Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Construction of CuCo2O4@CuCo2O4 hierarchical nanowire arrays grown on Ni foam for high-performance supercapacitors†
Yan Zhang,
Jie Xu *,
Yayun Zheng,
Yingjiu Zhang*,
Xing Hu and
Tingting Xu
*,
Yayun Zheng,
Yingjiu Zhang*,
Xing Hu and
Tingting Xu
School of Physical Engineering, Key Laboratory of Material Physics, Ministry of Education, Zhengzhou University, No. 75, Daxue Road, Zhengzhou 450052, China. E-mail: xujie@zzu.edu.cn; zhangyj2006@zzu.edu.cn
First published on 16th January 2017
Abstract
In this study, hierarchical CuCo2O4@CuCo2O4 nanowire arrays have been directly fabricated on Ni foam via a two-step method that involves hydrothermal and calcination processes. The CuCo2O4 nanowires are fully covered by porous CuCo2O4 nanosheets. The as-prepared double-metal oxide hierarchical nanowire arrays exhibit superior redox reactivity for supercapacitors. The unique hierarchical nanowire arrays own a specific capacitance of up to 888.9 F g−1 at 2 mA cm−2, which is much higher than the specific capacitance values of simple CuCo2O4 nanowire arrays. The hierarchical CuCo2O4 nanostructure shows superior cycling stability, retaining 101.77% of the initial capacitance after 2000 cycles. Moreover, by employing CuCo2O4@CuCo2O4 as the positive electrode and active carbon (AC) as the negative electrode, an asymmetric supercapacitor (ASC) device was fabricated and it displayed an excellent electrochemical performance. The enhanced electrochemical performance is mainly due to its unique hierarchical structure, which provides good ion and electron transfer, a large number of active sites, and the synergistic effect. In view of their outstanding electrochemical performance and the cost-effective fabrication process, this unique integrated nanoarchitecture may offer great promise as superior electrodes for high-performance supercapacitors.
1. Introduction
With the fossil fuels consumption and worsening environmental pollution problems, sustainable energy storage using clean and renewable energy sources is becoming one of the great challenges in the twenty-first century.1–4 Compared to the conventional rechargeable devices, such as NiMH batteries and Li-ion batteries, supercapacitor (SC) is considered as a preferable candidate energy storage device due to its high power density, fast charging/discharging rate, long steady cycle life, and being relatively environmentally-friendly.4–6 In recent years, scientists have made great efforts to study pseudocapacitors because their energy density is substantially higher than that of the electrical double layer capacitors (EDLCs). The performance of pseudocapacitors is largely determined by the faradaic reaction characteristics of the electrode materials,7,8 and the ternary cobalt-based spinel oxides have been actively pursued because they possess a higher electrical conductivity and better electrochemical performance than those of single cobalt oxides.9,10 Among the various ternary cobalt-based spinel oxide electrode materials, spinel copper cobaltite (CuCo2O4) has been widely used as a very promising electrode for supercapacitors,10,11 catalysts,12 and lithium batteries13 because it offers many advantages, such as low-cost, availability, and environmental compatibility.10–13 More significantly, CuCo2O4 possesses a greater electrochemical activity and a much better electronic conductivity, at least two orders of magnitude higher than those of the single components of copper oxides and cobalt oxides alone.10,14 Generally, an ideal supercapacitor should have the merits such as high power density, long cycle life, fast charging/discharging rate, good safety, and low maintenance cost. However, supercapacitors fabricated with one-dimensional (1D) nanomaterials as electrodes have been shown to have difficulties in meeting all these requirements.8,15–17 Therefore, it is necessary to prepare new nanostructured electrode materials to solve this problem. An efficient way is to synthesize three-dimensional (3D) hierarchical nanomaterials directly on Ni foam as binder- and additive-free electrodes for supercapacitors.18 The 3D structures and the synergistic effects of component nanomaterials may lead to improved electrochemical performance of the electrodes.18–20To further optimize the electrochemical performance, many hierarchical nanostructure electrode materials have been studied.21 In a typical hierarchical system, the increasing structural hierarchy could provide better conductivity and increase the active sites of the array structures. Additionally, a strong synergistic effect could also be realized due to the construction of the hierarchical structure.22,23 Moreover, the electrode materials would be able to realize a higher electrochemical performance. For instance, Chen et al. have reported that NiCo2S4 nanotube@NiCo2S4 nanosheet arrays on Ni foam display a superior specific capacity than that of the pure NiCo2S4 arrays and the hierarchical structure also exhibited an excellent cyclic stability.24 Zhang et al. have reported that MnCo2O2@MnO2 composites exhibit an increased capacitance and better cycling performance as compared to those of the pristine MnCo2O4 nanowire arrays.25
In the present study, hierarchical CuCo2O4@CuCo2O4 nanowire arrays were grown on Ni foam as binder- and additive-free electrodes via a two-step approach, which involves hydrothermal and calcination processes. Benefiting from the unique properties of the hierarchical nanowire arrays configuration, such as large numbers of active sites, good ion and electron transfer, and good strain accommodation, the as-prepared CuCo2O4@CuCo2O4 electrode exhibited a notable electrochemical performance with high capacitance, good rate capabilities, and desirable cycle stability at high current densities.
