Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Persistent luminescence in the self-activated K2Zr(BO3)2
Guifang Ju *,
Yihua Hu,
Li Chen,
Yahong Jin and
Yang Li
*,
Yihua Hu,
Li Chen,
Yahong Jin and
Yang Li
School of Physics and Optoelectronic Engineering, Guangdong University of Technology, Waihuan Xi Road, No.100, Guangzhou 510006, People's Republic of China. E-mail: jugf@gdut.edu.cn; Fax: +86-20-39322265; Tel: +86-20-39322262
First published on 16th January 2017
Abstract
This study reports a cyan emitting self-activated persistent phosphor K2Zr(BO3)2. The material is synthesized by solid state reaction method. The persistent phosphor was characterized in detail by X-ray powder diffraction, diffuse reflectance, photoluminescence, persistent luminescence and thermoluminescence spectra. After UV irradiation, the K2Zr(BO3)2 phosphor shows a cyan persistent luminescence dominating at ∼485 nm. Both the fluorescence and persistent luminescence are due to charge transfer emission from the central zirconium ion to oxygen in the ligand. The deconvolution of the thermoluminescence curve reveals that there are four traps responsible for the persistent luminescence. The depth of the dominant trap is 0.66 eV. Based on experimental results, the trapping and detrapping processes of the charge carriers are discussed. A rudimentary energy level scheme was proposed to explain the mechanisms of persistent luminescence as well as photoluminescence.
Introduction
Persistent luminescence is an optical phenomenon in which luminescence can persist for minutes and even days without energy sources.1–4 It is a special case of thermally stimulated luminescence at room temperature. The required energy is reserved from the excitation source earlier by either intrinsic or dopant induced traps.Matsuzawa et al. reported the persistent luminescence in SrAl2O4:Eu2+,Dy3+ in 1996,5 this aluminate was regarded as the first milestone of persistent phosphors. Thereafter, research of rare earth doped persistent phosphors boomed. Eu2+-activated aluminates and silicates, e.g., CaAl2O4:Eu2+,Nd3+,6 Sr4Al14O25:Eu2+,Dy3+ (ref. 7) and Sr2MgSi2O7:Eu2+,Dy3+,8 are particularly representative and the elites of these phosphors have been successfully commercialized and are widely used in a variety of fields, such as instruction signs, displays and decoration.9–11
Le Masne de Chermont et al. introduced persistent phosphors into the in vivo optical imaging field,12 because persistent phosphors do not require real-time excitation, which can avoid tissue autofluorescence. In order to fit in the biological window,13 near-infrared persistent phosphors have received considerable attention. In 2011, Pan et al. reported the near-infrared persistent luminescence from Cr3+-doped zinc gallogermanates.1 The after glow of this material can last amazing 360 h. Zn3Ga2Ge2O10:Cr3+ can be taken as the second milestone of persistent phosphors. Besides Zn3Ga2Ge2O10:Cr3+, a lot of Cr3+-doped near-infrared persistent phosphors were also reported, most of them are gallates or gallogermanates, e.g., ZnGa2O4:Cr3+,14 La3Ga5GeO14:Cr3+,15,16 and LiGa5O8:Cr3+.17
Most phosphors are compositions of an activator (usually several percent) and a host. The activators have an incompletely filled shell which can provide certain energy levels. The energy levels are responsible to the absorption and emission of the phosphors. On the other hand, the host constituent ions only have complete shells; therefore the host is inert to visible light. However, some phosphors contain no activator. In other words, the activator concentration is 100%. This kind of phosphors is known as self-activated phosphors.18,19 At present, some studies on self-activated persistent phosphors have been reported, including Ba2TiP2O9,20 Ca2Ge7O16,21 Ca2ZrSi4O12,22 Na5YZrSi6O18 (ref. 23) and CaZr4(PO4)6.24 Although tungstates and vanadates are the well known self-activated phosphors, when it comes to self-activated persistent phosphors, zirconates are the main players.
