DOI:
10.1039/C6RA25869G
(Paper)
RSC Adv., 2017,
7, 6523-6529
Synthesis of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of tomentosin†
Received
26th October 2016
, Accepted 5th January 2017
First published on 19th January 2017
Abstract
A series of novel enantiomerically pure spiro-isoxazolidines and spiro-isoxazolines were synthesized regioselectively by 1,3-dipolar cycloaddition using respectively two dipoles, nitrones and nitrile oxides, on the exocyclic double bond of the B ring of tomentosin (α-methylene-γ-butyrolactone), a sesquiterpene lactone extracted from Dittrichia viscosa.
Introduction
Plants have a long history as therapeutics in the treatment of human diseases and have been a continuous source of inspiration for the development of new medicines. Among them, the genus Inula (Asteraceae) comprises more than 100 species, many of which are widely used in traditional medicine for a variety of biological purposes including anti-inflammatory, anti-cancer and antibacterial activities.1–3 Numerous compounds of interest have been isolated and identified from these plants such as flavonoids, monoterpenes, triterpenoids, and polyphenols. This genus is also a rich source of sesquiterpene acids and lactones. Many studies have focused on sesquiterpene lactones since they exhibit a wide range of biological properties4–6 and have candidates in different phases of clinical trials such as parthenolide, costunolide, helenalin, and artemisinin (Fig. 1). The cytotoxicity of sesquiterpene lactones was partly attributed to the presence of potential alkylating agents such as the α-methylene-γ-lactone moiety, which are prone to covalently react with biological nucleophiles, e.g., L-cysteine, in a Michael-type addition.7–12 This highly electrophilic structure may also be the origin of a major contact allergen effect and plants that contain sesquiterpene lactones are held responsible for an increasing number of cases of contact dermatitis.13–15
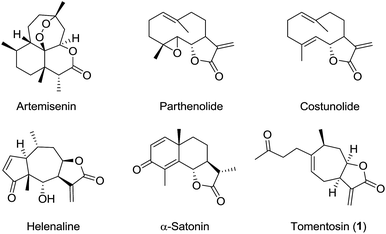 |
| | Fig. 1 Examples of biologically active sesquiterpene lactones. | |
Using this reactive site, various structural modifications have been carried out to obtain less toxic and more reactive candidates and lately the introduction of spiro-heterocyclic molecular frameworks has aroused particular interest among medicinal chemists.16,17 For example, the Ding and Kumar groups18–20 recently synthesized spiro-isoxazoline and spiro-isoxazolidine derivatives of parthenin, α-santonin and artemisinin and promising anti-cancer activities were obtained. As part of the Moroccan plant development program,21–25 Dittrichia viscosa L. Greuter, an invasive perennial weed, was particularly examined.26,27 This plant is used either as extracts or essential oil in traditional Moroccan medicine for its antipyretic, antiseptic and anti-inflammatory properties.28,29 Easily accessible, it is a renewable source of sesquiterpene lactones such as tomentosin (1),30,31 also known as xanthalongin. This molecule is straightforwardly isolated in respectively 1.5% with respect to the dry weight of the aerial part of the plant (Fig. 1).32,33 Tomentosin was already reported to act as a cytotoxic and anti-inflammatory agent34–36 but despite this biological potential, it has received little pharmacological attention so far. Therefore, we propose herein the introduction of an isoxazoline and an isoxazolidine functionality to form libraries of structurally original spiro-bicyclic analogues of tomentosin by 1,3-dipolar cycloaddition with two dipoles, nitrones and nitrile oxides on the exocyclic double bond of the B ring of tomentosin (Fig. 2).
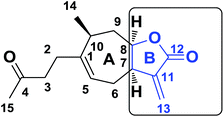 |
| | Fig. 2 Sesquiterpene lactones, ring B of tomentosin. | |
Results and discussion
The spiroisoxazoline derivatives of tomentosin were synthesized through a 1,3-dipolar cycloaddition of various aldoximes 2 with the exocyclic ring B double bond (Scheme 1).
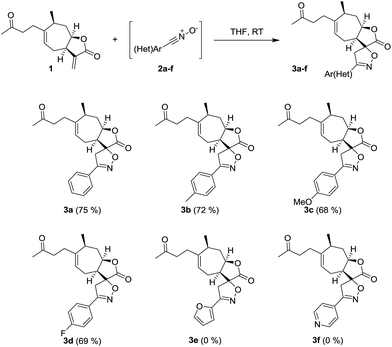 |
| | Scheme 1 Synthesis of spiro-isoxazoline derivatives of tomentosin. | |
Nitrile oxides 2 were prepared by converting various aromatic aldehydes to the corresponding oximes via the reaction with hypochlorite anion present in bleach. The bleach was used in two steps: initially to produce chlorooxime and then thanks to its basicity to induce dehydrohalogenation, leading to the nitrile oxide. Experimentally, aldoxime was mixed with the tomentosin in THF, and then a bleach solution (14.5% of chlorine) was added dropwise during 12 hours. Only one diastereoisomer was isolated and characterized by NMR spectroscopic analysis and mass spectrometry. The 1H NMR spectra of the spiroisoxazoline derivatives of tomentosin 3a showed the disappearance of the alkene protons along with the appearance of two doublets at respectively 3.42 and 3.58 ppm that confirm the selectivity of the nitrile oxide cycloaddition. The reaction took place whatever the substituent in the para position of the aryl entity, whether electron donating (CH3, OCH3) or electron-attracting (F). When a poor or electron-rich heteroaryl was used, the reaction did not take place and only the starting material was recovered.
