations of the molecule.29 Therefore, the enhancement of Raman intensity could be attributed to the charge transfer between Ag and the g-C3N4 molecules, originating from the plasma and Raman enhancement effects of Ag nanoparticles and g-C3N4 molecules,30 respectively. The results convincingly demonstrate that an interaction exists between Ag and g-C3N4 molecules to facilitate charge transfer.
N stretching vibration of g-C3N4, which is also defined as graphitic G band, indicating the formation of graphite like structure, while the 552 cm−1 belongs to in-plane symmetrical stretching vibration of heptazine heterocycles.27 The peaks located at 475 cm−1, and 1235 cm−1 stem from the vibration modes of CN heterocycles in g-C3N4.28 In general, Raman intensity can be greatly enhanced by the electromagnetic interaction or coupling Ag nanoparticles with proper shape and size. Ling et al. reported that graphite-like materials also have the potential enhancing the Raman effects and found that the Raman enhancement factors can be distinguished through three classes that correspond to the symmetry of vibrations of the molecule.29 Therefore, the enhancement of Raman intensity could be attributed to the charge transfer between Ag and the g-C3N4 molecules, originating from the plasma and Raman enhancement effects of Ag nanoparticles and g-C3N4 molecules,30 respectively. The results convincingly demonstrate that an interaction exists between Ag and g-C3N4 molecules to facilitate charge transfer.
XPS was carried out to determine the chemical composition of the Ag/G-g-C3N4 sample and the valence states of various species present therein. Fig. 4a presents the survey spectrum of Ag/G-g-C3N4. The results indicated the presence of C, N, Ag and a small amount of O, which may be due to the surface absorption and oxidation. The C1s (Fig. 4b) spectrum of Ag/G-g-C3N4 could be fitted to four peaks at 284.6, 285.1, 288.0, and 288.5 eV, corresponding to the sp2 C–C bonds, C–NH2 species, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N coordination and the N–C–O groups, respectively.31 In addition, the sample exhibited N1s in Fig. 4c profiles with core levels at around 398.5, 399.1, and 401.1 eV, which could be attributed to sp2-hybridized nitrogen (C–N
C–N coordination and the N–C–O groups, respectively.31 In addition, the sample exhibited N1s in Fig. 4c profiles with core levels at around 398.5, 399.1, and 401.1 eV, which could be attributed to sp2-hybridized nitrogen (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), sp3-hybridized nitrogen (N–C3), and amino functional groups with a hydrogen atom (C–NH2), respectively.32 Fig. 4d shows the characteristic Ag3d peak that has a 6.0 eV splitting of the 3d doublet, which is corresponding to the metallic Ag0 species.21 This result confirms the presence of metallic Ag in the Ag/G-g-C3N4.
C), sp3-hybridized nitrogen (N–C3), and amino functional groups with a hydrogen atom (C–NH2), respectively.32 Fig. 4d shows the characteristic Ag3d peak that has a 6.0 eV splitting of the 3d doublet, which is corresponding to the metallic Ag0 species.21 This result confirms the presence of metallic Ag in the Ag/G-g-C3N4.
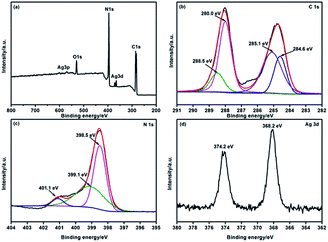 | ||
| Fig. 4 XPS survey spectrum (a), high-resolution C1s spectrum (b), N1s spectrum (c) and Ag3d spectrum (d) of Ag/G-g-C3N4. | ||
The morphology and micro structure of prepared samples were investigated with SEM and TEM as shown in Fig. 5. Compared to pristine g-C3N4 consisting of solid agglomerates with a size of several micrometers (Fig. 5a), the representative G-g-C3N4 appears as loose and soft agglomerates with a size of tens of micrometers (Fig. 5b). After photodeposition of Ag nanoparticles, Ag/G-g-C3N4 keeps the morphology of G-g-C3N4, and exhibits an exfoliated and wrinkled nanosheets texture characteristic (Fig. 5c). The high-magnification SEM image in Fig. 5d also confirms that the appearance of Ag/G-g-C3N4 is quite similar to that of graphene and Ag nanoparticles well dispersed on nanosheets. From the TEM image of Ag/G-g-C3N4 (Fig. 5e and f), Ag nanoparticles (black colored dots) with sizes about 10 nm can be found clearly, which are uniformly dispersed on the surface of the thin G-g-C3N4 nanosheets. Furthermore, we can observe that a very close connection existed between Ag and g-C3N4 to form the heterostructure, guaranteeing the charge transfer in the space smoothly. The inset in Fig. 5f is the HRTEM image of Ag/G-g-C3N4, which indicates that the lattice spacing is 0.236 nm, corresponding to the (111) lattice planes of metal Ag.25
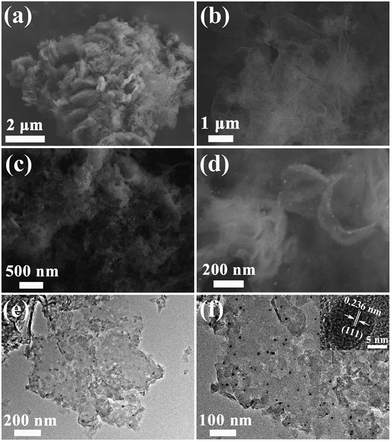 | ||
| Fig. 5 SEM images of pristine g-C3N4 (a) and G-g-C3N4 (b). Low-(c) and high-magnification (d) SEM images of Ag/G-g-C3N4. Low-(e) and high magnification (e) TEM images of Ag/G-g-C3N4. | ||
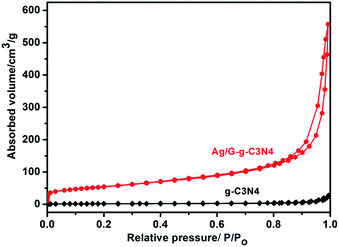
The nitrogen adsorption–desorption isotherms in Fig. 6 show type IV isotherms with distinct H3 hysteresis loops, which use to calculated the BET specific surface areas. The BET specific surface areas of g-C3N4, and Ag/G-g-C3N4 are 5.55 and 189.9 m2 g−1, respectively. The BET specific surface area was 34 times higher than that of pristine g-C3N4, which could provide abundant reactive sites and short bulk diffusion length for reducing the recombination probability of photoexcited charge carriers.20
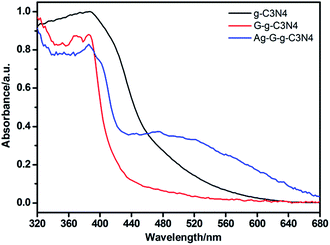
Optical absorption of the as-prepared g-C3N4, G-g-C3N4 and Ag/G-g-C3N4 were investigated using an UV-vis spectrometer. As shown in Fig. 7, an obvious blue shift of the intrinsic absorption edge in the G-g-C3N4 was observed with respect to the pristine g-C3N4. The derived band gaps are 2.95 and 2.54 eV for the G-g-C3N4 and the pristine g-C3N4. The reason for this larger bandgap is attributed to the well-known quantum confinement effect by shifting the conduction and valence band edges in opposite directions.33 The larger bandgap of resultant G-g-C3N4 hinders its visible light absorption. However, compared with G-g-C3N4, when G-g-C3N4 coupled Ag nanoparticles, the absorption intensity in the visible light region is significantly improved, and the absorption edge red shift about 20 nm (0.14 eV), while the powder colors shift from white to grey with Ag loading. This phenomenon could be attributed to the surface plasmon resonance effect of Ag nanoparticles.34
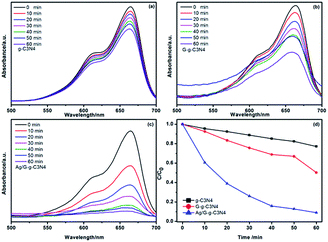
To investigate the photocatalytic activity of the prepared samples, MB was chosen as are presentative hazardous dye to evaluate the photocatalytic performance, which showed a major absorption band at 664 nm. A blank test (without any photocatalyst) exhibited almost no self-degradation of MB. As show in Fig. 8a–c, the absorbance of MB decreases with increase of light irradiation time, which indicates that all prepared samples have the photocatalytic degradation ability. Compared with g-C3N4 and G-g-C3N4, the Ag/G-g-C3N4 exhibits higher photocatalytic activity, which could completely degrade MB dye in 60 min under visible light irradiation. As shown in Fig. 8c, no absorbance peak is observed after visible light irradiation of 60 min, which indirectly proves that the MB molecules are destroyed or decomposed into small molecules completely. The degradation of dyes can be ascribed to a pseudo-first order reaction with a Langmuir–Hinshelwood model when C0 is small: ln(C0/C) = kat, where ka is the apparent first-order rate constant.35 By calculated correspondingly degradation dynamics curves (Fig. 8d), the apparent rate constants (ka, min−1) are 0.004 min−1 for pristine g-C3N4 and 0.04 min−1 for Ag/G-g-C3N4 nanocomposite. It is clearly shows that Ag/G-g-C3N4 displayed an enhanced photocatalytic activity for MB degradation and the degradation rate was 10 times higher than that of pristine g-C3N4 under visible light irradiation. The enhancement of photocatalytic activity could be attributed to the surface plasmon resonance effect of Ag and large surface area of G-g-C3N4. TOC during the photocatalytic reactions was monitored to investigate the mineralization of MB. After visible light irradiation for 60 min (Fig. S1†), about 85% of total organic carbon (TOC) are removed, indicating that MB has not only been decolorized but also efficiently mineralized under visible light irradiation for Ag–G-g-C3N4.
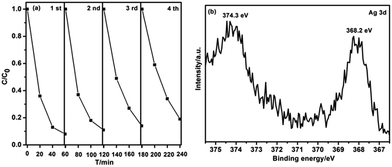
From the viewpoint of pratical applications, the photocatalytic stability of the sample was evaluated by measuring the degradation ratio of MB in the same condition. As shown in Fig. 9a, the as-prepared Ag–G-g-C3N4 composite shows a good catalytic stability, maintaining a similar level of reactivity after four cycles. The slight decrease should originate from the inescapable loss of catalyst during the recycling process. The composition of the recyclable composite was also characterized by XPS. Fig. 9b shows the characteristic Ag3d peak that has a 6.0 eV splitting of the 3d doublet, which is corresponding to the metallic Ag0 species. This result confirms that metallic Ag is stable after four cycles.
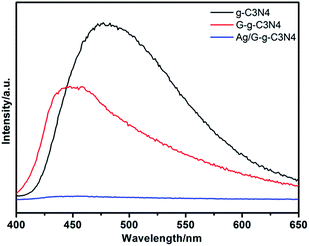
Photoluminescence analysis is useful in revealing the efficiency of carrier transfer and separation, and investigating the lifetime of charge carriers. Fig. 10 shows the spectra of g-C3N4, G-g-C3N4 and Ag/G-g-C3N4 excited by 365 nm. From Fig. 9, it can be observed that there is a significant decrease in the PL intensity of Ag/G-g-C3N4 composite in comparison with that of the pristine g-C3N4. A weaker intensity of the peak means a lower recombination probability of photogenerated charge carriers.17 Ag nanoparticles dispersed on the surface of G-g-C3N4, combined with the intimate interface between the two components, could effectively inhibit the recombination of photoinduced carriers, which is helpful for the separation of photogenerated electron–hole pairs in g-C3N4. Meanwhile, the Ag/G-g-C3N4 with enhanced visible light absorption should absorb more solar energy, the improved photogenerated carriers separation efficiency indicates that more charge carriers could be used by Ag/G-g-C3N4 composites. Therefore, the Ag/G-g-C3N4 photocatalyst possesses high photocatalytic performance. Fig. S2† show ESR spectra measured as the effect of light irradiation on the Ag–G-g-C3N4 composite. Just the signal of DMPO–·O2− are clearly observed when Ag–G-g-C3N4 composite are irradiated under light irradiation. Therefore, ·O2− radicals are the main active species which play important roles during the photo-degradation process. These are also confirmed in the photodegradation of phenol (Fig. S3†).
Conclusions
In summary, Ag/G-g-C3N4 photocatalyst was successfully synthesized by a simple thermal oxidation exfoliation-photo deposition technique. The monodispersed Ag nanoparticles were well dispersed on the surface of G-g-C3N4. The obtained Ag/G-g-C3N4 shows an enhanced photocatalytic activity than that of G-g-C3N4 and pristine g-C3N4 under visible light irradiation. Ag/G-g-C3N4 photocatalyst has the improved visible light absorption ability, abundant reactive sites as well as promoted photogenerated electron–hole pairs separation ability, which were derive from the synergic effect between the surface plasmon resonance effect of Ag nanoparticles and large surface area of G-g-C3N4. This work provides a promising approach to develop visible-light-response photocatalyst applied to pollutants degradation.Acknowledgements
This research was supported by the International Sci. & Tech. Cooperation Foundation of Gansu Provincial, China (Grant No. 1504WKCA088 and 1304WCGA177) and the Fundamental Research Funds for the Central Universities (No. lzujbky-2016-131).Notes and references
- K. Li, Z. Zeng, L. Yan, S. Luo, X. Luo, M. Huo and Y. Guo, Appl. Catal., B, 2015, 165, 428–437 CrossRef CAS.
- Q. Li, N. Zhang, Y. Yang, G. Wang and D. H. Ng, Langmuir, 2014, 30, 8965–8972 CrossRef CAS PubMed.
- M. Tahir, C. Cao, F. K. Butt, S. Butt, F. Idrees, Z. Ali, I. Aslam, M. Tanveer, A. Mahmood and N. Mahmood, CrystEngComm, 2014, 16, 1825 RSC.
- Z. Zhu, Z. Lu, D. Wang, X. Tang, Y. Yan, W. Shi, Y. Wang, N. Gao, X. Yao and H. Dong, Appl. Catal., B, 2016, 182, 115–122 CrossRef CAS.
- B. Liu, Y. Huang, Y. Wen, L. Du, W. Zeng, Y. Shi, F. Zhang, G. Zhu, X. Xu and Y. Wang, J. Mater. Chem., 2012, 22, 7484 RSC.
- C. Han, Z. Chen, N. Zhang, J. C. Colmenares and Y.-J. Xu, Adv. Funct. Mater., 2015, 25, 221–229 CrossRef CAS.
- P. Dong, Y. Wang, B. Cao, S. Xin, L. Guo, J. Zhang and F. Li, Appl. Catal., B, 2013, 132–133, 45–53 CrossRef CAS.
- J. Chen, S. Shen, P. Guo, M. Wang, J. Su, D. Zhao and L. Guo, J. Mater. Res., 2013, 29, 64–70 CrossRef.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- S. Samanta, S. Martha and K. Parida, ChemCatChem, 2014, 6, 1453–1462 CAS.
- J. Zhang, G. Zhang, X. Chen, S. Lin, L. Mohlmann, G. Dolega, G. Lipner, M. Antonietti, S. Blechert and X. Wang, Angew. Chem., Int. Ed. Engl., 2012, 51, 3183–3187 CrossRef CAS PubMed.
- X. Bai, S. Yan, J. Wang, L. Wang, W. Jiang, S. Wu, C. Sun and Y. Zhu, J. Mater. Chem. A, 2014, 2, 17521–17529 CAS.
- X. Wang, S. Wang, W. Hu, J. Cai, L. Zhang, L. Dong, L. Zhao and Y. He, Mater. Lett., 2014, 115, 53–56 CrossRef CAS.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed. Engl., 2010, 49, 3356–3359 CrossRef CAS PubMed.
- P. Niu, G. Liu and H.-M. Cheng, J. Phys. Chem. C, 2012, 116, 11013–11018 CAS.
- S. C. Yan, S. B. Lv, Z. S. Li and Z. G. Zou, Dalton Trans., 2010, 39, 1488–1491 RSC.
- J. Xue, S. Ma, Y. Zhou, Z. Zhang and M. He, ACS Appl. Mater. Interfaces, 2015, 7, 9630–9637 CAS.
- Y. Sun, T. Xiong, Z. Ni, J. Liu, F. Dong, W. Zhang and W.-K. Ho, Appl. Surf. Sci., 2015, 358, 356–362 CrossRef CAS.
- J. Sun, J. Zhang, M. Zhang, M. Antonietti, X. Fu and X. Wang, Nat. Commun., 2012, 1139 CrossRef.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- L. Ge, C. Han, J. Liu and Y. Li, Appl. Catal., A, 2011, 409–410, 215–222 CrossRef CAS.
- Y. Bu, Z. Chen and W. Li, Appl. Catal., B, 2014, 144, 622–630 CrossRef CAS.
- M. Groenewolt and M. Antonietti, Adv. Mater., 2005, 17, 1789–1792 CrossRef CAS.
- Z. Chen, P. Sun, B. Fan, Q. Liu, Z. Zhang and X. Fang, Appl. Catal., B, 2015, 170–171, 10–16 CrossRef CAS.
- J. Qin, J. Huo, P. Zhang, J. Zeng, T. Wang and H. Zeng, Nanoscale, 2016, 8, 2249–2259 RSC.
- W. Bing, Z. Chen, H. Sun, P. Shi, N. Gao, J. Ren and X. Qu, Nano Res., 2015, 8, 1648–1658 CrossRef CAS.
- J. Jiang, L. Zhu, J. Zou, L. Ou-yang, A. Zheng and H. Tang, Carbon, 2015, 87, 193–205 CrossRef CAS.
- J. Jiang, L. Ou-yang, L. Zhu, A. Zheng, J. Zou, X. Yi and H. Tang, Carbon, 2014, 80, 213–221 CrossRef CAS.
- X. Ling, L. Xie, Y. Fang, H. Xu, H. Zhang, J. Kong, M. S. Dresselhaus, J. Zhang and Z. Liu, Nano Lett., 2010, 10, 553–561 CrossRef CAS PubMed.
- X. Bai, R. Zong, C. Li, D. Liu, Y. Liu and Y. Zhu, Appl. Catal., B, 2014, 147, 82–91 CrossRef CAS.
- B. Long, J. Lin and X. Wang, J. Mater. Chem. A, 2014, 2, 2942 CAS.
- J. Li, B. Shen, Z. Hong, B. Lin, B. Gao and Y. Chen, Chem. Commun., 2012, 48, 12017–12019 RSC.
- A. P. Alivisatos, Science, 1996, 271, 933 CAS.
- F. Fina, H. Menard and J. T. Irvine, Phys. Chem. Chem. Phys., 2015, 17, 13929–13936 RSC.
- P. Dong, B. Liu, Y. Wang, H. Pei and S. Yin, J. Mater. Res., 2011, 25, 2392–2400 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26498k |
| This journal is © The Royal Society of Chemistry 2017 |