The borate compound K2Zr(BO3)2 (KZBO) was first identified by Akella and Keszler.26 They determined the structure of the material and measured its refractive indices. Since then the compound was forgotten. In this paper, we present an insightful investigation of the luminescence of KZBO. The luminescence properties, including excitation and emission spectra, luminescence decay were reported. The photoluminescence and the persistent luminescence mechanisms were discussed based on the luminescence properties and thermoluminescence (TL) glow curve.
Experimental
Powder samples of KZBO were synthesized using a high temperature solid state reaction method. Stoichiometric amounts of A.R. grade starting materials K2CO3, ZrO2 and H3BO3 were weighed and thoroughly mixed in an agate mortar. In order to prevent spewing and also to decompose the boric acid and potassium carbonate, the mixture was first heated up to 600 °C very slowly and kept at this temperature for 1 h. After regrinding, the samples were sintered at 750 °C for 10 h. After cooling to room temperature inside the furnace, the obtained samples were ground again for next characterizations.Phase purity of the prepared samples were checked by powder X-ray diffraction (XRD) using a diffractometer with Cu Kα radiation (λ = 1.5406 Å) at 36 kV tube voltage and 20 mA tube current (Beijing PGENERAL, XD-2). Diffuse reflection spectra were obtained by a UV-visible spectrophotometer (Thermo scientific, EVOLUTION 220) using BaSO4 as a reference. The photoluminescence excitation (PLE), emission (PL) spectra and fluorescence decay curve of the samples were recorded by a fluorescence spectrophotometer (Edinburgh, FLS980). A thermoluminescent dosimeter (Guangzhou-radiation science and technology, SL08-L) was applied to measure the thermoluminescence (TL) glow curve. The persistent luminescence isothermal decay curve was obtained with a single-photon counter system (Tianjin Tuopu Instrument, WSZ-5A). Prior to the TL glow and persistent luminescence isothermal decay curve measurements, the sample was excited for 1 min by a 15 W low-pressure mercury discharge lamp (254 nm). For the TL glow curve measurement, the heating rate was 5 °C s−1.
Results and discussion
The XRD pattern of KZBO together with the powder diffraction file (PDF) #80-0204 is shown in Fig. 1. The XRD lines of the KZBO sample are well coincident with the standard PDF. No extra line was observed, and the entire pattern could be well indexed to KZBO single phase.The compound KZBO is isostructural to the mineral buetschliite with a trigonal unit cell and space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 5.2830 Å, c = 17.5180 Å, V = 423.42 Å3 and Z = 3.25 The unit cell structure of KZBO as well as the coordination polyhedrons of KO9 and ZrO6 is illustrated in Fig. 2.25 In this structure, parallel (orthogonal to the crystallographic c axis) layers of flat trigonal BO3 groups build up the skeleton, and single sheets of Zr4+ and double sheets of K+ are alternately interleaved into the BO3 layers. Each ZrO6 octahedron is connected with two KO9 octahedrons by sharing face which is orthogonal to the c axis. The ZrO6 octahedrons within a same layer are bridged by BO3 triangles by sharing vertexes. The KO9 octahedrons within the same sheet are also bridged by BO3 triangle but there are common O vertices shared between the KO9 octahedrons from different sheets.
m, a = 5.2830 Å, c = 17.5180 Å, V = 423.42 Å3 and Z = 3.25 The unit cell structure of KZBO as well as the coordination polyhedrons of KO9 and ZrO6 is illustrated in Fig. 2.25 In this structure, parallel (orthogonal to the crystallographic c axis) layers of flat trigonal BO3 groups build up the skeleton, and single sheets of Zr4+ and double sheets of K+ are alternately interleaved into the BO3 layers. Each ZrO6 octahedron is connected with two KO9 octahedrons by sharing face which is orthogonal to the c axis. The ZrO6 octahedrons within a same layer are bridged by BO3 triangles by sharing vertexes. The KO9 octahedrons within the same sheet are also bridged by BO3 triangle but there are common O vertices shared between the KO9 octahedrons from different sheets.