2. Experimental
The nickel foam was purchased from Suzhou jiashide metal foam Co., Ltd (China) with a 0.1–0.2 mm pore size, 98% porosity, and through-hole rate greater than or equal to 98%. The filter paper was bought from Fushun Dongyang Industry and Trade Co., Ltd (China) of qualitative filter paper with the type of 102. All the reagents used in the experiments were of analytical grade and used as received without further purification.2.1 Synthesis of the CuCo2O4 nanowire arrays (NWAs)
In a typical procedure, 1 mmol Cu(NO3)2, 2 mmol Co(NO3)2, 12 mmol urea, and 3 mmol CTAB were dissolved in a 70 mL deionized water and stirred to form a clear solution. Fresh Ni foam of a 1 × 4 cm size was used as the substrate, and it was carefully cleaned with 3 mol L−1 HCl solution, deionized water, and ethanol for 20 min each to remove the impurities and oxide layer. The aqueous solution and the Ni foam were transferred to a 100 mL Teflon-lined stainless-steel autoclave, which was sealed, maintained at 120 °C for 12 h, and then naturally cooled down to room temperature. The product was collected and sonicated in deionized water for 10 s, and then washed with deionized water several times. The precursors were dried in a vacuum oven at 60 °C for 3 h and finally annealed in a quartz tube at 300 °C in air for 2 h. The mass loading of CuCo2O4 NWAs on Ni foam was about 1.1 mg cm−2.2.2 Preparation of the CuCo2O4@CuCo2O4 nanowire arrays (NWAs)
Typically, 1 mmol of CuCl2, 2 mmol CoCl2, 12 mmol urea, and 3 mmol CTAB were dissolved in 70 mL deionized water under stirring. Then, the solution was transferred into a Teflon-lined stainless-steel autoclave. The as-synthesized CuCo2O4 nanowire array was placed in the autoclave. The autoclave was sealed and heated at 120 °C for 12 h, and then cooled down to room temperature. Subsequently, the substrate was taken out and washed with deionized water several times and dried in vacuum at 60 °C for 3 h. Finally, the precursor was annealed at 300 °C in air for 2 h. After calcination, the average mass loading of the final product was approximately 2 mg cm−2.2.3 Characterization of the CuCo2O4@CuCo2O4 NWAs
The structural characterizations of the synthesized products were performed by X-ray diffraction (XRD, Netherlands Philip X'Pert) using Cu Kα radiation (λ = 0.154056 nm). The morphologies and microstructures of the as-prepared products were characterized using a scanning electron microscope (SEM, JEOL-JSM-6700F, Japan Electronics), transmission electron microscopy (TEM), and high-resolution transmission electron microscopy (HRTEM) (TEM, JEM-2100, Japan Electronics). The specific surface area and pore size distribution of the materials were determined using the Brunauer–Emmett–Teller (BET) method and Barrett–Joyner–Halenda (BJH) method, respectively (Micromeritics TriStar II 3020 instrument). The mass changes of the products were determined by a microbalance with a readability of 0.1 mg (Mettler, AL204).2.4 Electrochemical measurements
The electrochemical measurements were carried out using a three-electrode electrochemical cell containing a 2 mol L−1 KOH aqueous solution as the electrolyte. The CuCo2O4 NWAs and CuCo2O4@CuCo2O4 NWAs were directly used as the working electrodes, and the area of electrode used in the electrochemical measurement was 1 cm2. The electrochemical measurements were performed using an electrochemical workstation (CS2350, Wuhan). A standard calomel electrode and Pt foil were used as the reference and counter electrodes, respectively, and all of the experiments were performed at ambient temperature. Electrochemical impedance spectroscopy (EIS) measurements were evaluated using an alternating-current voltage with a 5 mV amplitude in the frequency range from 0.01 Hz to 100 kHz.For the assembly of the CuCo2O4@CuCo2O4//AC device, an AC electrode was prepared by mixing active carbon, acetylene black, and polyvinylidene difluoride at a weight ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. The slurry was directly coated on a porous nickel foam and dried overnight in a vacuum oven at 60 °C. The ASC device fabricated by separating two electrodes (AC as the negative electrode and CuCo2O4@CuCo2O4 as positive electrode) with filter paper was tested in a two-electrode system and using 2 mol L−1 KOH as the electrolyte. Based on the three-electrode electrochemical measurement results of both the CuCo2O4@CuCo2O4 and AC electrodes, cell balance was achieved by setting the electrode mass ratio of positive/negative to 0.23 (the negative loading mass was around 8.6 mg cm−2). The total mass loading of the active materials was about 10.6 mg.
5, respectively. The slurry was directly coated on a porous nickel foam and dried overnight in a vacuum oven at 60 °C. The ASC device fabricated by separating two electrodes (AC as the negative electrode and CuCo2O4@CuCo2O4 as positive electrode) with filter paper was tested in a two-electrode system and using 2 mol L−1 KOH as the electrolyte. Based on the three-electrode electrochemical measurement results of both the CuCo2O4@CuCo2O4 and AC electrodes, cell balance was achieved by setting the electrode mass ratio of positive/negative to 0.23 (the negative loading mass was around 8.6 mg cm−2). The total mass loading of the active materials was about 10.6 mg.