The spiroisoxazolidine derivatives of tomentosin were obtained through 1,3-dipolar cycloaddition of various nitrones 4 in refluxing dry benzene (Scheme 2).37–39 Nitrones 4 were straightforwardly obtained according to the literature procedure in which nitroaryls were first reduced in the presence of zinc and acetic acid to obtain the corresponding aryl hydroxylamines that were condensed with various aromatic aldehydes.40
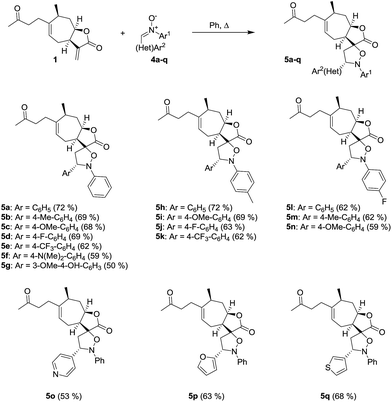 |
| | Scheme 2 Synthesis of spiro-isoxazolidine derivatives of tomentosin. | |
The spiro-isoxazolidines 5 were obtained as one diastereomer after purification by flash chromatography. The operating conditions are compatible with the introduction of nitrones with aryl entities bearing electron-donating (CH3, OCH3) or electron-attracting (CF3, F) substituents. The use of nitrones with heteroaryl entities was carried out successfully. It should be noted that the use of toluene instead of benzene, for environmental reasons, did not unfortunately allow us to obtain the products. The structures of the spiroisoxazolidines were confirmed by their 1H, 13C and 2 D NMR spectroscopic data as described for 5q (Fig. 3). The 1H and 1H-COSY data showed the correlation of H-7 with H-8 and H-8 with H-16. Further, the HMBC experiment showed the correlation of H-7 and H-8 with C-17 and H-7 with C-16 (Fig. 3).
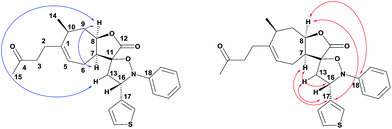 |
| | Fig. 3 Selected 1H, 1H–COSY and HMBC correlations of 5q. | |
In the 1H NMR comparison with the literature data, Reddy et al.18 obtained two diastereomers. Each diastereomer was isolated and the clear chemical shift deviation of the benzylic proton adjacent to the nitrogen atom in the isoxazolidine ring between two diastereomers was observed in 1H NMR. In the major isomer this proton appeared at 5 ppm, but in the minor isomer this signal shifted toward a more shielding region and appeared approximately at 4 ppm.
Experimental section
General information
All reagents were purchased from commercial suppliers and were used without further purification. The reactions were monitored by thin-layer chromatography (TLC) analysis using silica gel (60 F254) plates. Compounds were visualized by UV irradiation. Flash column chromatography was performed on silica gel 60 (230–400 mesh, 0.040–0.063 mm). 1H and 13C NMR spectra were recorded on a Bruker AVANCE II spectrometer at 250 MHz (13C, 62.9 MHz) and on a Bruker AVANCE III HD nanobay at 400 MHz (13C 101 MHz). Chemical shifts are given in parts per million from tetramethylsilane (TMS) or deuterated solvents (MeOH-d4, CDCl3) as internal standard. The following abbreviations were used for the proton spectra multiplicities: b: broad, s: singlet, d: doublet, t: triplet, q: quartet, p: pentuplet, m: multiplet. Coupling constants (J) are reported in hertz (Hz). High-resolution mass spectra (HRMS (ESI)) were performed on a Maxis Bruker 4G.
General procedure for the synthesis of spiro-isoxazolines
The appropriate aldehyde (1 equiv.) was diluted in CH2Cl2 (10 mL) and stirred at room temperature. Hydroxylamine (2 equiv.) and NaOH (2 equiv.) were added and the mixture was heated to reflux for 4 h. Ethanol was evaporated under reduced pressure then the crude mixture was diluted with water (10 mL) and AcOEt (10 mL) then extracted with AcOEt (3 × 10 mL). The combined organic layers were dried with MgSO4, filtered and concentrated under reduced pressure to lead to the aldoxime intermediate. The aldoxime was diluted in THF (10 mL) then tomentosin (0.2 equiv.) and bleach (5 mL) were added dropwise. The reaction mixture was stirred at room temperature for 12 h. The mixture was diluted with water (10 mL) and extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried with MgSO4, filtered and concentrated under reduced pressure providing a crude product, which was purified by flash chromatography on silica gel.
13-[Amino, phenyl-methyl]-11, N-epoxy-tomentosin (3a). Following the general procedure, using tomotensin (80 mg, 0.32 mmol), column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 3a (88 mg, 0.24 mmol, 75%) as a yellow oil; [α]20D −38.8 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.66 (dt, J = 7.6, 1.4 Hz, 2H), 7.46–7.36 (m, 3H), 5.43 (dd, J = 9.4, 3.5 Hz, 1H), 4.91 (ddd, J = 11.3, 6.5, 4.5 Hz, 1H), 3.58 (d, J = 16.8 Hz, 1H), 3.42 (d, J = 16.9 Hz, 1H), 2.84 (ddd, J = 13.1, 6.7, 3.1 Hz, 1H), 2.59–2.15 (m, 7H), 2.13 (s, 3H), 1.94 (ddd, J = 23.2, 11.4, 7.4 Hz, 2H), 1.16 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 173.7 (C), 156.1 (C), 145.1 (C), 130.8 (C), 128.9 (2 CH), 128.5 (CH), 127.0 (2 CH), 120.7 (CH), 89.0 (C), 80.1 (CH), 46.1 (CH), 42.6 (CH2), 37.6 (CH2), 36.6 (CH2), 33.1 (CH), 30.8 (CH2), 30.1 (CH3), 22.7 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C22H25NO4 [M + H]+ 368.1856; found 368.1856.
13-[Amino, (p-tolyl)-methyl]-11, N-epoxy-tomentosin (3b). Following the general procedure, using tomotensin (80 mg, 0.32 mmol), column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 3b (89 mg, 0.23 mmol, 72%) as a colorless oil; [α]20D +46.0 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 7.9 Hz, 2H), 5.43 (dd, J = 9.4, 3.5 Hz, 1H), 4.90 (ddd, J = 11.4, 6.5, 4.6 Hz, 1H), 3.56 (d, J = 16.8 Hz, 1H), 3.40 (d, J = 17.0 Hz, 1H), 2.84 (ddd, J = 13.0, 6.6, 3.0 Hz, 1H), 2.59–2.39 (m, 3H), 2.38 (s, 3H), 2.36–2.15 (m, 4H), 2.13 (s, 3H), 2.02–1.86 (m, 2H), 1.16 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 173.8 (C), 156.1 (C), 145.1 (C), 141.1 (C), 129.6 (2 CH), 127.0 (2 CH), 125.7 (C), 120.7 (CH), 88.8 (C), 80.1 (C), 46.2 (CH), 42.6 (CH2), 37.7 (CH2), 36.6 (CH2), 33.1 (CH), 30.8 (CH2), 30.1 (CH3), 22.7 (CH2), 21.6 (CH3), 21.2 (CH3); HRMS (ESI+): calc. For C23H27NO4 [M + H]+ 382.2012; found 382.2012.