 | ||
| Fig. 2 Schematic view of the crystallographic structure of the unit cell of KZBO (left) and the coordination polyhedrons of KO9 and ZrO6 (right).25 | ||
In order to investigate the energy absorption of the KZBO phosphor, diffuse reflectance spectrum is shown in Fig. 3a. The diffuse reflectance spectrum shows a near plateau of ∼80% reflectance in the wavelength range of 400–700 nm and starts to decrease from 400 nm to higher energy. The absorption can be attributed to the ligand to metal charge transfer (LMCT) absorption, an electron from oxygen ion is transferred to a molecular orbital which is largely localized on the zirconium ions.
 | ||
| Fig. 3 (a) Diffuse reflectance spectrum of KZBO; (b) determination of the optical bandgap (Eg) using Tauc plot for KZBO. | ||
The near edge relation between absorption coefficient α and optical band gap Eg can be described by the equation:26,27
| (hνα)2 = A(hν − Eg) | (1) |
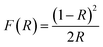 | (2) |
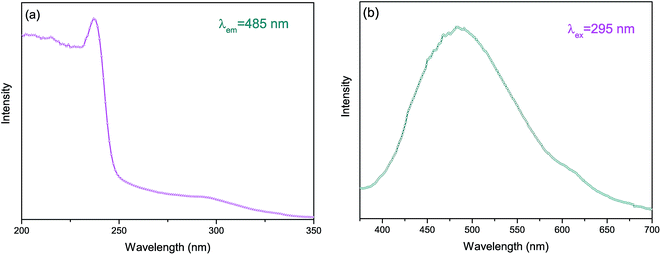
Fig. 4 shows the PLE and PL spectra of the LZBO sample at room temperature. Upon 295 nm excitation, the LZBO sample shows cyan luminescence with a maximum wavelength at about 485 nm. Based on the PL spectrum, Commission Internationale de l'Eclairage (CIE) chromaticity coordinate of the sample is calculated to be (0.232, 0.313), which is in the cyan region. It can be ascribed to the charge transfer transition from central zirconium ion to oxygen ligands. The full width at half maximum (FWHM) of the emission band and the Stokes shift are about 5600 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 cm−1, respectively. The large Stokes shift results in the mismatch between PLE and PL spectra, therefore concentration quenching is missing. The excitation spectrum by monitoring at 485 nm consists of two broad bands with double maxima at around 295 and 237 nm, respectively. The excitation spectrum agrees with the reflectance spectrum. The band at 295 nm can be attributed to electron–hole pair creation, while the higher band at 237 nm is attributed to valence–conduction band charge transfer. In the former case, the electron (on the zirconium) and hole (on the oxygen) remain together. In the latter case, however, free electrons and holes are created.
100 cm−1, respectively. The large Stokes shift results in the mismatch between PLE and PL spectra, therefore concentration quenching is missing. The excitation spectrum by monitoring at 485 nm consists of two broad bands with double maxima at around 295 and 237 nm, respectively. The excitation spectrum agrees with the reflectance spectrum. The band at 295 nm can be attributed to electron–hole pair creation, while the higher band at 237 nm is attributed to valence–conduction band charge transfer. In the former case, the electron (on the zirconium) and hole (on the oxygen) remain together. In the latter case, however, free electrons and holes are created.
Under 237 nm excitation, the luminescence decay curve of KZBO was measured (λem = 485 nm), and the results are shown in Fig. 5. The decay curve can be well fitted well by single exponential function (R2 = 0.998). The life time value was determined to be 5.7 μs. The value is in line with similar systems.24,30
After ceasing the irradiation light source of 254 nm at room temperature, persistent luminescence was observed. Fig. 6 shows the decay curves of the persistent luminescence in the time range of 1–1000 s recorded at room temperature. The decay curve fits to single exponential functions in 0–40 s (τ = 17 s), but to t−1.4 power function afterwards. The decay is very fast during the first minute, but slows down later.