3. Results and discussion
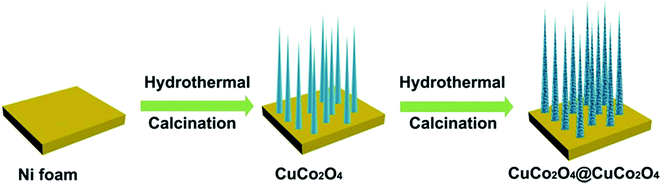
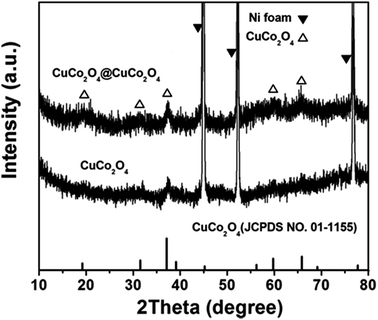
In this study, the construction of the hierarchical CuCo2O4@CuCo2O4 NWAs for pseudocapacitive electrode was successfully obtained by a facile synthetic strategy including two steps, as schematically illustrated in Fig. 1. Initially, the aligned pristine CuCo2O4 NWAs were grown on the Ni foam through the hydrothermal and post-annealing processes. Subsequently, a rough porous layer was coated on the surface of CuCo2O4 NWAs as CuCo2O4 layers were deposited on the backbone of CuCo2O4 NWAs through a secondary hydrothermal process, finally resulting in the formation of hierarchical CuCo2O4@CuCo2O4 nanostructures on the conductive substrate as a superior binder- and additive-free electrode.The XRD patterns of the CuCo2O4 NWAs and CuCo2O4@CuCo2O4 hierarchical NWAs supported on Ni foam are shown in Fig. 2. The three marked strong peaks are typical peaks of the Ni foam substrate. The diffraction peaks at around 19.07°, 31.36°, 36.96°, 59.59°, and 65.70° can be clearly observed and are well indexed to the (111), (220), (311), (511), and (440) planes of the cubic CuCo2O4 phase, respectively (JCPDS card no. 01-1155). For the hierarchical nanowire arrays, the pattern shows little difference with that of the bare one. There are no extra peaks in the pattern, demonstrating that the outer layer material was also CuCo2O4. However, the diffraction peaks of the CuCo2O4@CuCo2O4 hierarchical NWAs are broader than those of the bare one, which may be due to the smaller size of the outer layer CuCo2O4 crystals or the lower crystallinity.23
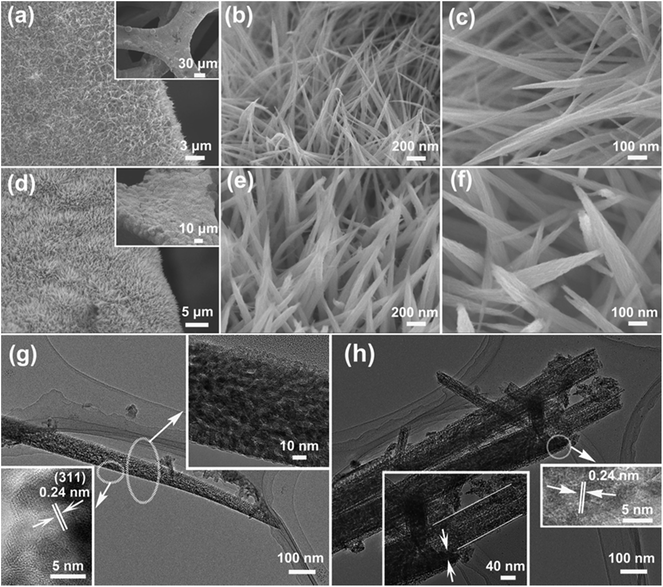
The morphologies and nanoarchitectures of the CuCo2O4 NWAs and CuCo2O4@CuCo2O4 hierarchical NWAs on Ni foam are shown in Fig. 3. After the hydrothermal and post-annealing processes, large-scale and aligned CuCo2O4 NWAs uniformly grew on the Ni foam skeletons (Fig. 3a). The needle-structure CuCo2O4 NWAs appear like numerous “grass”, standing on the Ni foam substrate (Fig. 3b). The magnified image shows that CuCo2O4 NWAs possessed rough surfaces (Fig. 3c). Fig. 3d–f show the typical SEM images of the CuCo2O4@CuCo2O4 hierarchical NWAs obtained through the hydrothermal and post-annealing processes with the needle-like CuCo2O4 NWAs as the substrate. The as-prepared CuCo2O4@CuCo2O4 hierarchical NWAs were much denser than the CuCo2O4 NWAs grown on the substrate (Fig. 3d). Note that the uniformity of the nanoneedle structure was still well retained. The surfaces of the CuCo2O4@CuCo2O4 hierarchical NWAs became rougher (Fig. 3e and f). The diameter of the CuCo2O4@CuCo2O4 nanowire was increased, indicating the successful growth of a uniform CuCo2O4 layer (Fig. 3f and S1†). The inner CuCo2O4 nanowire and the outer CuCo2O4 layer have different aspect ratios and dimensions, which may be due to the different substrates used during the reaction process (the CuCo2O4 nanowire backbone and Ni foam). Obviously, no CuCo2O4 was packed in the interspace of the nanowires, suggesting that the CuCo2O4 layer was preferentially grown on the surface of the inner CuCo2O4 nanowires. TEM analysis provides the detailed morphology and nanoarchitectures of the as-obtained electrodes. Fig. 3g shows the TEM images of the CuCo2O4 NWAs and the diameter of the CuCo2O4 nanowire is about 90 nm at the bottom of the needle-like structure. On closer observation (the inset of Fig. 3g at the upper right), the CuCo2O4 nanowire shows a mesoporous structure. The measured lattice spacing of 0.24 nm in the HRTEM image of the inset in Fig. 3g at the lower left corresponds to the (311) planes of the cubic CuCo2O4 nanowire. Afterwards, the needle-like CuCo2O4 NWAs were employed as the backbone and provide a vast number of sites for the growth of CuCo2O4 outer layer. The TEM images in Fig. 3f show that the uniform layer of the CuCo2O4 nanoparticles is covered on the whole surface of the needle-structure CuCo2O4 nanowires in the formation of a hierarchical structure. The magnified image inset in Fig. 3h at the lower left shows that the thickness of the CuCo2O4 nanowires is about 90 nm and that of the CuCo2O4 layer is around 20 nm; the inset at the right shows visible lattice fringes with equal interplanar distance of 0.24 nm, which correspond to the (311) plane of cubic CuCo2O4 and confirms that the outer layer has the same crystal structure as that of the CuCo2O4 nanowires.24 This unique hierarchical nanoarchitecture has a large number of interspaces, which could improve the utilization rate of the active materials and due to this, the electrolytes are highly accessible to all the electrode materials.26 The specific surface area and the average pore size of CuCo2O4@CuCo2O4 and CuCo2O4 were 30.95 m2 g−1 and 22.36 m2 g−1, and 7.5 nm and 8.2 nm, respectively. The CuCo2O4 outer layer with the porous structure was uniformly deposited on the CuCo2O4 nanowire backbone, which could develop the hierarchical structure with an ameliorative pore size distribution as compared to that of the original CuCo2O4 nanowire.
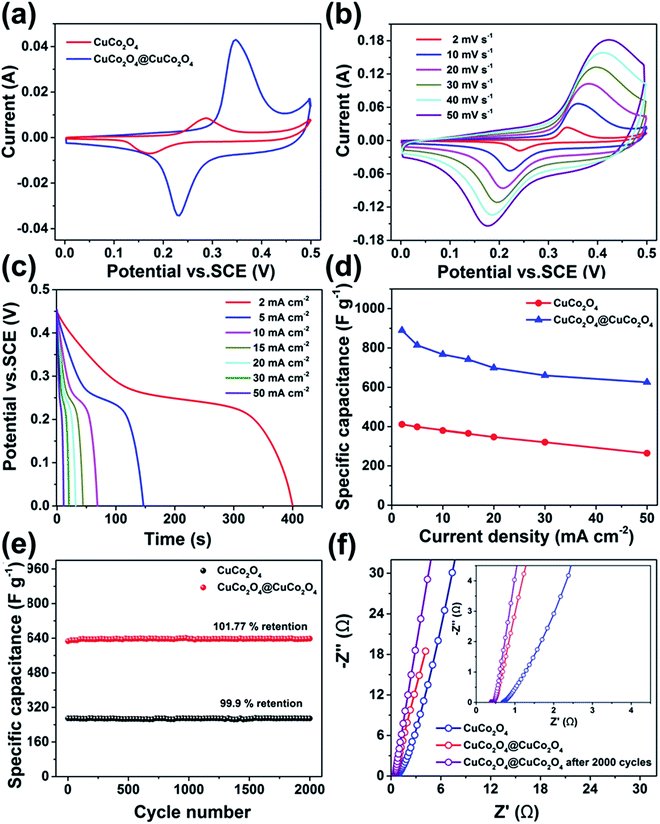
Three-electrode measurements were conducted in 2 mol L−1 KOH electrolytes to investigate the electrochemical performance of the electrodes. For comparison, Fig. 4a shows the typical cyclic voltammetry (CV) curves for both the CuCo2O4 and CuCo2O4@CuCo2O4 electrodes within the potential window of 0–0.5 V at the same scan rate of 5 mV s−1. It could be observed that all the CV curves have obvious redox peaks, indicating that the faradic nature of the battery-type electrodes and the redox peaks were mainly governed by the faradaic reactions related to M–O/M–O–OH (M refers to Cu or Co) associated with OH− anions.2 Furthermore, the enclosed area of the CV curve for the CuCo2O4@CuCo2O4 hierarchical NWAs electrode was much larger than that for the original CuCo2O4 NWAs electrode, revealing that the hierarchical nanowire arrays have higher electrochemical reaction activity.27,28 It should be attributed to the following reasons: on one hand, the porous CuCo2O4 outer layer with a huge surface area is beneficial for ion diffusion, intimate electrode/electrolyte contact, and it can provide a large electrochemically active surface area for the redox reactions; on the other hand, it could be because the rational construction of the CuCo2O4@CuCo2O4 hierarchical NWAs can make the best use of each component resulting in a synergistic effect. Note that the redox peak positions of the two electrode materials were obviously different, possibly because the polarization behavior during the CV tests were different in the electrode.29,30 The polarization behavior is closely associated to the morphology and structure of the electrode material.30 Therefore, the potential difference for the redox peaks of the two electrodes may be attributed to the fact that the CuCo2O4@CuCo2O4 hierarchical nanowire electrode having a porous CuCo2O4 layer with a larger surface area is beneficial for ion diffusion, intimate electrode/electrolyte contact, and it can provide a large number of electroactive sites for the redox reactions. Moreover, the synergistic effect of the CuCo2O4@CuCo2O4 hierarchical architecture also provided efficient pathways for ion and electron transport. Fig. 4b shows the typical CV curves of the hierarchical CuCo2O4@CuCo2O4 electrode obtained at the scan rates of 2, 10, 20, 30, 40, and 50 mV s−1 in the potential range of 0–0.5 V. As the scan rate increased, the anodic peaks shifted in the anodic direction and the cathodic peaks shifted in the cathodic direction,31 showing that the faradaic reactions occurred at the interface of the active material and electrolyte.6,32 Then, the potential difference between the redox peaks (ΔEp) was generally used to characterize the reversibility of the faradaic reaction, and a smaller ΔEp reveals a higher reversibility. The CV curves in Fig. 4b exhibit that the ΔEp is much higher than the theoretical value of 59.2 mV, illustrating that the electrode was not reversible during the electrochemical process.6 Moreover, based on Fig. 4b, both cathodic and anodic peak currents (ip) showed a nearly linear relationship with the square root of the scan rate (ν1/2), which is shown in Fig. S2,† revealing that the electrode reaction corresponds to the quasi-reversible and diffusion-controlled process with the electrolyte involved.6 The CV curves of the CuCo2O4 electrode at different scan rates are shown in Fig. S3a.†
Galvanostatic charge–discharge (GCD) measurements were conducted in the potential range from 0 to 0.45 V with various current densities ranging from 2 to 50 mA cm−2. Fig. 4c and S1b† exhibited the GCD curves of the CuCo2O4@CuCo2O4 and CuCo2O4 electrodes, respectively. The specific capacitance Cs (F g−1) can be calculated from the GCD curves by the equation Cs = IΔt/(mΔV), where I (A) is the discharge current, Δt (s) is the discharge time, m (g) represents the mass of the electroactive materials, and ΔV (V) is the discharge potential range. According to the equation, the specific capacitances of the electrodes were calculated from the discharge curves and the corresponding results are plotted in Fig. 4d. Impressively, the CuCo2O4@CuCo2O4 electrode delivered high specific capacitances of 888.9, 814.1, 767.1, 741.4, 698.2, 660.0, and 625.4 F g−1 at the current densities of 2, 5, 10, 15, 20, 30, and 50 mA cm−2, respectively (Fig. 4d). It can be seen that even by a 25-fold increase in the scan rate, the capacitance retention of the CuCo2O4@CuCo2O4 electrode was about 70.3% of its initial capacitance value. This good rate capability is ascribed to the highly porous structure of the CuCo2O4@CuCo2O4 electrode. The specific capacitance of the CuCo2O4@CuCo2O4 NWAs electrode still retained a value of 625.4 F g−1 at 50 mA cm−2. This is because of the diffusion effect of the proton within the electrode, where the inner active sites cannot completely precede the redox transitions at higher current densities.25 Moreover, the hierarchical nanowire arrays electrode delivered a specific capacitance of 888.9 F g−1 at the current density of 2 mA cm−2, which is much higher than that of the pristine CuCo2O4 NWAs electrode (about 411.4 F g−1).
The long-life cycling performance plays a key role for supercapacitor applications. The long-term cycling stabilities of the CuCo2O4 and CuCo2O4@CuCo2O4 electrodes were investigated by repeating the GCD tests at 50 mA cm−2 for 2000 cycles, as shown in Fig. 4e. For comparison, the original CuCo2O4 electrode displayed a specific capacitance of 269.3 F g−1 (∼99.9% capacitance retention). Impressively, even at a very high discharge current density, the CuCo2O4@CuCo2O4 hierarchical electrode still exhibited a high specific capacitance of 626.5 F g−1 (∼101.77% capacitance retention) after 2000 cycles. The coulombic efficiency during the 2000 charge/discharge cycles was more than 101%, which revealed the electrochemical suitability of the hierarchical CuCo2O4@CuCo2O4 electrode, whose redox reactions were quite feasible. As expected, the CuCo2O4@CuCo2O4 electrode showed high specific capacity, good rate capability, and cycling stability, which can be mainly due to their multiple merits: first, the direct growth of the CuCo2O4@CuCo2O4 NWAs on the current collector ensures good mechanical adhesion and a fast charge transfer pathway between the active material and current collector; second, the CuCo2O4 nanowires that were directly grown on the Ni foam allow for good electron transport, and the porous CuCo2O4 outer layer with a large surface area is beneficial for fast ion diffusion, intimate electrode/electrolyte contact, and it can provide a large number of electroactive sites for the redox reactions; and third, the entire electrode architecture provided efficient pathways for ion and electron transport. Note that the cycling stability of the hierarchical structured CuCo2O4@CuCo2O4 electrode was better than that of the other reported electrodes, such as ZnCo2O4@NixCo2x(OH)6x NWAs on Ni foam (81.4% capacitance retention after 2000 cycles at 20 mA cm−2),33 NiMoO4@Ni(OH)2 core/shell nanorods on Ni foam (72% capacitance retention after 2000 cycles at 8 mA cm−2),34 Co3O4@Ni(OH)2 NWAs on Ni foam (81.1% capacitance retention after 1500 cycles at 10 A g−1).35 In addition, EIS measurement was further performed to explain the excellent electrochemical behaviour of the CuCo2O4@CuCo2O4 electrode. Fig. 4f shows the impedance Nyquist plots of the CuCo2O4 electrode and the CuCo2O4@CuCo2O4 electrode before and after 2000 cycles. In the low-frequency region, the CuCo2O4@CuCo2O4 electrode has a more ideal straight line, revealing that they have a lower diffusion resistance. This can be due to the porous CuCo2O4 outer layer with a large surface area that has increased the active material utilization and thus facilitated the supply of OH− to the inner CuCo2O4 nanowires.36,37 Obviously, the CuCo2O4@CuCo2O4 electrode also displayed a low bulk resistance and charge-transfer resistance and exhibited high electrochemical activity for the CuCo2O4@CuCo2O4 hierarchical electrode for supercapacitors. This may be because the synergistic effects of the hierarchical architecture of the CuCo2O4@CuCo2O4 nanowires arrays electrode, which can increase the conductivity and improve the ion transfer during the charge/discharge process as compared to those of the bare CuCo2O4 nanowires arrays electrode.38 After 2000 cycles, the impedance spectra in a low-frequency region of the CuCo2O4@CuCo2O4 electrode almost remained unchanged. However, in the low-frequency region, the CuCo2O4@CuCo2O4 electrode after 2000 cycles had a more ideal straight line (compared with the CuCo2O4@CuCo2O4 electrode before 2000 cycles), representing more efficient electrolyte and proton diffusion after cycling. This may be because of the full activation of the electrode. The SEM images of the CuCo2O4 and CuCo2O4@CuCo2O4 electrodes after 2000 cycles at different magnifications are shown in Fig. S4 and S5.† According to the SEM images, it can be observed that the nanowire arrays almost had no change after 2000 cycles, the overall morphology of the product was maintained and the 3D nanowire arrays were still found to densely cover the Ni surface of Ni foam substrate. Therefore, using CuCo2O4 to design the hierarchical arrays can reach excellent cycling stability.