13-[Amino, (4-methoxyphenyl)-methyl]-11, N-epoxy-tomentosin (3c). Following the general procedure, using tomotensin (80 mg, 0.32 mmol), column chromatography on silica gel (petroleum ether/ethyl acetate 5/5) provided 3c (87 mg, 0.22 mmol, 68%) as a colorless oil; [α]20D +41.9 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.63–7.57 (m, 2H), 6.95–6.90 (m, 2H), 5.43 (d, J = 3.7 Hz, 1H), 4.92 (ddd, J = 11.3, 6.6, 4.4 Hz, 1H), 3.85 (s, 3H), 3.56 (d, J = 16.8 Hz, 1H), 3.39 (d, J = 16.8 Hz, 1H), 2.84 (ddd, J = 13.0, 6.6, 3.1 Hz, 1H), 2.61–2.15 (m, 7H), 2.14 (s, 3H), 2.03–1.87 (m, 2H), 1.17 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 173.9 (C), 161.6 (C), 155.7 (C), 145.2 (C), 128.6 (2 CH), 121.1 (C), 120.8 (CH), 114.4 (2 CH), 88.7 (C), 80.1 (CH), 55.5 (CH3), 46.3 (CH), 42.6 (CH2), 37.9 (CH2), 36.6 (CH2), 33.1 (CH), 30.8 (CH2), 30.1 (CH3), 22.8 (CH2), 21.3 (CH3); HRMS (ESI+): calc. For C23H27NO5 [M + H]+ 398.1962; found 398.1961.
13-[Amino, (4-fluorophenyl)-methyl]-11, N-epoxy-tomentosin (3d). Following the general procedure, using tomotensin (80 mg, 0.32 mmol), column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 3d (86 mg, 0.22 mmol, 69%) as a colorless oil; [α]20D +76.0 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 8.6, 5.3 Hz, 2H), 7.10 (t, J = 8.5 Hz, 2H), 5.43 (dd, J = 9.4, 3.5 Hz, 1H), 4.91 (ddd, J = 11.3, 6.6, 4.5 Hz, 1H), 3.54 (d, J = 16.8 Hz, 1H), 3.40 (d, J = 16.9 Hz, 1H), 2.84 (ddd, J = 13.0, 6.6, 3.0 Hz, 1H), 2.60–2.14 (m, 7H), 2.13 (s, 3H), 2.01–1.87 (m, 2H), 1.16 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 207.9 (C), 173.6 (C), 164.1 (d, J = 251.7 Hz, C), 155.2 (C), 145.2 (C), 129.0 (d, J = 8.5 Hz, 2 CH), 124.9 (d, J = 3.5 Hz, CH), 120.6 (CH), 116.1 (d, J = 22.0 Hz, 2 CH), 89.1 (C), 80.14 (CH), 46.1 (CH), 42.6 (CH2), 37.6 (CH2), 36.6 (CH2), 33.1 (CH), 30.8 (CH2), 30.1 (CH3), 22.7 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C22H24FNO4 [M + H]+ 386.1762; found 386.1762.
General procedure for the synthesis of spiro-isoxazolidines
The appropriate nitrone (1.1 equiv.) was added to a solution of tomentosin (1 equiv.) in benzene (2 mL). The resulting suspension was heated to reflux for 12 h. Then the reaction mixture was concentrated under reduced pressure and the crude material was purified by flash chromatography on silica gel to provide the expected spiro-isoxasolidine.
13-[(Phenylamine), phenyl-methyl]-11, N-epoxy-tomentosin (5a). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (70 mg, 0.35 mmol), column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5a (104 mg, 0.23 mmol, 72%) as a yellowish oil; [α]20D +45.6 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 7.8 Hz, 2H), 7.41–7.35 (m, 2H), 7.34–7.28 (m, 1H), 7.21–7.12 (m, 2H), 6.96–6.86 (m, 3H), 5.44–5.37 (m, 1H), 5.14–5.07 (m, 1H), 4.96–4.89 (m, 1H), 2.85 (td, J = 12.3, 11.0, 5.4 Hz, 2H), 2.60–2.17 (m, 7H), 2.15 (s, 3H), 2.13–2.07 (m, 1H), 1.92 (ddd, J = 19.2, 12.8, 7.4 Hz, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.2 (C), 174.9 (C), 151.4 (C), 145.0 (C), 140.7 (C), 129.3 (2 CH), 128.9 (2 CH), 128.2 (CH), 126.9 (2 CH), 122.8 (CH), 121.4 (CH), 115.8 (2 CH), 85.6 (C), 80.0 (CH), 70.4 (CH), 46.0 (CH), 43.9 (CH2), 42.9 (CH2), 36.9 (CH2), 33.4 (CH), 31.0 (CH2), 30.3 (CH3), 23.1 (CH2), 21.4 (CH3); HRMS (ESI+): calc. For C28H31NO4 [M + H]+ 446.2325; found 446.2325.
13-[(Phenylamine), (p-tolyl)-methyl]-11, N-epoxy-tomentosin (5b). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (74 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5b (104 mg, 0.22 mmol, 69%) as a yellowish oil; [α]20D +59.7 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 7.7 Hz, 2H), 7.17 (ddd, J = 13.8, 6.8, 2.7 Hz, 4H), 6.96–6.87 (m, 3H), 5.42 (dd, J = 9.4, 3.5 Hz, 1H), 5.06 (dd, J = 9.5, 6.7 Hz, 1H), 4.91 (ddd, J = 11.4, 6.5, 4.4 Hz, 1H), 2.91–2.76 (m, 2H), 2.60–2.37 (m, 4H), 2.36 (s, 3H), 2.34–2.17 (m, 3H), 2.15 (s, 3H), 2.13–2.06 (m, 1H), 1.93 (dt, J = 14.5, 11.5 Hz, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.8 (C), 151.3 (C), 144.7 (C), 137.8 (C), 137.4 (C), 129.8 (2 CH), 128.7 (2 CH), 126.7 (2 CH), 122.6 (CH), 121.1 (CH), 115.7 (2 CH), 85.3 (C), 79.7 (CH), 70.1 (CH), 45.8 (CH), 43.8 (CH2), 42.7 (CH2), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.1 (CH3), 22.9 (CH2), 21.2 (CH3), 21.2 (CH3); HRMS (ESI+): calc. For C29H33NO4 [M + H]+ 460.2480; found 460.2482.