Persistent luminescence is a special case of thermally stimulated luminescence. The TL technique may be the only available tool for revealing the nature of traps in persistent phosphors. Fig. 7a shows the TL curve of KZBO. The TL curve consist of two main band (66 and 123 °C, T1 and T2) and two minor contributions at the higher side (187 and 257 °C, T3 and T4). Apparently, T1 and T2 are mainly responsible for the room temperature persistent luminescence. The band shape is asymmetric with the low-temperature side narrower than the high-temperature one, indicating retrapping dominates and therefore the process is of the second order kinetics. The persistent luminescence decay profiles of these phosphors follow power law after the initial period. This also suggests second order kinetics.
 | ||
| Fig. 7 The TL curves of the KZBO undergoing different decay times (0, 10, 30 and 70 min) and the general order deconvolution results. | ||
In order to achieve a quantitative analysis of the TL curve, the TL curve was deconvoluted into general order components:31
 | (3) |
| Traps | E (eV) | s (s−1) | n0 | b | Tm (°C) | Height (a. u.) | Area |
|---|---|---|---|---|---|---|---|
| 1st | 0.66 | 2.23 × 109 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2 | 65.8 | 4400 | 2.3 × 105 |
| 2nd | 0.82 | 7.76 × 109 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.74 | 122.8 | 1060 | 5.97 × 104 |
| 3rd | 0.72 | 1.64 × 107 | 1700 | 1.45 | 186.5 | 110 | 8500 |
| 4th | 0.98 | 4.43 × 108 | 920 | 1 | 256.7 | 70 | 4400 |
To investigate the detrapping process in KZBO, the TL glow curves with different delay times (0, 10, 30, and 70 min) after excitation are recorded in Fig. 7. With the increasing of decay time, the amplitudes of T1 and T2 decreases sharply, resulting in the centroid of the whole TL band moving to higher temperature side. On the other side, however, T3 and T4 are almost intact. These traps require higher temperature to be emptied. At this stage, it is no ground to speculate the nature of the traps.
On the basis of the above results, a tentative mechanism by using an energy level scheme was proposed to illustrate the persistent luminescence in KZBO, as shown in Fig. 8. In Fig. 8, the band gap of KZBO and the trap depth are drawn according to the determined values. For the clarity to the readers, only T1 is depicted. Upon UV excitation, electrons in the valence band (VB) are excited to the conduction band (CB) and holes are left behind in VB. However, this process does not necessarily yield free carriers.19 In strong-coupled systems likes KZBO, a considerable relaxation occurs after excitation and giving up excess energy to the lattice, the electrons and holes are localized. They remain together and cannot transfer their energy to a neighbor, that why concentration quenching is absence in this phosphor. The recombination of the electrons and holes results in the emission of KZBO. Before they can fluoresce, the carriers (electrons and/or holes) may have chances to escape to the energy bands with or without the aid of phonons. The free carriers can be captured by traps with opposite charges which are from lattice defects. If the trap density is too high the carriers may migrate from one trap to another by tunneling until to a killer site. Therefore suitable trap density as well as trap depth is crucial for a persistent phosphor. The trapped carriers have chances to be free again by the thermal energy at ambient temperature. Finally the freed carriers go back to a zirconium complex and subsequently emission occurs.
Conclusions
In conclusion, a novel cyan emitting self-activated persistent phosphor KZBO has been successfully prepared via a high-temperature solid-state reaction method. The absorption properties, emission properties, persistent luminescence and TL of the sample are characterized and analyzed in detail. The bandgap of KZBO is determined to be 5.05 eV. The persistent luminescence originates from the charge transfer in the zirconium complex. Under UV light excitation, the phosphor shows a broad emission band located at ∼485 nm. The depth of dominating trap responsible for the room temperature persistent luminescence is 0.66 eV. Although the physical nature of the traps is still unclear, the trap type can be revealed in future conductivity and Hall measurements.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 21271049).References
- Z. Pan, Y. Y. Lu and F. Liu, Nat. Mater., 2011, 11, 58–63 CrossRef PubMed.
- H. F. Brito, J. Hölsä, T. Laamanen, M. Lastusaari, M. Malkamäki and L. C. V. Rodrigues, Opt. Mater. Express, 2012, 2, 371–381 CrossRef CAS.
- K. Van den Eeckhout, P. F. Smet and D. Poelman, Materials, 2010, 3, 2536–2566 CrossRef CAS.
- A. Jain, A. Kumar, S. J. Dhoble and D. R. Peshwe, Renewable Sustainable Energy Rev., 2016, 65, 135–153 CrossRef CAS.