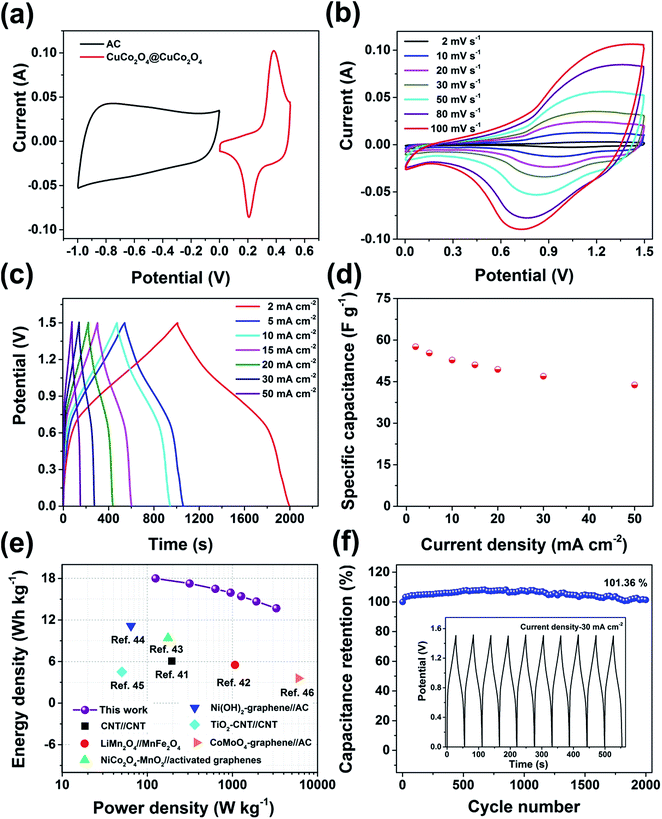
Furthermore, to evaluate the CuCo2O4@CuCo2O4 hierarchical electrode for practical applications, an asymmetric supercapacitor cell was assembled using AC as the negative electrode and CuCo2O4@CuCo2O4 as the positive electrode. This configuration benefits from a high energy redox-based (CuCo2O4@CuCo2O4) and a high power EDLC-based (AC) electrode, ensuring high performance of the final device. Fig. 5a shows the individual CV curves of the positive and negative electrodes at a scan rate of 20 mV s−1, obtained separately in a three-electrode configuration. In the case of the AC electrode, the CV curve is almost rectangular in the potential window of −1.0–0 V, revealing the EDLC charge storage mechanism of the carbon-based electrodes. Unlike the AC electrode, the CuCo2O4@CuCo2O4 product had a distorted shape, which showed clear redox peaks in the voltage range of 0–0.5 V, demonstrating the faradaic nature of the charge storage mechanism. Therefore, the total cell voltage of the ASC device could be as large as 1.5 V, which is based on the working potential windows of the negative and positive electrodes. This operation voltage window value is higher than the thermodynamic decomposition voltage limit of water in aqueous solutions (e.g. >1.2 V) and AC-based symmetric supercapacitor devices (0.8–1.0 V).39,40 Fig. 5b shows the CV curves of the CuCo2O4@CuCo2O4//AC supercapacitor at different scan rates, illustrating the contribution of both EDLC (0–1 V) and faradaic redox (1–1.5 V) charge storage mechanism. With the increasing scan rates, the CV curves showed no obvious distortion and also showed the fast charge–discharge reversibility of the device. The charge–discharge profiles of the CuCo2O4@CuCo2O4//AC asymmetric supercapacitor were also evaluated for the applied current densities ranging from 2 to 50 mA cm−2, as shown in Fig. 5c. The specific capacitances of the device calculated from the galvanostatic discharge curves were 57.60, 55.27, 52.76, 51.01, 49.39, 46.93, and 43.78 F g−1 at 2, 5, 10, 15, 20, 30, and 50 mA cm−2, respectively (Fig. 5d). Note that the CuCo2O4@CuCo2O4//AC supercapacitor exhibited excellent rate capability with a high retention rate of 76.0%, even when operated under a high current density of 50 mA cm−2, indicating the good rate performance of the ASC device used in this study.
According to the specific capacitance of the asymmetric supercapacitors, the energy density (E, in W h kg−1) and power density (P, in W kg−1) could be further calculated using the equations E = 1/2CU2 and P = E/t, respectively. With the current density increasing from 2 to 50 mA cm−2, the Ragone plot of the estimated energy densities and power densities for the ASC device are shown in Fig. 5e, which displays a high energy density of 18.0 W h kg−1 at a power density of 125 W kg−1, whereas can still maintain 13.7 W h kg−1, even at a power density of 3283 W kg−1. As it can be observed in this figure, the values show a much improved energy density at high power density compared with a CNT//CNT symmetric device (6.1 W h kg−1 at 195 W kg−1),41 a LiMn2O4//MnFe2O4 asymmetric device (5.5 W h kg−1 at 1080 W kg−1),42 a NiCo2O4–MnO2//activated graphene asymmetric device (9.4 W h kg−1 at 175 W kg−1),43 a Ni(OH)2–graphene//AC asymmetric device (11.11 W h kg−1 at 64 W kg−1),44 a TiO2–CNT//CNT asymmetric device (4.47 W h kg−1 at 50 W kg−1),45 and a CoMoO4–graphene//AC asymmetric device (3.59 W h kg−1 at 6000 W kg−1).46 Moreover, the CuCo2O4@CuCo2O4//AC ASC device showed a high cycling stability of about 101.36% capacity retention after 2000 cycles even at a high current density of 30 mA cm−2 (Fig. 5f), indicating that it has great potential in practical applications as one of the most attractive candidates for energy storage.
4. Conclusion
In conclusion, we have facilely synthesized uniform hierarchical nanowire arrays CuCo2O4@CuCo2O4 supported on Ni foam by a two-step approach, which involves hydrothermal and calcination methods. The as-prepared product has been directly used as a binder- and additive-free electrode in the electrochemical investigation for supercapacitors. According to the obtained results, the CuCo2O4@CuCo2O4 NWAs exhibited a high specific capacitance of 888.9 F g−1 at a scan rate of 2 mA cm−2 (much higher than that of the CuCo2O4 NWAs synthesized via a similar method) and excellent cycle life with 101.77% capacitance retention at 50 mA cm−2 after 2000 cycles. Moreover, the as-fabricated ASC device based on the CuCo2O4@CuCo2O4//AC hierarchical nanowires exhibited a maximum energy density of 18.0 W h kg−1, which gradually decreased to 13.7 W h kg−1 as the power density increased from 125 W kg−1 to 3283 W kg−1. Our study reveals that this unique integrated nanostructure shall have great promise as superior electrochemical electrodes for the next generation electrochemical energy storage devices.Acknowledgements
The present work was financially supported by the Startup Research Fund of Zhengzhou University (51090104) and the National Natural Science Foundation of China (51602289, 11504331).References
- E. T. Mombeshora and V. O. Nyamori, Int. J. Energy Res., 2015, 39, 1955–1980 CrossRef CAS.
- J. B. Cheng, H. L. Yan, Y. Lu, K. W. Qiu, X. Y. Hou, J. Y. Xu, L. Han, X. M. Liu, J. K. Kim and Y. S. Luo, J. Mater. Chem. A, 2015, 3, 9769–9776 CAS.
- Q. Zhang, Y. H. Deng, Z. H. Hu, Y. F. Liu, M. M. Yao and P. P. Liu, Phys. Chem. Chem. Phys., 2014, 16, 23451–23460 RSC.
- Y. Zhang, J. Xu, Y. Y. Zheng, X. Y. Hu, Y. Y. Shang and Y. J. Zhang, RSC Adv., 2016, 6, 59976–59983 RSC.
- D. P. Cai, D. D. Wang, B. Liu, L. L. Wang, Y. Liu, H. Li, Y. R. Wang, Q. H. Li and T. H. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 5050–5055 CAS.
- J. Xu, S. L. Gai, F. He, N. Niu, P. Gao, Y. J. Chen and P. P. Yang, J. Mater. Chem. A, 2014, 2, 1022–1031 CAS.
- L. Y. Lin, T. M. Liu, J. L. Liu, R. Sun, J. H. Hao, K. M. Ji and Z. C. Wang, Appl. Surf. Sci., 2016, 360, 234–239 CrossRef CAS.
- R. J. Zou, M. F. Yuen, Z. Y. Zhang, J. Q. Hu and W. J. Zhang, J. Mater. Chem. A, 2015, 3, 1717–1723 CAS.
- S. Abouali, M. A. Garakani, Z. L. Xu and J. K. Kim, Carbon, 2016, 102, 262–272 CrossRef CAS.
- Q. F. Wang, D. Chen and D. H. Zhang, RSC Adv., 2015, 5, 96448–96454 RSC.
- A. Shanmugavani and R. K. Selvan, Electrochim. Acta, 2015, 188, 852–862 CrossRef.
- W. M. Zhang, Z. W. Zhou, X. Y. Shan, R. Xu, Q. Chen, G. Y. He, X. Q. Sun and H. Q. Chen, New J. Chem., 2016, 40, 4769–4774 RSC.
- J. L. Cheng, X. H. Li, Z. X. Wang, H. J. Guo, W. J. Peng and Q. Y. Hu, Ceram. Int., 2016, 42, 2871–2875 CrossRef CAS.
- Q. F. Wang, J. Xu, X. F. Wang, B. Liu, X. J. Hou, G. Yu, P. Wang, D. Chen and G. Z. Shen, ChemElectroChem, 2014, 1, 559–564 CrossRef.
- L. Q. Mai, F. Dong, X. Xu, Y. Z. Luo, Q. Y. An, Y. L. Zhao, J. Pan and J. N. Yang, Nano Lett., 2013, 13, 740–745 CrossRef CAS PubMed.
- F. X. Wang, Y. X. Zeng, D. Z. Zheng, C. Li, P. Liu, X. H. Lu and Y. X. Tong, Carbon, 2016, 103, 56–62 CrossRef CAS.
- Y. B. He, Y. L. Bai, X. F. Yang, J. Y. Zhang, L. P. Kang, H. Xu, F. Shi, Z. B. Lei and Z. H. Liu, J. Power Sources, 2016, 317, 10–18 CrossRef CAS.
- L. J. Zhang, K. N. Hui, K. S. Hui and H. Lee, J. Power Sources, 2016, 318, 76–85 CrossRef CAS.
- Y. Zhang, J. Xu, Y. J. Zhang and X. Y. Hu, J. Mater. Sci.: Mater. Electron., 2016, 27, 1–7 CrossRef.
- D. Z. Kong, C. W. Cheng, Y. Wang, J. I. Wong, Y. P. Yang and H. Y. Yang, J. Mater. Chem. A, 2015, 3, 16150–16161 CAS.
- X. Z. Wang, Y. H. Xiao, D. C. Su, L. M. Zhou, S. D. Wu, L. F. Han, S. M. Fang and S. K. Cao, Electrochim. Acta, 2016, 194, 377–384 CrossRef CAS.
- W. J. Ren, D. Guo, M. Zhou, B. K. Guan, D. Zhang and Q. H. Li, RSC Adv., 2015, 5, 21881–21887 RSC.
- X. Y. Liu, S. J. Shi, Q. Q. Xiong, L. Li, Y. J. Zhang, H. Tang, C. D. Gu, X. L. Wang and J. P. Tu, ACS Appl. Mater. Interfaces, 2013, 5, 8790–8795 CAS.
- H. C. Chen, S. Chen, H. Y. Shao, C. Li, M. Q. Fan, D. Chen, G. L. Tian and K. Y. Shu, Chem.–Asian J., 2016, 11, 248–255 CrossRef CAS PubMed.
- X. T. Zheng, Y. L. Ye, Q. Yang, B. Y. Geng and X. J. Zhang, Dalton Trans., 2016, 45, 572–578 RSC.
- D. P. Cai, B. Liu, D. D. Wang, L. L. Wang, Y. Liu, H. Li, Y. R. Wang, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2014, 2, 1954–4960 Search PubMed.
- T. Liu, H. Chai, D. Z. Jia, Y. Su, T. Wang and W. Y. Zhou, Electrochim. Acta, 2015, 180, 998–1006 CrossRef CAS.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed.
- C. Wu, J. J. Cai, Q. B. Zhang, X. Zhou, Y. Zhu, P. K. Shen and K. L. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 26512–26521 CAS.
- W. W. Zhou, D. Z. Kong, X. T. Jia, C. Y. Ding, C. W. Cheng and G. W. Wen, J. Mater. Chem. A, 2014, 2, 6310–6315 CAS.
- W. Zeng, G. H. Zhang, X. Wu, K. Zhang, H. Zhang, S. C. Hou, C. C. Li, T. H. Wang and H. G. Duan, J. Mater. Chem. A, 2015, 3, 24033–24040 CAS.
- G. L. Wang, J. C. Huang, S. L. Chen, Y. Y. Gao and D. X. Cao, J. Power Sources, 2011, 196, 5756–5760 CrossRef CAS.
- W. B. Fu, Y. L. Wang, W. H. Han, Z. M. Zhang, H. M. Zha and E. Q. Xie, J. Mater. Chem. A, 2016, 4, 173–182 CAS.
- G. Jiang, M. Y. Zhang, X. Q. Li and H. Gao, RSC Adv., 2015, 5, 69365–69370 RSC.
- X. D. Zhang, J. L. Xiao, X. Y. Zhang, Y. Meng and D. Xiao, Electrochim. Acta, 2016, 191, 758–766 CrossRef CAS.
- W. Chen, C. Xia and N. H. Alshareef, ACS Nano, 2014, 8, 9531–9541 CrossRef CAS PubMed.
- D. Cheng, Y. F. Yang, J. L. Xie, C. J. Fang, G. Q. Zhang and J. Xiong, J. Mater. Chem. A, 2015, 3, 14348–14357 CAS.
- C. C. Tu, L. Y. Lin, B. C. Xiao and Y. S. Chen, J. Power Sources, 2016, 320, 78–85 CrossRef CAS.
- A. Pendashteh, J. Palma, M. Anderson and R. Marcilla, J. Mater. Chem. A, 2015, 3, 16849–16859 CAS.
- Z. X. Gu, R. F. Wang, H. H. Nan, B. Y. Geng and X. J. Zhang, J. Mater. Chem. A, 2015, 3, 14578–14584 CAS.
- B. Q. Wang, Z. H. Wen and J. H. Li, Adv. Funct. Mater., 2006, 16, 2141–2146 CrossRef.
- Y. P. Lin and N. L. Wu, J. Power Sources, 2011, 196, 851–854 CrossRef CAS.
- M. Kuang, Z. Q. Wen, X. L. Guo, S. M. Zhang and Y. X. Zhang, J. Power Sources, 2014, 270, 426–433 CrossRef CAS.
- X. Wang, J. Y. Liu, Y. Y. Wang, C. M. Zhao and W. T. Zheng, Mater. Res. Bull., 2014, 52, 89–95 CrossRef CAS.
- X. Sun, M. Xie, J. J. Travis, G. K. Wang, H. T. Sun, J. Lian and S. M. George, J. Phys. Chem. C, 2013, 117, 22497–22508 CAS.
- X. Z. Yu, B. G. Lu and Z. Xu, Adv. Mater., 2014, 26, 1044–1051 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The electrochemical characterizations of CuCo2O4 and CuCo2O4@CuCo2O4 electrode; the high magnification SEM image of CuCo2O4@CuCo2O4 and typical SEM images of CuCo2O4 and CuCo2O4@CuCo2O4 electrode after cycling performance at different magnifications. See DOI: 10.1039/c6ra25970g |
| This journal is © The Royal Society of Chemistry 2017 |