13-[(Phenylamine), (4-methoxyphenyl)-methyl]-11, N-epoxy-tomentosin (5c). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (80 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 5/5) provided 5c (103 mg, 0.22 mmol, 68%) as a yellow oil; [α]20D +38.0 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.43–7.35 (m, 2H), 7.16 (t, J = 7.4 Hz, 2H), 6.97–6.87 (m, 5H), 5.42 (dd, J = 9.3, 3.5 Hz, 1H), 5.03 (dd, J = 9.5, 6.7 Hz, 1H), 4.90 (ddd, J = 11.4, 6.7, 4.5 Hz, 1H), 3.81 (s, 3H), 2.87–2.78 (m, 2H), 2.60–2.17 (m, 7H), 2.15 (s, 3H), 2.10 (dt, J = 13.9, 4.2 Hz, 1H), 1.99–1.84 (m, 2H), 1.14 (d, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.9 (C), 159.4 (C), 151.2 (C), 144.7 (C), 132.1 (C), 128.7 (2 CH), 128.0 (2 CH), 122.8 (CH), 121.1 (CH), 116.0 (2 CH), 114.5 (2 CH), 85.2 (C), 79.7 (CH), 70.0 (CH), 55.4 (CH3), 45.8 (CH), 43.7 (CH2), 42.7 (CH2), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.1 (CH3), 22.9 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C29H33NO5 [M + H]+ 476.2429; found 476.2431.
13-[(Phenylamine), (4-fluorophenyl)-methyl]-11, N-epoxy-tomentosin (5d). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (76 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5d (103 mg, 0.22 mmol, 69%) as a yellow oil; [α]20D −78.6 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.47 (dd, J = 8.3, 5.3 Hz, 2H), 7.24–7.17 (m, 2H), 7.09 (t, J = 8.4 Hz, 2H), 6.99–6.89 (m, 3H), 5.44 (dd, J = 9.4, 3.4 Hz, 1H), 5.11 (dd, J = 9.4, 6.7 Hz, 1H), 4.94 (ddd, J = 11.4, 6.6, 4.5 Hz, 1H), 2.86 (ddd, J = 19.2, 9.8, 4.8 Hz, 2H), 2.62–2.20 (m, 7H), 2.17 (s, 3H), 2.11 (t, J = 4.2 Hz, 1H), 2.01–1.86 (m, 2H), 1.17 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.7 (C), 162.5 (d, J = 246.4 Hz, C), 151.0 (C), 144.8 (C), 136.1 (d, J = 3.2 Hz, C), 128.8 (2 CH), 128.4 (d, J = 8.0 Hz, 2 CH), 122.9 (CH), 121.1 (CH), 116.0 (d, J = 21.6 Hz, 2 CH), 115.8 (2 CH), 85.4 (C), 79.8 (CH), 69.6 (CH), 45.8 (CH), 43.6 (CH2), 42.6 (CH2), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.0 (CH3), 22.9 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C28H30FNO4 [M + H]+ 464.2228; found 464.2231.
13-[(Phenylamine), (4-trifluoromethylphenyl)-methyl]-11, N-epoxy-tomentosin (5e). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (94 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 5/5) provided 5e (102 mg, 0.20 mmol, 62%) as a colorless oil; [α]20D +85.6 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.68–7.59 (m, 4H), 7.24–7.15 (m, 2H), 6.98–6.92 (m, 1H), 6.88 (d, J = 7.9 Hz, 2H), 5.40 (dd, J = 9.4, 3.4 Hz, 1H), 5.23–5.16 (m, 1H), 4.94 (ddd, J = 11.4, 6.3, 4.6 Hz, 1H), 2.91–2.80 (m, 2H), 2.59–2.17 (m, 7H), 2.15 (s, 3H), 2.10 (q, J = 4.7, 4.1 Hz, 1H), 2.00–1.82 (m, 2H), 1.15 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.5 (C), 151.0 (C), 144.9 (C), 145.0 (C), 130.4 (d, J = 32.4 Hz, C), 128.9 (2 CH), 127.1 (2 CH), 126.1 (q, J = 3.8 Hz, C), 124.1 (d, J = 273.2 Hz, 2 CH), 122.9 (CH), 121.0 (CH), 115.4 (2 CH), 85.6 (C), 79.9 (CH), 69.6 (CH), 45.8 (CH), 43.4 (CH2), 42.6 (CH2), 36.7 (CH2), 33.1 (CH), 30.8 (CH2), 30.1 (CH3), 23.0 (CH2), 21.3 (CH3); HRMS (ESI+): calc. For C29H30F3NO4 [M + H]+ 514.2197; found 514.2199.
13-[(Phenylamine), (4-dimethylaminophenyl)-methyl]-11, N-epoxy-tomentosin (5f). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (85 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 3/7) provided 5f (93 mg, 0.19 mmol, 59%) as a brown oil; [α]20D +48.9 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.4 Hz, 2H), 7.16 (t, J = 7.7 Hz, 2H), 6.98–6.89 (m, 3H), 6.73 (d, J = 8.4 Hz, 2H), 5.43 (dd, J = 9.3, 3.5 Hz, 1H), 4.98 (dd, J = 9.7, 6.5 Hz, 1H), 4.89 (ddd, J = 11.4, 6.9, 4.3 Hz, 1H), 2.96 (s, 6H), 2.89–2.75 (m, 2H), 2.60–2.18 (m, 7H), 2.15 (s, 3H), 2.10 (dd, J = 10.5, 3.4 Hz, 1H), 1.95 (ddd, J = 21.0, 9.5, 2.7 Hz, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 175.0 (C), 151.3 (C), 150.4 (C), 144.7 (C), 128.6 (2 CH), 127.7 (2 CH), 127.3 (C), 122.7 (CH), 121.2 (CH), 116.2 (2 CH), 112.9 (2 CH), 85.1 (C), 79.6 (CH), 70.2 (CH), 45.8 (C), 43.8 (CH2), 42.7 (CH2), 40.7 (2 CH3), 36.7 (CH2), 33.3 (CH), 30.8 (CH2), 30.1 (CH3), 22.8 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C30H36N2O4 [M + H]+ 489.2744; found 489.2747.
13-[(Phenylamine), ((4-hydroxy-3-methoxy)phenyl)-methyl]-11, N-epoxy-tomentosin (5g). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (86 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 3/7) provided 5g (78 mg, 0.16 mmol, 50%) as an orange oil; [α]20D −68.0 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.21–7.14 (m, 2H), 7.01 (d, J = 1.9 Hz, 1H), 6.98–6.89 (m, 5H), 5.67 (s, 1H), 5.45–5.39 (m, 1H), 5.03 (dd, J = 9.6, 6.5 Hz, 1H), 4.91 (ddd, J = 11.3, 6.7, 4.3 Hz, 1H), 3.88 (s, 3H), 2.87–2.77 (m, 2H), 2.60–2.17 (m, 7H), 2.14 (s, 3H), 2.10 (dt, J = 13.7, 4.1 Hz, 1H), 1.99–1.88 (m, 2H), 1.14 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.1 (C), 174.8 (C), 151.3 (C), 147.1 (C), 145.4 (C), 144.8 (C), 132.1 (C), 128.7 (2 CH), 122.7 (CH), 121.2 (CH), 119.7 (CH), 115.8 (2 CH), 114.8 (CH), 108.9 (CH), 85.3 (C), 79.7 (CH), 70.3 (CH), 56.2 (CH3), 45.8 (CH), 43.8 (CH2), 42.6 (CH2), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.1 (CH3), 22.9 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C29H33NO6 [M + H]+ 492.2381; found 492.2380.
13-[(p-Tolylamine), phenyl-methyl]-11, N-epoxy-tomentosin (5h). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (74 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5h (105 mg, 0.23 mmol, 72%) as a yellowish oil; [α]20D −34.8 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.4 Hz, 2H), 7.40–7.33 (m, 2H), 7.33–7.27 (m, 1H), 6.98 (d, J = 8.1 Hz, 2H), 6.86 (d, J = 8.2 Hz, 2H), 5.41 (dd, J = 9.4, 3.5 Hz, 1H), 5.04 (dd, J = 9.5, 6.6 Hz, 1H), 4.90 (ddd, J = 11.3, 6.8, 4.4 Hz, 1H), 2.89–2.78 (m, 2H), 2.60–2.25 (m, 7H), 2.23 (s, 3H), 2.15 (s, 3H), 2.10 (dt, J = 13.8, 4.1 Hz, 1H), 1.99–1.87 (m, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.9 (C), 148.6 (C), 144.7 (C), 140.1 (C), 132.6 (C), 129.3 (2 CH), 129.0 (2 CH), 128.0 (CH), 126.9 (2 CH), 121.1 (CH), 116.6 (2 CH), 85.2 (C), 79.7 (CH), 70.5 (CH), 45.9 (CH), 43.7 (CH2), 42.7 (CH2), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.0 (CH3), 22.8 (CH2), 21.2 (CH3), 20.8 (CH3); HRMS (ESI+): calc. For C29H33NO4 [M + H]+ 460.2481; found 460.2482.
13-[(p-Tolylamine), (4-methoxyphenyl)-methyl]-11, N-epoxy-tomentosin (5i). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (85 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 5/5) provided 5i (108 mg, 0.22 mmol, 69%) as a brown oil; [α]20D +94.3 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 8.2 Hz, 2H), 6.88 (dd, J = 10.0, 8.3 Hz, 4H), 5.42 (dd, J = 9.4, 3.5 Hz, 1H), 4.96 (dd, J = 9.7, 6.4 Hz, 1H), 4.88 (ddd, J = 11.4, 6.8, 4.3 Hz, 1H), 3.80 (d, J = 1.1 Hz, 3H), 2.86–2.75 (m, 2H), 2.60–2.25 (m, 7H), 2.23 (s, 3H), 2.15 (s, 3H), 2.09 (dt, J = 13.7, 4.3 Hz, 1H), 1.99–1.86 (m, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 175.1 (C), 159.4 (C), 148.4 (C), 144.7 (C), 132.8 (C), 131.6 (C), 129.3 (2 CH), 128.2 (2 CH), 121.1 (CH), 117.1 (2 CH), 114.4 (2 CH), 85.0 (C), 79.7 (CH), 70.3 (CH), 55.4 (CH3), 46.0 (CH), 43.7 (CH2), 42.7 (CH2), 36.7 (CH2), 33.3 (CH), 30.8 (CH2), 30.0 (CH3), 22.8 (CH2), 21.2 (CH3), 20.8 (CH3); HRMS (ESI+): calc. For C30H35NO5 [M + H]+ 490.2586; found 490.2588.
13-[(p-Tolylamine), (4-fluorophenyl)-methyl]-11, N-epoxy-tomentosin (5j). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (81 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5j (96 mg, 0.20 mmol, 63%) as a yellowish oil; [α]20D +55.8 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.43 (dd, J = 8.4, 5.3 Hz, 2H), 7.05 (t, J = 8.5 Hz, 2H), 6.98 (d, J = 8.2 Hz, 2H), 6.84 (d, J = 8.2 Hz, 2H), 5.42 (dd, J = 9.4, 3.5 Hz, 1H), 5.02 (dd, J = 9.6, 6.4 Hz, 1H), 4.89 (ddd, J = 11.4, 6.8, 4.4 Hz, 1H), 2.85–2.78 (m, 2H), 2.60–2.25 (m, 7H), 2.23 (s, 3H), 2.15 (s, 3H), 2.10 (dt, J = 13.7, 4.1 Hz, 1H), 1.98–1.86 (m, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.9 (C), 162.5 (d, J = 246.4 Hz, C), 148.3 (C), 144.8 (C), 135.7 (d, J = 3.2 Hz, C), 133.0 (C), 129.4 (2 CH), 128.6 (d, J = 8.1 Hz, 2 CH), 121.1 (CH), 116.9 (2 CH), 115.9 (d, J = 21.5 Hz, 2 CH), 85.2 (C), 79.8 (CH), 70.0 (CH), 46.0 (CH), 43.6 (CH2), 42.7 (CH2), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.0 (CH3), 22.9 (CH2), 21.2 (CH3), 20.8 (CH3); HRMS (ESI+): calc. For C29H32FNO4 [M + H]+ 478.2387; found 478.2388.
13-[(p-Tolylamine), (4-trifluoromethylphenyl)-methyl]-11, N-epoxy-tomentosin (5k). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (98 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 5/5) provided 5k (104 mg, 0.20 mmol, 62%) as a yellowish oil; [α]20D +98.7 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.63 (t, J = 6.1 Hz, 4H), 7.00 (d, J = 8.1 Hz, 2H), 6.83 (d, J = 8.1 Hz, 2H), 5.40 (dd, J = 9.5, 3.4 Hz, 1H), 5.13 (dd, J = 9.4, 6.7 Hz, 1H), 4.91 (ddd, J = 11.3, 6.7, 4.5 Hz, 1H), 2.84 (dd, J = 12.6, 6.6 Hz, 2H), 2.59–2.25 (m, 7H), 2.24 (s, 3H), 2.15 (s, 3H), 2.13–2.06 (m, 1H), 1.91 (dd, J = 13.7, 11.3 Hz, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.7 (C), 148.3 (C), 144.9 (C), 144.5 (C), 133.0 (C), 130.3 (d, J = 32.5 Hz, C), 129.5 (2 CH), 127.2 (2 CH), 126.0 (q, J = 3.7 Hz, 2 CH), 124.1 (d, J = 272.1 Hz, C), 121.0 (CH), 116.5 (2 CH), 85.4 (C), 79.9 (CH), 69.9 (CH), 45.9 (CH), 43.5 (CH), 42.6 (CH), 36.7 (CH2), 33.2 (CH), 30.8 (CH2), 30.0 (CH3), 22.9 (CH2), 21.2 (CH3), 20.8 (CH3); HRMS (ESI+): calc. For C30H32F3NO4 [M + H]+ 528.2355; found 528.2356.
13-[(4-Fluorophenylamine), phenyl-methyl]-11, N-epoxy-tomentosin (5l). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (76 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5l (102 mg, 0.22 mmol, 68%) as a yellowish oil; [α]20D +57.9 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.48–7.43 (m, 2H), 7.40–7.28 (m, 3H), 6.96–6.83 (m, 4H), 5.45–5.37 (m, 1H), 4.99 (dd, J = 9.8, 6.4 Hz, 1H), 4.87 (ddd, J = 11.4, 7.0, 4.1 Hz, 1H), 2.89–2.78 (m, 2H), 2.62–2.18 (m, 7H), 2.15 (s, 3H), 2.10 (dt, J = 13.8, 4.2 Hz, 1H), 2.00–1.83 (m, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 175.1 (C), 159.3 (d, J = 241.6 Hz, C), 147.0 (d, J = 2.5 Hz, C), 144.8 (C), 139.5 (C), 129.1 (2 CH), 128.3 (CH), 127.0 (2 CH), 120.9 (CH), 118.6 (d, J = 8.0 Hz, 2 CH), 115.4 (d, J = 22.6 Hz, 2 CH), 85.1 (C), 79.7 (CH), 71.1 (CH), 45.7 (CH), 43.9 (CH2), 42.7 (CH2), 36.7 (CH2), 33.5 (CH), 30.8 (CH2), 30.1 (CH3), 22.8 (CH2), 21.2 (CH3); HRMS (ESI+): calc. For C28H30FNO4 [M + H]+ 464.2231; found 464.2231.
13-[(4-Fluorophenylamine), (p-tolyl)-methyl]-11, N-epoxy-tomentosin (5m). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (81 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5m (95 mg, 0.20 mmol, 62%) as a yellowish oil; [α]20D +66.4 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.36–7.30 (m, 2H), 7.17 (d, J = 7.8 Hz, 2H), 6.97–6.82 (m, 4H), 5.42 (dd, J = 9.2, 3.7 Hz, 1H), 4.94 (dd, J = 9.9, 6.3 Hz, 1H), 4.85 (ddd, J = 11.4, 7.0, 4.1 Hz, 1H), 2.90–2.75 (m, 2H), 2.60–2.40 (m, 5H), 2.35 (s, 3H), 2.32–2.19 (m, 2H), 2.15 (s, 3H), 2.10 (dt, J = 13.7, 4.2 Hz, 1H), 2.02–1.85 (m, 2H), 1.14 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 175.2 (C), 159.3 (d, J = 241.5 Hz, C), 147.0 (d, J = 2.5 Hz, C) 144.8 (C), 138.1 (C), 136.3 (C), 129.8 (2 CH), 127.0 (2 CH), 120.9 (CH), 118.8 (d, J = 7.9 Hz, 2 CH), 115.3 (d, J = 22.5 Hz, 2 CH), 85.0 (C), 79.7 (CH), 70.9 (CH), 45.7 (CH), 44.0 (CH2), 42.7 (CH2), 36.7 (CH2), 33.5 (CH), 30.8 (CH2), 30.1 (CH3), 22.7 (CH2), 21.3 (CH3), 21.1 (CH3); HRMS (ESI+): calc. For C29H32FNO4 [M + H]+ 478.2386; found 478.2388.
13-[(4-Fluorophenylamine), (4-methoxyphenyl)-methyl]-11, N-epoxy-tomentosin (5n). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (86 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 5/5) provided 5n (94 mg, 0.19 mmol, 59%) as a yellowish oil; [α]20D +71.0 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.38–7.32 (m, 2H), 6.98–6.82 (m, 6H), 5.46–5.40 (m, 1H), 4.93–4.80 (m, 2H), 3.81 (s, 3H), 2.89–2.72 (m, 2H), 2.61–2.18 (m, 7H), 2.15 (s, 3H), 2.10 (dt, J = 13.7, 4.2 Hz, 1H), 2.03–1.85 (m, 2H), 1.14 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 175.3 (C), 159.6 (C), 159.4 (d, J = 241.7 Hz, C), 146.9 (d, J = 2.5 Hz, C), 144.8 (C), 130.9 (C), 128.3 (2 CH), 120.9 (CH), 119.2 (d, J = 8.0 Hz, 2 CH), 115.3 (d, J = 22.5 Hz, 2 CH), 114.5 (2 CH), 84.9 (C), 79.6 (CH), 70.9 (CH), 55.4 (CH3), 45.7 (CH), 43.9 (CH2), 42.7 (CH2), 36.7 (CH2), 33.6 (CH), 30.8 (CH2), 30.1 (CH3), 22.7 (CH2), 21.1 (CH3); HRMS (ESI+): calc. For C29H32FNO5 [M + H]+ 494.2336; found 494.2337.
13-[(Phenylamine), (pyridin-4-yl)-methyl]-11, N-epoxy-tomentosin (5o). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (70 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 2/8) provided 5o (76 mg, 0.17 mmol, 53%) as a yellowish oil; [α]20D +15.1 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 8.66–8.58 (m, 2H), 7.52–7.39 (m, 2H), 7.23–7.15 (m, 2H), 7.01–6.82 (m, 3H), 5.40 (dd, J = 9.2, 3.4 Hz, 1H), 5.14 (dd, J = 9.1, 7.0 Hz, 1H), 4.95 (ddd, J = 11.3, 6.6, 4.5 Hz, 1H), 2.89–2.82 (m, 2H), 2.66–2.17 (m, 7H), 2.14 (s, 3H), 2.13–2.07 (m, 1H), 2.00–1.80 (m, 2H), 1.14 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 207.9 (C), 174.3 (C), 150.8 (C), 150.6 (2 CH), 150.0 (C), 145.0 (C), 129.0 (2 CH), 122.9 (CH), 121.6 (2 CH), 121.0 (CH), 115.1 (2 CH), 85.8 (C), 80.0 (CH), 68.9 (CH), 45.7 (CH), 42.8 (CH2), 42.6 (CH2), 36.7 (CH2), 33.1 (CH), 30.8 (CH2), 30.1 (CH3), 23.0 (CH2), 21.3 (CH3); HRMS (ESI+): calc. For C27H30N2O4 [M + H]+ 447.2277; found 447.2278.
13-[(Phenylamine), (furan-2-yl)-methyl]-11, N-epoxy-tomentosin (5p). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (66 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5p (87 mg, 0.20 mmol, 63%) as a yellowish oil; [α]20D +71.3 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 1.8, 0.9 Hz, 1H), 7.25–7.18 (m, 2H), 6.99 (dd, J = 8.4, 7.1 Hz, 3H), 6.35 (dd, J = 3.3, 1.8 Hz, 1H), 6.31 (dt, J = 3.3, 0.7 Hz, 1H), 5.45 (ddd, J = 7.9, 2.5, 1.2 Hz, 1H), 5.10 (dd, J = 8.2, 7.0 Hz, 1H), 4.93 (ddd, J = 11.3, 6.6, 4.6 Hz, 1H), 2.88–2.72 (m, 2H), 2.60–2.18 (m, 7H), 2.15 (s, 3H), 2.14–2.07 (m, 1H), 2.03–1.90 (m, 2H), 1.15 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.0 (C), 174.6 (C), 151.6 (C), 150.5 (C), 144.7 (C), 142.9 (CH), 128.8 (2 CH), 123.4 (CH), 121.4 (CH), 116.5 (2 CH), 110.7 (CH), 108.5 (CH), 85.7 (C), 80.1 (CH), 64.7 (CH), 46.3 (CH), 42.7 (CH2), 38.5 (CH2), 36.7 (CH2), 33.0 (CH), 30.9 (CH2), 30.1 (CH3), 23.0 (CH2), 21.3 (CH3); HRMS (ESI+): calc. For C26H29NO5 [M + H]+ 436.2118; found 436.2118.
13-[(Phenylamine), (thiophen-3-yl)-methyl]-11, N-epoxy-tomentosin (5q). Following the general procedure, using tomotensin (80 mg, 0.32 mmol) and corresponding nitrone (72 mg, 0.35 mmol) column chromatography on silica gel (petroleum ether/ethyl acetate 6/4) provided 5q (99 mg, 0.22 mmol, 68%) as a yellowish oil; [α]20D −17.0 (c 1.0, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.36 (dd, J = 5.0, 3.0 Hz, 1H), 7.28 (dt, J = 3.0, 1.0 Hz, 1H), 7.22–7.14 (m, 3H), 6.96 (ddt, J = 8.3, 3.3, 1.7 Hz, 3H), 5.41 (dt, J = 7.8, 2.4 Hz, 1H), 5.16 (dd, J = 9.0, 6.6 Hz, 1H), 4.91 (ddd, J = 11.3, 6.7, 4.4 Hz, 1H), 2.82 (ddd, J = 19.3, 11.2, 4.9 Hz, 2H), 2.60–2.17 (m, 7H), 2.15 (s, 3H), 2.10 (dt, J = 13.8, 4.1 Hz, 1H), 2.00–1.85 (m, 2H), 1.14 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.2 (C), 175.0 (C), 151.2 (C), 145.0 (C), 141.3 (C), 129.0 (2 CH), 127.2 (CH), 126.2 (CH), 123.3 (CH), 122.4 (CH), 121.4 (CH), 116.5 (2 CH), 85.6 (C), 80.1 (CH), 67.0 (CH), 46.3 (CH), 42.9 (CH2), 42.4 (CH2), 36.9 (CH2), 33.4 (CH), 31.0 (CH2), 30.3 (CH3), 23.2 (CH2), 21.4 (CH3); HRMS (ESI+): calc. For C26H29NO4S [M + H]+ 452.1887; found 452.1890.
Conclusions
In summary, we described here the synthesis of interesting spiro-isoxazolidine and isoxazoline derivatives of tomentosin by a 1,3-dipolar cycloaddition of respectively nitrones and nitrile oxides to the natural compound. We used an enantiomerically pure and natural starting material, thereby limiting the chemical impact on the environment. This procedure allowed us to generate enantiomerically pure spiro compounds in one diastereoisomer form with a limited number of steps.
Acknowledgements
This work was supported by the Region Centre, France in the ValPAMMeT Program and was carried out in collaboration with the Meknes-Tafilalet area in Morocco.
Notes and references
- Jiangsu New Medical College, in A comprehensive dictionary of traditional Chinese material medica, Shanghai People’s Press, Shanghai, 1977, vol. 1, p. 81 Search PubMed.
- X. Han, L. Yin, L. Xu, X. Wang and J. Peng, Anal. Lett., 2010, 43, 545 CrossRef.
- A. M. Seca, A. Grigore, D. C. Pinto and A. M. Silva, J. Ethnopharmacol., 2014, 154, 286 CrossRef PubMed.
- N. J. Lawrence, A. T. McGown, J. Nduka, J. A. Hadfield and R. G. Pritcharda, Bioorg. Med. Chem. Lett., 2011, 11, 429 CrossRef.
- S. Nasim and P. A. Crooks, Bioorg. Med. Chem. Lett., 2008, 18, 3870 CrossRef CAS PubMed.
- J. R. Woods, H. Mo, A. A. Bieberich, T. Alavanja and D. A. Colby, J. Med. Chem., 2011, 54, 7934 CrossRef CAS PubMed.
- S. M. Kupchan, D. C. Fessler, M. A. Eakin and T. J. Giacobbe, Science, 1970, 168, 376–378 CAS.
- S. M. Kupchan, M. A. Eakin and A. M. Thomas, J. Med. Chem., 1971, 14, 1147 CrossRef CAS PubMed.
- I. H. Hall, K.-H. Lee, E. C. Mar, C. O. Starnes and T. G. Waddell, J. Med. Chem., 1977, 20, 333 CrossRef CAS PubMed.
- K.-H. Lee, Y.-S. Wu and I. H. Hall, J. Med. Chem., 1977, 20, 911 CrossRef CAS PubMed.
- W.-H. Shao, B.-Y. Chen, X.-R. Cheng, H. Yuan, H. Chen, W.-L. Chang, I. Ye, S. Lin, Q.-Y. Sun and W.-D. Zhang, Eur. J. Med. Chem., 2015, 93, 274 CrossRef CAS PubMed.
- M. G. Hyldgaard, S. Purup, A. D. Bond, X. C. Frette, H. Qu, K. T. Jansen and L. P. Christensen, J. Nat. Prod., 2015, 78, 1877 CrossRef CAS PubMed.
- J. P. Lepoittevin, V. Berl and E. Gimeńez-Arnau, Chem. Rec., 2009, 9, 258 CrossRef CAS PubMed.
- M. Jacob, J. Brinkmann and T. J. Schmidt, Contact Dermatitis, 2012, 66, 233 CrossRef CAS PubMed.
- E. Paulsen, L. P. Christensen, M. Hindseń and K. E. Andersen, Contact Dermatitis, 2013, 69, 303 CAS.
- F. Perron and K. F. Albizati, Chem. Rev., 1989, 89, 1617 CrossRef CAS.
- S. Dadiboyena, Eur. J. Med. Chem., 2013, 63, 347 CrossRef CAS PubMed.
- D. M. Reddy, N. A. Qazi, S. D. Sawant, A. H. Bandey, J. Srinivas, M. Shankar, S. K. Singh, M. Verma, G. Chashoo, A. Saxena, D. Mondhe, A. K. V. Saxena, V. K. Sethi, S. C. Taneja, G. N. Qazi and H. M. S. Kumar, Eur. J. Med. Chem., 2011, 46, 3210 CrossRef CAS PubMed.
- J. Khazir, P. P. Singh, D. M. Reddy, I. Hyder, S. Shafi, S. D. Sawant, G. Chashoo, A. Mahajan, M. S. Alam, A. K. Saxena, S. Arvinda, B. D. Gupta and H. M. S. Kumar, Eur. J. Med. Chem., 2013, 63, 279 CrossRef CAS PubMed.
- G. Liu, S. Song, S. Shu, Z. Miao, A. Zhang and C. Ding, Eur. J. Med. Chem., 2015, 103, 17 CrossRef CAS PubMed.
- J. J. Rubal, F. M. Guerra, F. J. Moreno-Dorado, M. Akssira, F. Mellouki, A. J. Pujadas, Z. D. Jorge and G. M. Massanet, Tetrahedron, 2004, 60, 159 CrossRef CAS.
- M. Akssira, F. Mellouki, A. Salhi, H. Alilou, A. Saouf, F. El Hanbali, J. F. Arteaga and A. F. Barrero, Tetrahedron Lett., 2006, 47, 6719 CrossRef CAS.
- M. Tebbaa, A. El Hakmaoui, A. Benharref and M. Akssira, Tetrahedron Lett., 2011, 52, 3769 CrossRef CAS.
- M. Moumou, A. El Hakmaoui, A. Benhrref and M. Akssira, Tetrahedron Lett., 2012, 53, 3000 CrossRef CAS.
- M. Zaki, M. Tebbaa, M.-A. Hiebel, A. Benharref, M. Akssira and S. Berteina-Raboin, Tetrahedron, 2015, 71, 2035 CrossRef CAS.
- T. G. Tutin, V. H. Heywood, N. A. Burges, D. M. Moore, D. H. Valentine, S. M. Walters and D. A. Webb, Flora Europea, Cambridge University Press, London, 1976, vol. 4 Search PubMed.
- I. Chiappini, G. Fardella, A. Mengini and C. Rossi, Planta Med., 1982, 44, 159 CrossRef CAS PubMed.
- N.-A. Zeggwagh, M.-L. Ouahidi, A. Lemhadri and M. Eddouks, J. Ethnopharmacol., 2006, 108, 223 CrossRef PubMed (and references cited therein).
- P. Barbetti, I. Chiappini, G. Fardella and A. Menghini, Planta Med., 1985, 51, 471 CrossRef CAS PubMed.
- F. Bohlmann, P. K. Mahanta, J. Jakupovic, R. C. Rastogi and A. A. Natu, Phytochemistry, 1978, 17, 1165 CrossRef CAS.
- C. Willuhn, A. Skibinski and T. J. Schmidt, Planta Med., 1998, 64, 635 CrossRef PubMed.
- Y. Cohen, W. Wang, B.-H. Ben-Daniel and Y. Ben-Daniel, Phytopathology, 2006, 96, 417 CrossRef CAS PubMed.
- G. Fontana, S. La Rocca, S. Passannanti and M. P. Paternostro, Nat. Prod. Res., 2007, 21, 824 CrossRef CAS PubMed.
- S. Rozenblat, S. Grossman, M. Bergman, H. Gottlieb, Y. Cohen and S. Dovrat, Biochem. Pharmacol., 2008, 75, 369 CrossRef CAS PubMed.
- A. Vasas and J. Hohmann, Nat. Prod. Rep., 2011, 28, 824 RSC.
- H.-H. Park, S. G. Kim, M. J. Kim, J. Lee, B.-K. Choi, M.-H. Jin and E. Lee, Biol. Pharm. Bull., 2014, 37, 1177 CAS.
- R. Huisgen, Angew. Chem., Int. Ed., 1963, 2, 565 CrossRef.
- J. J. Tufariello, Acc. Chem. Res., 1979, 12, 396 CrossRef CAS.
- I. N. N. Namboothiri and N. Rastogi, Top. Heterocycl. Chem., Synthesis of Heterocycles via Cycloadditions I, ed. A. Hassner, Springer-Verlag, Germany, 2008, vol. 12, p. 1 Search PubMed.
- F. Henry, Nitrile oxide, nitrones and nitronates in organic synthesis: novel strategies in synthesis, New Jersey, 2nd edn, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra25869g |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a
*a