- T. Matsuzawa, Y. Aoki, N. Takeuchi and Y. Murayama, J. Electrochem. Soc., 1996, 143, 2670–2673 CrossRef CAS.
- H. Yamamoto and T. Matsuzawa, J. Lumin., 1997, 72–74, 287–289 CrossRef CAS.
- Y. Lin, Z. Tang and Z. Zhang, Mater. Lett., 2001, 51, 14–18 CrossRef CAS.
- Y. Lin, Z. Tang, Z. Zhang, X. Wang and J. Zhang, J. Mater. Sci. Lett., 2001, 20, 1505–1506 CrossRef CAS.
- C. C. S. Pedroso, J. M. Carvalho, L. C. V. Rodrigues, J. Hölsä and H. F. Brito, ACS Appl. Mater. Interfaces, 2016, 8, 19593–19604 Search PubMed.
- Y. Pan, L. Li, J. Lu, R. Pang, L. Wan and S. Huang, Dalton Trans., 2016, 45, 9506–9512 RSC.
- H. Guo, Y. Wang, W. Chen, W. Zeng, G. Li and Y. Li, New J. Chem., 2016, 40, 613–618 RSC.
- Q. le Masne de Chermont, C. Chanéac, J. Seguin, F. Pellé, S. Maîtrejean, J. P. Jolivet, D. Gourier, M. Bessodes and D. Scherman, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9266–9271 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- A. Bessière, S. Jacquart, K. Priolkar, A. Lecointre, B. Viana and D. Gourier, Opt. Express, 2011, 19, 10131–10137 CrossRef PubMed.
- W. Yan, F. Liu, Y.-Y. Lu, X.-J. Wang, M. Yin and Z. Pan, Opt. Express, 2010, 18, 20215–20221 CrossRef CAS PubMed.
- D. Jia, L. A. Lewis and X.-J. Wang, Electrochem. Solid-State Lett., 2010, 13, J32–J34 CrossRef CAS.
- F. Liu, W. Yan, Y.-J. Chuang, Z. Zhen, J. Xie and Z. Pan, Sci. Rep., 2013, 3, 1554 Search PubMed.
- C. R. Ronda, Luminescence: from theory to applications, Wiley-VCH, Weinheim, 2008 Search PubMed.
- G. Blasse and B. C. Grabmaier, Luminescent materials, Springer-Verlag, Berlin, New York, 1994 Search PubMed.
- C. B. Liu and G. B. Che, Chin. J. Inorg. Chem., 2006, 22, 503–506 CAS.
- T. Wang, J. Gou, X. Xu, D. Zhou, J. Qiu and X. Yu, Opt. Express, 2015, 23, 12595–12604 CrossRef PubMed.
- P. Feng, J. Zhang, C. Wu, X. Liu and Y. Wang, Mater. Chem. Phys., 2013, 141, 495–501 CrossRef CAS.
- Y. Guan, L. Qin, Y. Huang, C. Qin, D. Wei and H. J. Seo, Mater. Res. Bull., 2014, 54, 24–27 CrossRef CAS.
- G. Ju, Y. Hu, L. Chen, Y. Jin, S. Zhang, F. Xue and H. Chen, Mater. Res. Bull., 2016, 83, 211–216 CrossRef CAS.
- A. Akella and D. A. Keszler, Inorg. Chem., 1994, 33, 1554–1555 CrossRef CAS.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627–637 CrossRef CAS.
- M. A. Omar, Elementary solid state physics : principles and applications, Addison-Wesley Pub. Co., Reading, Mass., 1975 Search PubMed.
- E. L. Simmons, Appl. Opt., 1975, 14, 1380–1386 CrossRef CAS PubMed.
- J. H. Nobbs, Rev. Prog. Color. Relat. Top., 1985, 15, 66–75 CrossRef.
- C. W. Park and H. J. Seo, Ceram. Interfaces, 2014, 40, 2495–2499 CrossRef CAS.
- S. W. S. McKeever, Thermoluminescence of solids, Cambridge University Press, New York, 1985 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |