Open Access Article
Open Access ArticleEfficient conversion of fructose into 5-hydroxymethylfurfural over WO3/reduced graphene oxide catalysts†
Huatao Han,
Hongyan Zhao,
Yang Liu,
Zhuofei Li,
Jinyi Song,
Wenyi Chu * and
Zhizhong Sun*
* and
Zhizhong Sun*
School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, P. R. China. E-mail: wenyichu@hlju.edu.cn; sunzz@hlju.edu.cn
First published on 13th January 2017
Abstract
A sustainable and efficient catalyst for converting carbohydrates to a renewable platform chemical 5-hydroxymethylfurfural (HMF) is the goal in the study of biomass recycling. Reduced graphene oxide-supported tungsten trioxide (WO3/RGO) as an acidic catalyst was synthesized through a one-step hydrothermal method, characterized via TEM, XPS, XRD and Raman spectroscopy and applied to the conversion of fructose to HMF. The WO3/RGO catalyst showed a highly efficient catalytic activity, and the yield of HMF could reach up to 84.2% with complete conversion of fructose. The catalyst could be reused five times with a slight decrease in activity. Further study indicated that WO3/RGO could also catalyze the conversion of cellulose, glucose and sucrose to HMF.
Introduction
The burning of fossil fuels does great harm to the ecological environment, and the depletion of non-renewable energy makes humans face a great challenge. Biomass as the sole renewable carbon source can guarantee the sustainable use of energy.1,2 Therefore, the conversion of biomass into fuels, chemicals and materials has received broad attention.3–6 5-Hydroxymethylfurfural (HMF), produced from the acid-catalyzed dehydration of hexose, is an important platform chemical between carbohydrate biomass and the oil industry.7–10Although formation of HMF from abundant renewable carbohydrates, such as glucose and fructose, has been achieved, scientists are still aiming to research and develop a type of green and efficient catalyst. A series of acid catalysts, such as various mineral acids,11,12 metal Lewis acids,13–15 organic acids,16 and acidic ionic liquids,7,17 were synthesized and used in HMF production from fructose, glucose and other sugars. Supported transition metal oxide composites, the same as solid acid catalysts used in HMF formation, form sugars due to their Brønsted or Lewis acid sites.18–20 For several acid-catalyzed conversions of biomass, the acidity of the supported transition metal oxide plays a crucial role in the reaction. Nevertheless, the structural instability under severe conditions and lower dispersability of a traditional solvent in a carrier material remains a concern.
Graphene, as one of the most promising carbon nanomaterials, provides a template to anchor active species for catalysis due to its unique two-dimensional structure, strong surface area, and superior mechanical and electrical transmission performance.21–23 Therefore, graphene-based materials have become the focal points in the growing field of carbocatalysis in recent years.24–26 Our previous study proved that Pd@PdO–NDG27 and Cu NPs@RGO28 could act as an effective catalyst in organic reactions. In addition, tungsten oxide (WO3), as an acidic catalyst, had been used for biomass conversion. Prasenjit Bhaumik et al.29 reported silica-supported WO3 as a solid acid catalyst in the synthesis of furfural, directly from lignocellulosic biomass. Yue Liu et al.30 reported that WO3 catalyzed the conversion of cellulose into propylene glycol and ethylene glycol. Therefore, WO3/RGO, which was prepared by tungsten trioxide loaded onto the surface of reduced graphene oxide, could be used as an acidic catalyst with considerable value and research prospects for conversion of biomass.
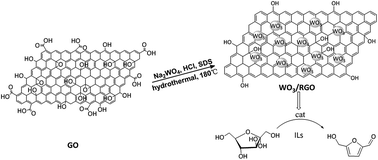
In Scheme 1, reduced graphene oxide-supported tungsten trioxide (WO3/RGO) was designed and synthesized through a simple one-step hydrothermal method. WO3/RGO, as an acid catalyst, was studied for conversion of sugars to HMF due to its acid-catalytic performance. A high HMF yield (up to 84.2%) was obtained using WO3/RGO catalyst in ionic liquids.
Experimental
Synthesis of WO3/RGO composites
Graphene oxide (GO) was synthesized by the modified Hummer's31 method. Exfoliate graphite, as the starting material, was oxidized by potassium permanganate and concentrated sulfuric acid. WO3/RGO composites were synthesized through a one-step hydrothermal method. GO was dispersed into deionized water and then sonicated for 0.5 hours to create a homogeneous dispersion (1 mg mL−1). Then, 0.3 g Na2WO4·2H2O, 0.05 g sodium dodecyl sulfate (SDS) and 0.05 g NaCl were dissolved in 20 mL GO dispersion and kept stirring for 1 hour. The pH of the dispersion was adjusted to about 1.5 using HCl solution. After stirring for 3 hours, the suspension was transferred to a 25 mL Teflon-lined stainless steel autoclave and heated to 180 °C. The suspension was maintained for 24 hours and naturally cooled to room temperature. The final product was washed with deionized water and ethanol, dried at 50 °C for 12 hours and heated at 350 °C in a muffle furnace under nitrogen atmosphere for 6 hours with a heating rate of 15 °C min−1 under nitrogen atmosphere. The catalyst was obtained with an 8.7% RGO mass content in the WO3/RGO.Changing the amount of GO resulted in 4.5% and 12.5% of RGO obtained in the WO3/RGO. Pure WO3 was obtained under the same condition without adding GO in the process.
Catalytic reaction procedure
In a typical experiment for the synthesis of HMF from fructose, 1 mmol fructose was added in 2.0 g of 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) in a small reaction vessel, and 10 mg catalyst of WO3/RGO was subsequently added. The reaction mixture was stirred and kept 120 °C. After the reaction, the mixture was transferred into 5 mL distilled water. The catalyst was separated via filtration and recycled; the filtrate was extracted with ethyl acetate (5 mL × 3). The organic phase was collected, dried with anhydrous sodium sulphate, and the solvent was evaporated by rotary evaporator to obtain pure HMF. The production and aqueous phase were analyzed via high-pressure liquid chromatography (HPLC).9,10 The yield of HMF and fructose conversion rate were calculated from a calibration curve.HMF was identified via 1H NMR spectroscopy. 1H NMR (400 MHz, CDCl3): δH (400 MHz, CDCl3) 9.56 (1H, s), 7.23 (1H, d, J 3.5), 6.52 (1H, d, J 3.5), 4.71 (2H, s).
Results and discussion
Characterization of WO3/RGO catalyst
The morphology and microstructure of the obtained WO3/RGO were characterized via transmission electron microscopy (TEM) and are shown in Fig. 1. Fig. 1(a) shows the TEM image of RGO to be a 2D sheet structure with crinkles. As shown in Fig. 1(b), the WO3 nanoparticles, dispersed on graphene, had a 12–15 nm average diameter. The HRTEM image in Fig. 1(c) revealed lattice fringes with a 0.342 and 0.314 nm uniform interlayer distance corresponding to the (001) and (200) WO3 lattice planes.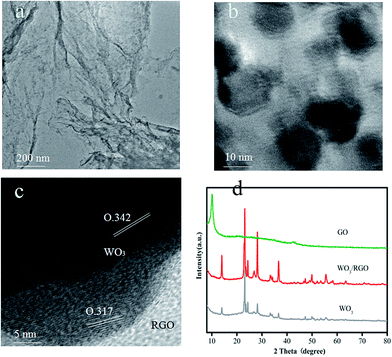 | ||
| Fig. 1 TEM images of (a) RGO and (b) WO3/RGO, (c) HRTEM images of WO3/RGO, (d) XRD patterns of the GO, WO3/RGO and pure WO3 samples. | ||
X-ray diffraction (XRD) was used to characterize the sample structure. The XRD of GO, WO3/RGO and pure WO3 are shown in Fig. 1(d). The diffraction peaks of WO3/RGO and pure WO3 correspond well to a standard XRD spectrum (JCPDS: 33-1387), which proved their excellent crystalline form. The disappearance of the strong diffraction peak around 10.2° of graphene oxide in the diffraction peak of WO3/RGO proved that graphene oxide was reduced to graphene. There is no evident diffraction peak of graphene in the WO3/RGO diffraction pattern because the weak graphene diffraction peaks may be masked by the WO3 peak.
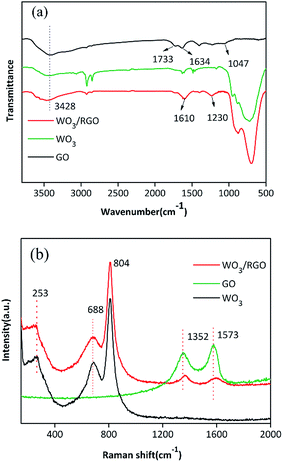
The FTIR spectra of WO3 and WO3/RGO catalyst are shown in Fig. 2(a). The prominent broad and strong absorption band at a high frequency of 3428 cm−1 is ascribed to the O–H stretching vibration. The characteristic absorption bands of GO were observed at 1047 cm−1 (C–OH), 1634 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 1733 cm−1 (C
C) and 1733 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The broad absorption band of the WO3/RGO composite at low frequencies is ascribed to the W–O–W bond vibration. Moreover, as demonstrated in the WO3/RGO FTIR spectrum, the GO peaks corresponding to C
O). The broad absorption band of the WO3/RGO composite at low frequencies is ascribed to the W–O–W bond vibration. Moreover, as demonstrated in the WO3/RGO FTIR spectrum, the GO peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–OH vibrations at 1733 cm−1 and 1047 cm−1 were not observed. The absorption band at 1610 cm−1 might be attributed to the skeletal vibration of the RGO sheets. These results indicated that the original GO was reduced to RGO in the process of preparing WO3/RGO.
O and C–OH vibrations at 1733 cm−1 and 1047 cm−1 were not observed. The absorption band at 1610 cm−1 might be attributed to the skeletal vibration of the RGO sheets. These results indicated that the original GO was reduced to RGO in the process of preparing WO3/RGO.
The Raman spectra of WO3, GO and WO3/RGO are shown in Fig. 2(b). The D and G peaks of GO and WO3/RGO appear at 1352 cm−1 and 1573 cm−1. In the Raman spectrum of WO3/RGO, sharp peaks belonging to WO3 could be observed. Raman bands of 804 cm−1 and 688 cm−1 correspond to the O–W–O stretching modes, which are the main characteristic peaks of WO3 crystallites. The band located at 253 cm−1 is related to the W–O–W bending mode for WO3. Compared with that of pure WO3, the peak at 688 cm−1 was slightly lower, probably due to the formation of C–O–W bonds between the graphene and WO3 nanoparticles.32 This chemical bond indicated that WO3 was chemically bonded to the surface of graphene layer via C–O–W bonds rather than physically interacting with graphene.
X-ray photoelectron spectroscopy (XPS) was used to investigate the surface element composition of catalysts and the corresponding valence state. As shown in Fig. 3(a), the C 1s XPS spectrum of GO was defined by four peaks, corresponding to C atoms in several representative functional groups of C–C (284.6 eV), C–O (hydroxyl and epoxy groups, 286.6 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.7 eV) and the carbonyl C at 288.9 eV. Fig. 3(b) shows the full spectrum of WO3/RGO catalyst. It could be clearly observed that the sample consisted of W, O, and C elements without impurities. The peaks of W 4f, O 1s, C 1s were very close to the XPS results provided by the literature reported for WO3.33 According to the C 1s XPS spectrum of WO3/RGO in Fig. 3(c), the carbonyl C at 288.9 eV had disappeared, the C
O (287.7 eV) and the carbonyl C at 288.9 eV. Fig. 3(b) shows the full spectrum of WO3/RGO catalyst. It could be clearly observed that the sample consisted of W, O, and C elements without impurities. The peaks of W 4f, O 1s, C 1s were very close to the XPS results provided by the literature reported for WO3.33 According to the C 1s XPS spectrum of WO3/RGO in Fig. 3(c), the carbonyl C at 288.9 eV had disappeared, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288 eV) and the C–O (286.1 eV) signal decreased markedly in intensity compared with C 1s of GO. It proved that GO had been reduced to graphene accompanied by elimination of the oxygen-containing group. The spectrum W 4f of WO3/RGO in Fig. 3(d) shows two peaks at 35.1 eV and 38.2 eV that could be ascribed to W 4f7/2 and W 4f5/2, respectively. These results completely correspond to the valence of W6+. However, the binding energy value of W 4f7/2 located at 35.1 eV was slightly lower than that of pure WO3;33,34 such a shift might be attributed to the interaction between WO3 and graphene (Scheme 2).
O (288 eV) and the C–O (286.1 eV) signal decreased markedly in intensity compared with C 1s of GO. It proved that GO had been reduced to graphene accompanied by elimination of the oxygen-containing group. The spectrum W 4f of WO3/RGO in Fig. 3(d) shows two peaks at 35.1 eV and 38.2 eV that could be ascribed to W 4f7/2 and W 4f5/2, respectively. These results completely correspond to the valence of W6+. However, the binding energy value of W 4f7/2 located at 35.1 eV was slightly lower than that of pure WO3;33,34 such a shift might be attributed to the interaction between WO3 and graphene (Scheme 2).
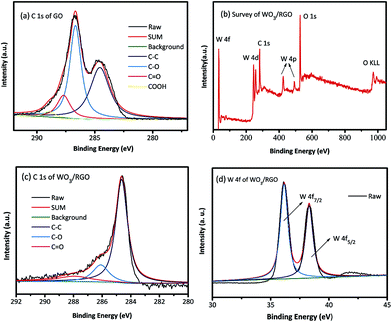 | ||
| Fig. 3 (a) C 1s XPS of GO, (b) the full spectra of WO3/RGO, (c) C 1s of WO3/RGO, (d) W 4f of WO3/RGO. | ||
Catalytic reactions
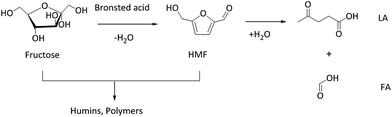
To evaluate the catalytic performance of WO3/RGO, various catalysts were used under identical reaction conditions, and the results are summarized in Table 1. Fructose was converted to HMF in experimental conditions including 1 mmol fructose, 10 mg catalyst, 2.0 g [BMIM]Cl and 2 hours reaction time. According to comparison (Table 1, entries 1–3), 8.7% RGO content in the catalyst obtained the highest fructose conversion (100%) and HMF yield (82.9%). Humins and polymers were be formed during the reaction, and the generated HMF was further hydrolyzed to levulinic acid (LA) and formic acid (FA),9,10 which would lead to a decrease in the HMF selectivity (Scheme 1). HMF was hydrolysed into equimolar amounts of LA and FA. The yield of FA was lower than LA due to the decomposition of FA during the reaction.6 The reaction without catalyst gave a much lower HMF yield (Table 1, entry 4). The yield of HMF with GO as the catalyst was 67.4% (Table 1, entry 5). GO possessed catalytic activity because it contains large amounts of carboxylic acid functional groups. When used WO3 with no carrier materials, the HMF yield was 62.5% (Table 1, entry 6). WO3/RGO exhibited excellent catalytic performance because the presence of RGO can increase the contact area between the catalyst and the substrate. Moreover, large amounts of hydroxyl groups on the surface of RGO promoted the catalyst dispersion in the solvent. H3PW12O40 and H2SO4, with strong Brønsted acid sites, could participate in homogeneous catalysis. With H2SO4 as the catalyst, a lower yield of HMF (41.8%) was obtained with more formation of LA (9.4%) and FA (5.2%) (Table 1, entry 7). With H3PW12O40 as the catalyst, a relatively higher HMF yield of up to 99.0% was obtained (Table 1, entry 8). Unfortunately, it brought great trouble in the separation of production and recycling of catalyst. Compared with WO3/RGO, WO3/ZrO2 as a catalyst gave a higher HMP yield (94.0%) (Table 1, entry 9). With SBA-15 and ZSM-5, the typical solid-acid catalysts, the yields of HMF were 41.9% and 75.0%, respectively (Table 1, entries 10 and 11). Glucose, sucrose and cellulose could also be converted to HMF (Table 1, entries 12–14) and the yields were 36.4%, 51.2% and 18.8%, respectively. With cellulose as a substrate, increasing the temperature increased the cellulose conversion, but the selectivity of HMF decreased greatly. Cellulose conversions were determined by measuring the difference in the weight of cellulose before and after the reaction.30 The molar quantities of cellulose were calculated based on the molecular weight of the C6H10O5 unit.| Entry | Substrate | Catalyst | T (°C) | Conversionb (%) | Yieldb (%) | ||
|---|---|---|---|---|---|---|---|
| HMF[Ref] | LA | FA | |||||
a Reaction conditions: substrate (1 mmol), catalyst 10 mg, [BMIM]Cl 2.0 g, 2 hours.b Determined by HPLC analysis.c Sucrose 0.5 mmol, catalyst 10 mg, [BMIM]Cl 2.0 g, 2 hours.d Cellulose 1.5 g, catalyst 100 mg, [BMIM]Cl/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (10 mL), closed system 8 hours, the conversion (wt%) was calculated by the weight of cellulose before and after reaction. 4 (10 mL), closed system 8 hours, the conversion (wt%) was calculated by the weight of cellulose before and after reaction. |
|||||||
| 1 | Fructose | WO3/RGO (4.5%) | 120 | 87.2 | 67.8 | 2.1 | 0.8 |
| 2 | Fructose | WO3/RGO (8.7%) | 120 | 100 | 84.2 | 5.2 | 3.1 |
| 3 | Fructose | WO3/RGO (12.5%) | 120 | 94.2 | 74.3 | 3.8 | 1.6 |
| 4 | Fructose | — | 120 | 57.0 | 22.6 | 0.5 | <0.1 |
| 5 | Fructose | GO | 120 | 86.6 | 67.4 | 3.4 | 1.3 |
| 6 | Fructose | WO3 | 120 | 88.0 | 62.5 | 1.9 | 0.6 |
| 7 | Fructose | H2SO4 | 120 | 69.2 | 41.8 | 9.4 | 5.2 |
| 8 | Fructose | H3PW12O40 | 80 | 100 | 99.0[12] | — | — |
| 9 | Fructose | WO3/ZrO2 | 120 | 100 | 94.0[14] | 2 | |
| 10 | Fructose | SBA-15 | 140 | 89.2 | 41.9[18] | — | — |
| 11 | Fructose | ZSM-5 | 170 | 89.0 | 75.0[11] | — | — |
| 12 | Glucose | WO3/RGO | 140 | 58.6 | 36.4 | 0.8 | <0.1 |
| 13 | Sucrosec | WO3/RGO | 140 | 70.2 | 51.2 | 1.1 | <0.1 |
| 14 | Cellulosed | WO3/RGO | 180 | 44.6 | 18.8 | 8.7 | 4.9 |
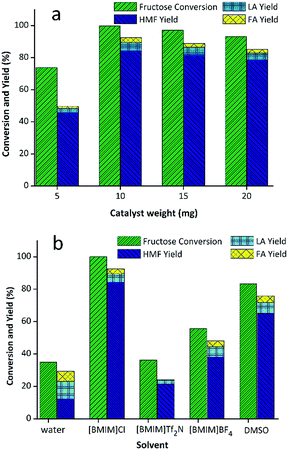
The abovementioned results unequivocally demonstrated that WO3/RGO was an outstanding acidic catalyst. In order to further investigate the influence of the catalyst in the reaction, WO3/RGO was used as a catalyst to optimize the reaction conditions including catalyst weight, solvents and temperature. First, different loadings of catalyst including 20 mg, 15 mg, 10 mg and 5 mg were screened (Fig. 4(a)). The use of 5 mg WO3/RGO catalyst obtained HMF in only 44.3% yield with 72.5% fructose conversion. The catalyst loading was 10 mg to give the highest yield of HMF up to 84.2% with complete conversion of fructose. The yields would reduce slightly with increased catalyst weight. The solvent, as an important influential factor for the catalytic reaction, was also screened. As shown in the Fig. 4(b), it was clear that the highest yield of the reaction that could be achieved was 84.2% with a complete conversion rate of fructose in [BMIM]Cl. In water, the obtained yield of HMF (16.4%) was low, whereas the fructose conversion (38.2%) and the yield of LA was much higher than others. Only 21% and 38% HMF yields could be obtained using [BMIM]Tf2N, [BMIM]BF4 as solvents, respectively. As is known, DMSO is a common solvent that could be used in HMF production, but with this solvent only a 64.8% yield was obtained.
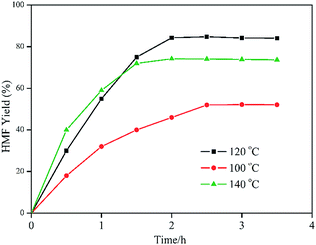
Fig. 5 exhibits the effects of reaction temperature and reaction time on the fructose to HMF conversion. Following the reaction progresses, HMF was constantly generated. When the reaction was performed at 100 °C, the rate of HMF formation was rather slow. Even with a further prolonged reaction time to 2.5 hours, the yield was only 52.2%. The maximum HMF yield of 84.2% with a full fructose conversion was obtained at 120 °C after a 2 hour reaction time. When the reaction temperature was raised to 140 °C, the reaction rate increased. While the yield of HMF decreased to 74.1% because of the further hydrolysis of HMF at an excessively high temperature.
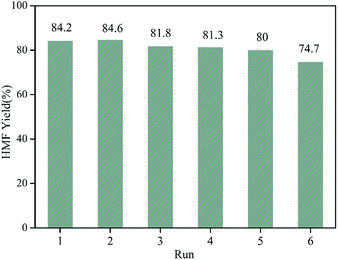
Apart from efficient catalytic activity, the recoverability and stability are significant criteria for solid catalysts. In order to attest the reusability of WO3/RGO catalyst, a six-cycle experiment was performed, and the results are shown in Fig. 6. The catalyst retained considerable activity after five cycles. Five reuse cycles showed that the yield of HMF was always over 80%, although it gradually decreased. The yield of HMF at the sixth cycle decreased to 74.7% due to the depressed activity of catalyst with frequent use.
Conclusions
In conclusion, the reduced graphene oxide supported tungsten trioxide (WO3/RGO) was prepared via a one-step hydrothermal method and used as an active acid catalyst for fructose to HMF conversion. A high yield of HMF up to 84.2% with a full fructose conversion was obtained with the catalyst under the optimum reaction conditions: 1 mmol fructose, 10 mg WO3/RGO, 2.0 g [BMIM]Cl, 120 °C for 2 hours. Moreover, it could also transform cellulose, glucose and sucrose to HMF despite having a relatively lower yield. WO3/RGO was proven to be a more suitable catalyst for fructose conversion. The catalyst could be reused five times with a slight decrease in activity.Acknowledgements
The authors thank the financial support from the Research Project of the Natural Science Foundation of Heilongjiang Province of China (No. B201207 and No. B201208).Notes and references
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS PubMed.
- J. J. Bozell, Science, 2010, 329, 522–523 CrossRef CAS PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610–626 CAS.
- D. Ding, J. Wang, J. Xi, X. Liu, G. Lu and Y. Wang, Green Chem., 2014, 16, 3846–3853 RSC.
- P. P. Upare, J.-W. Yoon, M. Y. Kim, H.-Y. Kong, D. W. Hwang, Y. K. Hwang, H. H. Kung and J.-S. Chang, Green Chem., 2013, 15, 2935–2943 RSC.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- Y. Jiang, L. Yang, C. M. Bohn, G. Li, D. Han, N. S. Mosier, J. T. Miller, H. I. Kenttämaa and M. M. Abu-Omar, Org. Chem. Front., 2015, 2, 1388–1396 RSC.
- A. Ranoux, K. Djanashvili, I. W. C. E. Arends and U. Hanefeld, ACS Catal., 2013, 3, 760–763 CrossRef CAS.
- D. W. Gardner, J. Huo, T. C. Hoff, R. L. Johnson, B. H. Shanks and J.-P. Tessonnier, ACS Catal., 2015, 5, 4418–4422 CrossRef CAS.
- Y. Xiao and Y.-F. Song, Appl. Catal., A, 2014, 484, 74–78 CrossRef CAS.
- A. Chinnappan, A. H. Jadhav, W.-J. Chung and H. Kim, Ind. Crops Prod., 2015, 76, 12–17 CrossRef CAS.
- K. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849–1853 CrossRef CAS.
- J. B. Binder, A. V. Cefali, J. J. Blank and R. T. Raines, Energy Environ. Sci., 2010, 3, 765–771 CAS.
- S. Hu, Z. Zhang, Y. Zhou, J. Song, H. Fan and B. Han, Green Chem., 2009, 11, 873–877 RSC.
- D. D. J. Liu and E. Y.-X. Chen, Appl. Catal., A, 2012, 435–436, 78–85 CAS.
- X. Guo, Q. Cao, Y. Jiang, J. Guan, X. Wang and X. Mu, Carbohydr. Res., 2012, 351, 35–41 CrossRef CAS PubMed.
- B. Liu and Z. Zhang, ACS Catal., 2016, 6, 326–338 CrossRef CAS.
- A. Chareonlimkun, V. Champreda, A. Shotipruk and N. Laosiripojana, Bioresour. Technol., 2010, 101, 4179–4186 CrossRef CAS PubMed.
- P. Goli, H. Ning, X. Li, C. Y. Lu, K. S. Novoselov and A. A. Balandin, Nano Lett., 2014, 14, 1497–1503 CrossRef CAS PubMed.
- Q. Huang, L. Zhou, X. Jiang, Y. Zhou, H. Fan and W. Lang, ACS Appl. Mater. Interfaces, 2014, 6, 13502–13509 CAS.
- B. F. Machado and P. Serp, Catal. Sci. Technol., 2012, 2, 54–75 CAS.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem. Rev., 2014, 114, 6179–6212 CrossRef CAS PubMed.
- H. Huang, Z. Yue, G. Li, X. Wang, J. Huang, Y. Du and P. Yang, J. Mater. Chem. A, 2013, 1, 15110–15116 CAS.
- L. Wang, X. Lu, S. Lei and Y. Song, J. Mater. Chem. A, 2014, 2, 4491–4509 CAS.
- B. Jiang, S. Song, J. Wang, Y. Xie, W. Chu, H. Li, H. Xu, C. Tian and H. Fu, Nano Res., 2014, 7, 1280–1290 CrossRef CAS.
- H. Zhao, G. Mao, H. Han, J. Song, Y. Liu, W. Chu and Z. Sun, RSC Adv., 2016, 6, 41108–41113 RSC.
- P. Bhaumik and P. L. Dhepe, ChemCatChem, 2016, 8, 1–9 CrossRef.
- Y. Liu, C. Luo and H. Liu, Angew. Chem., Int. Ed., 2012, 51, 3249–3253 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. Guo, Y. Li, S. Zhu, Z. Chen, Q. Liu, D. Zhang, W.-J. Moon and D.-M. Song, RSC Adv., 2012, 2, 1356–1363 RSC.
- B. Weng, J. Wu, N. Zhang and Y.-J. Xu, Langmuir, 2014, 30, 5574–5584 CrossRef CAS PubMed.
- L. Huang, H. Xu, Y. Li, H. Li, X. Cheng, J. Xia, Y. Xu and G. Cai, Dalton Trans., 2013, 42, 8606–8616 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26309g |
| This journal is © The Royal Society of Chemistry 2017 |