Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Promising advances of thiacalix[4]arene in crystal structures
Mei
Zhao
,
Jing
Lv
and
Dian-Shun
Guo
 *
*
College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Shandong Normal University, Jinan 250014, P. R. China. E-mail: chdsguo@sdnu.edu.cn; Fax: +86 531 86928773; Tel: +86 531 86180743
First published on 3rd February 2017
Abstract
Thiacalix[4]arenes are one kind of robust scaffolds and extensively applied in supramolecular chemistry and materials science owing to their novel features. This article reviews the research progress of thiacalix[4]arene derivatives in crystal and organic supramolecular structures. The actual morphological parameters of various conformers and their binding patterns as well as typical supramolecular assemblies are briefly summarized. Versatile interactions involving hydrogen bonds, C–H⋯π, π–π, halogen⋯π and ancillary electrostatic contacts between heteroatoms were found to play an important role in governing the conformational and supramolecular structures of thiacalix[4]arenes in the solid state. In some cases, the solvent molecules also participate in regulating the conformer and the packing of thiacalix[4]arenes.
1. Introduction
Thiacalix[4]arenes, new members of the calixarene family,1,2 have now become one kind of robust scaffolds and have attracted considerable attention in supramolecular chemistry and materials science.3–14 Compared with calix[4]arene, the four S bridges replacing the CH2 linkers endow many novel features such as larger cavity, better flexibility, richer conformational behavior, and the possibility of multiple chemical modifications.5 Moreover, the introduction of S atoms makes thiacalix[4]arenes possess the additional affinity for binding desired substrates in their supramolecular systems.Thiacalix[4]arenes, as versatile building blocks for highly organized receptors, have attracted much interest for more than two decades largely because of their specific affinity and selectivity in molecular recognition,6–9 and supramolecular assembly.10–14 Meanwhile, great advances have also been made to describe the precise morphology of various conformers and binding patterns of thiacalix[4]arene derivatives in the solid state, which are difficult to accurately deal with in solution owing to the flexibility of the thiacalix[4]arene platform. The crystal structures of thiacalix[4]arene derivatives as important ligands in coordination chemistry have been highlighted in recent reviews.11,12 However, as of now, the progress of thiacalix[4]arene derivatives in the crystal structures and organic supramolecular assemblies has not been reviewed. This article is intended to summarize the major advances in this field, according to cone, 1,3-alternate, 1,2-alternate and partial cone conformers (Fig. 1), respectively.
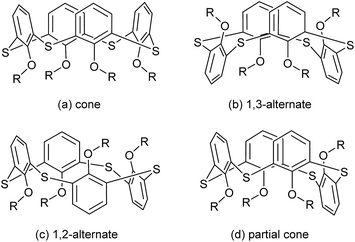 | ||
| Fig. 1 Four main conformers of thiacalix[4]arene: (a) cone, (b) 1,3-alternate, (c) 1,2-alternate, and (d) partial cone. | ||
In this review, we will focus on the precise conformations, binding patterns, and some typical supramolecular assemblies of thiacalix[4]arene derivatives mainly found in the solid state. The exactly conformational shape of thiacalix[4]arene core is characterized with the dihedral angles (termed as θ, interior angle) between the phenolic rings and the virtual plane (R) defined by the four S bridges. The difference amongst θ angles is significant for the shape of the thiacalix[4]arene core, the bigger the θ range, the more distorted the conformation.
2. Cone structures of thiacalix[4]arenes
Thiacalix[4]arenes in a cone conformation are easily available, thus many crystal structures of such conformers are proved by X-ray diffraction analysis. In this section, they will be discussed mainly according to the O-substituted extent at the lower rim.2.1 Parent thiacalix[4]arenes and their analogues
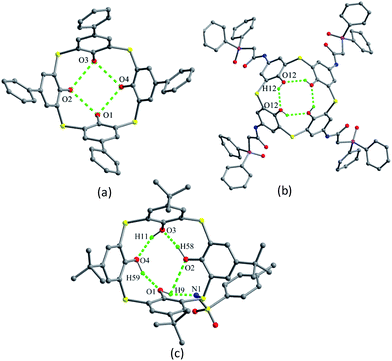
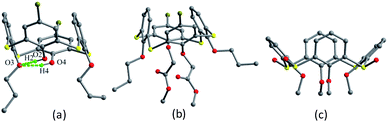
As demonstrated by Miyano et al.,3 the parent p-t-butylthiacalix[4]arene 1 (Fig. 2) shows very simple 1H NMR spectra in solution. Although the 1H NMR chemical shift for the OH groups of 1 suggested the formation of intramolecular H-bonds, their actual morphology is difficult to give. Alternatively, in the solid state, the parameters of a conformer can be exactly described by the X-ray diffraction analysis. And the shape of the thiacalix[4]arene core can be characterized by the θ angles.15 The crystal structure of 1 (Fig. 3) shows a perfect C4-symmetric cone conformation identified by the same θ value of 119.0° (Table 1).16 This may be ascribed to the formation of an intramolecular cyclic hydrogen bonding array involving four identical O–H⋯O H-bonds between the phenolic OH groups.17| Compd. | θ (°) | |||
|---|---|---|---|---|
| a Data obtained by calculation with Diamond Version 3.0. b Data obtained from refs. | ||||
| 1 | 119.0 | 119.0 | 119.0 | 119.0 |
| 2 | 114.4 | 136.9 | 117.8 | 136.9 |
| 3A | 108.2 | 142.9 | 111.4 | 143.2 |
| 3B | 107.9 | 144.5 | 108.7 | 139.8 |
| 4 | 100.0 | 141.4 | 112.1 | 146.3 |
| 5 | 120.4 | 133.1 | 130.1 | 126.7 |
| 6 | 115.7 | 126.2 | 121.1 | 126.0 |
| 7 | 123.3 | 123.3 | 123.3 | 123.3 |
| 8 | 108.1 | 142.2 | 120.3 | 132.6 |
| 9 | 112.0 | 112.6 | 112.0 | 112.6 |
| 10 | 115.7 | 115.7 | 115.7 | 115.7 |
| 11 | 103.6 | 125.8 | 103.6 | 125.8 |
| 12 | 60.0 | 139.3 | 120.5 | 139.5 |
| 13 | 65.5 | 139.8 | 129.8 | 133.6 |
| 14A | 66.2 | 148.1 | 116.4 | 139.3 |
| 14B | 65.5 | 149.3 | 116.4 | 138.9 |
| 15 | 108.2 | 128.3 | 112.2 | 122.0 |
| 16 | 68.9 | 125.9 | 137.3 | 139.8 |
| 17 | 104.7 | 147.0 | 106.3 | 146.4 |
| 18 | 92.9 | 148.7 | 111.6 | 143.6 |
| 20 | 86.5 | 147.9 | 62.2 | 154.2 |
| 21 | 112.1 | 123.5 | 115.5 | 131.1 |
| 22 | 71.2 | 146.2 | 102.4 | 147.3 |
| 23 | 74.3 | 135.1 | 103.0 | 140.0 |
| 24A | 69.9 | 140.1 | 109.2 | 135.6 |
| 24B | 71.3 | 138.4 | 115.2 | 134.7 |
| 25 | 77.0 | 133.4 | 108.8 | 124.1 |
| 26 | 68.3 | 139.5 | 108.2 | 138.1 |
| 27A | 93.0 | 136.4 | 96.6 | 138.6 |
| 27B | 94.3 | 135.5 | 102.1 | 138.2 |
| 28 | 71.0 | 157.4 | 109.2 | 143.1 |
| 29 | 108.8 | 149.4 | 109.7 | 144.3 |
| 30 | 106.5 | 142.1 | 108.9 | 146.5 |
| 31 | 102.9 | 135.6 | 114.1 | 147.3 |
| 32 | 111.3 | 136.0 | 111.3 | 136.0 |
| 33 | 69.8 | 159.3 | 70.8 | 151.6 |
| 34A | 54.3 | 104.3 | 43.1 | 75.7 |
| 34B | 41.5 | 94.0 | 51.9 | 87.5 |
| 35 | 79.2 | 126.7 | 95.8 | 141.1 |
| 36 | 78.7 | 121.3 | 91.5 | 123.8 |
| 37 | 65.2 | 136.0 | 65.4 | 136.7 |
| 38 | 106.0 | 108.2 | 115.0 | 116.9 |
| 39 b | 67.5 | 146.5 | 69.2 | 146.0 |
| 40 | 92.6 | 131.7 | 93.1 | 126.3 |
| 41 | 90.2 | 128.8 | 91.5 | 132.8 |
| 42 | 90.3 | 130.6 | 96.0 | 132.5 |
| 43 | 87.4 | 142.8 | 91.2 | 140.8 |
| 44 | 75.0 | 141.9 | 107.0 | 143.5 |
| 45 | 65.6 | 139.7 | 81.2 | 153.9 |
| 46 | 106.7 | 134.9 | 110.9 | 132.0 |
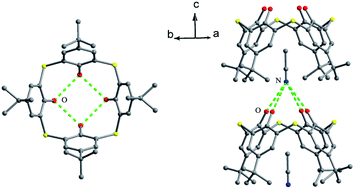
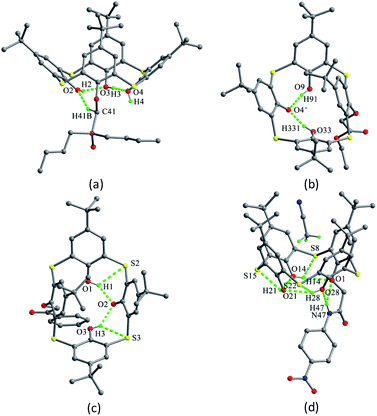
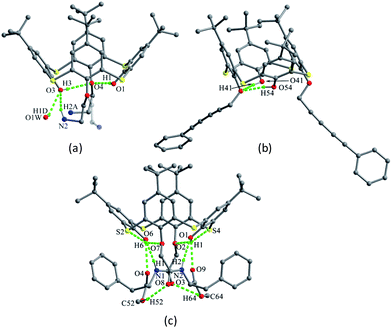
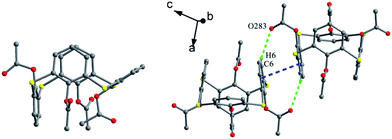
The skeleton of 1 is flexible enough to preorganize and include various small organic molecules into its cavity forming inclusion crystals.18–21 It was found that molecule 1 still retains a perfect C4-symmetric structure when some small organic molecules incorporated into its cavity, while it will adopt a slightly distorted conformation upon inclusion of some bigger molecules. This tailors the need for producing stable crystals through conformational interchanges between host and guest molecules. For instance, the cone conformer of 1 in complex 1·DCE (DCE is 1,2-dichloroethane, a bigger molecule) is slightly distorted, with different θ values of 125.9, 118.7, 136.5 and 124.1°.18 When MeCN (a smaller molecule) is included into the cavity of 1, it still keeps the perfect C4 symmetry (Fig. 3).20 The Me group of MeCN directs inside the cavity and its C–H bonds are perpendicular to the aromatic rings, where C–H⋯π contacts exist to stabilize the complex 1·MeCN. Moreover, the CN lobe of MeCN connects to the lower rim of the vicinal molecule 1 by O–H⋯N H-bonds. Thus a head-to-tail type of infinite columnar structure is created by these interactions.
Compounds 2–5 (Fig. 2),16,22 various de-t-butylation derivatives of 1, all adopt a cone conformation (Fig. 4). However, they exhibit two different modes: one has near C2 symmetry (2, 3 and 4), and the other shows near C4 symmetry (5), with four varied θ angles (Table 1). In the conformers of 2–4, O atoms of the OH groups are not arranged on one plane but deviate up and down, which would obstruct the formation of the intramolecular cyclic H-bonded array. Especially in 4, such tendency appears clearly with disappearance of the circular O–H⋯O H-bonds (Fig. 4c), due to the asymmetrical situations of the p-substituted groups. On the other hand, for 2, 3 and 5, the cyclic H-bonded array is remained (Fig. 4).
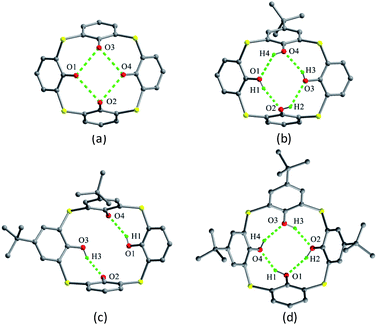 | ||
| Fig. 4 Crystal structures of 2 (a), 3 (b), 4 (c) and 5 (d). All protons of the OH groups were not found in the crystal of 2. | ||
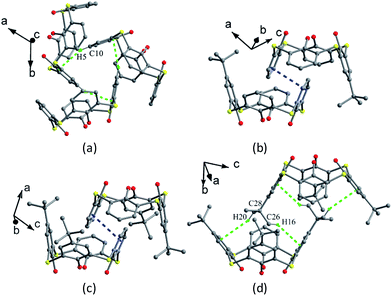
In the packing, a self-inclusion trimer of 2 is formed by C–H⋯π interactions, owing to removal of all four t-butyl groups (Fig. 5a). In the cases of 3–5, all give self-inclusion dimers in such a manner that the phenolic moiety of one molecule is inserted into the cavity of the other one. In 3 (Fig. 5b) and 4 (Fig. 5c), the phenolic rings with t-butyl-free are inserted into each other with face-to-face π–π interactions. In 5 (Fig. 5d), a t-butyl group enters into the cavity, accompanied by some C–H⋯π interactions. Moreover, these dimers in 3–5 further associate each other by the face-to-face overlap between the t-butyl-free phenol rings.
Thiacalix[4]arene 6 (Fig. 6),23 with four phenyl groups at the upper rim, adopts a cone conformation with four different θ values (Table 1). This conformer is also governed by the intramolecular cyclic H-bonding array at the lower rim, creating a deeper cavity (Fig. 7a). Such a deep-cavity thiacalix[4]arene possesses an extended π-aromatic system, and is potentially useful for solid state inclusion of suitable molecules.
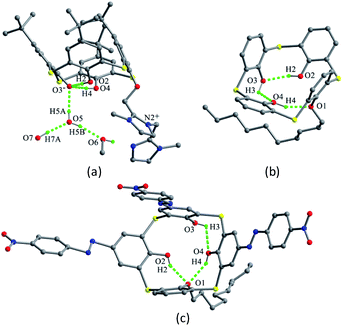
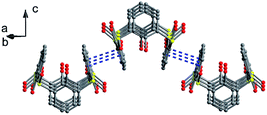
Thiacalix[4]arene 7 (Fig. 6),24 with four CMPO (carbamoyl-methylphosphineoxide) units at the upper rim, shows a perfect cone conformation with the same θ value of 123.3°. In its crystal structure (Fig. 7b), the typical circular hydrogen bonding array is also found, indicating that the CMPO units are large but cannot disrupt the H-bonded array formation. In the stacking (Fig. 8), the CMPO moieties of two molecules are interlocked to create a capsule-like dimer with S8 symmetry by intermolecular N–H⋯O H-bonds between the CONH and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. This capsule-like dimmer can exist in apolar solvents and be applied for inclusion of some guests in solution.
O groups. This capsule-like dimmer can exist in apolar solvents and be applied for inclusion of some guests in solution.
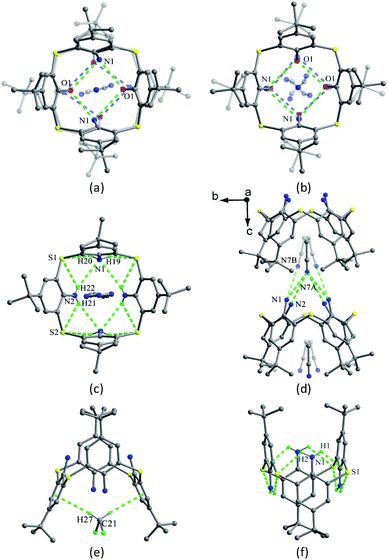
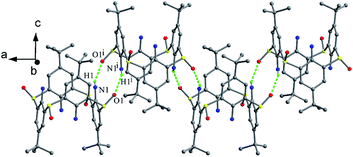
Compound 8 (ref. 25) (Fig. 6) is a derivative of 1 with a S bridge modified by sulfilimine moiety, and takes a distorted cone conformation with four different θ values (Table 1), in which the sulfilimine group directs toward the axial orientation (Fig. 7c). This conformer is stabilized by an intramolecular cyclic H-bonded array and an O1–H9⋯N1 H-bond between one OH group and N atom of the sulfilimine unit.
Thiacalix[4]arene analogues 9 and 10 (Fig. 6),26 with two amino groups at the lower rim, are also in a cone conformation with a circular H-bonded array in their crystals (Fig. 9a and b). However, the averaged distance between adjacent N, O atoms of 9 and 10 falls in the order of O⋯O < O⋯N < N⋯N, revealing that the strength of intramolecular H-bonds is weaker than those of 1. For this reason, thiacalix[4]arene analogue 11 (Fig. 6), with four NH2 groups, takes in either a cone or a 1,3-alternative conformation based on inclusion with different solvent molecules.27,28 It adopts a C2 cone conformation when a MeCN molecule is included into its cavity with two pairing θ angles of 103.6 and 125.8°, resulting from the co-operation of intramolecular N–H⋯N and N–H⋯S H-bonds (Fig. 9c), as well as intermolecular N–H⋯N H-bonds with MeCN molecule (Fig. 9d). Whereas, it takes a 1,3-alternate conformation when a CH2Cl2 molecule occupies its cavity with two pairs of θ values (100.2 and 112.4°) as they only form weak C–H⋯π contacts (Fig. 9e). In the case of guest-free, 11 shows a typical 1,3-alternate conformer with the same θ value of 95.3°, creating eight intramolecular N–H⋯S H-bonds (Fig. 9f).
2.2 O-Monosubstituted thiacalix[4]arenes
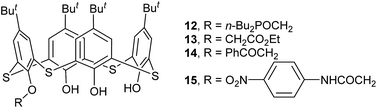
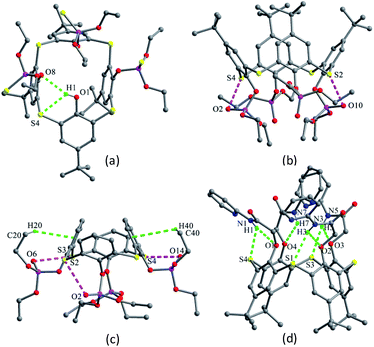
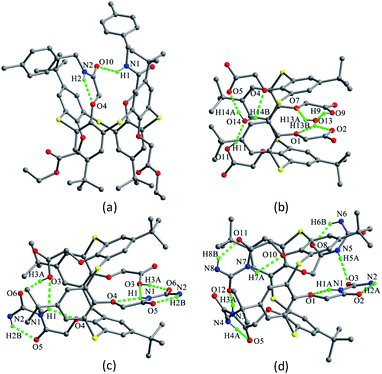
The chemical modification of the OH groups at the lower rim of thiacalix[4]arenes by selective etherification or esterification could provide mono- to tetra-O-substituted thiacalix[4]arene derivatives with some novel features.Compounds 12–14 (Fig. 10), mono ethereal derivatives of 1, all display a pinched cone conformation typically with four different θ angles (Table 1). The substituted phenolic ring is almost parallel to its opposite one and forms the smallest θ angle with the R plane. In 12 (Fig. 11a),29 such a conformer is fixed by intramolecular sequential O–H⋯O H-bonds between OH groups. Additionally, an intramolecular C–H⋯O H-bond between the P(O)CH2O moiety and one neighboring OH group results in the CH2 unit orienting inside the cavity. In the packing, a centrosymmetric dimer is produced by two intermolecular O4–H4⋯O5 bonds between the remained OH group and the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit.
O unit.
Molecule 13,30 bearing an ester unit at the lower rim, crystallizes in the form of its tetrabutylammonium salt. One interesting feature of the crystal is O4 with a negative charge, which is stabilized by two strong H-bonds from the two adjacent phenolic groups (Fig. 11b).
In the asymmetric unit of 14,31 there are two independent thiacalix[4]arene molecules A and B, with two Et4N+ and two Cl− ions. Both molecules give the same type of intramolecular O–H⋯O H-bonds as in 13. This may be ascribed to the H atom of the middle OH group creating an intermolecular O–H⋯Cl H-bond with Cl− ion. Dissimilar to 12 and 13, the conformers of A and B are further fixed by intramolecular O–H⋯S H-bonds (Fig. 11c).
Monosubstituted thiacalix[4]arene 15 (ref. 32) (Fig. 10) exhibits a different cone conformation with a θ angle of 108.2° between the substituted phenolic ring and the R plane, much larger than those in 12–14. This can be rationalized by the formation of a cyclic H-bonding array involving N–H⋯O and O–H⋯O H-bonds (Fig. 11d). The MeCN molecule was found in the calix cavity, with the Me group directing inside the cavity and its C–H bonds being perpendicular to the aromatic rings, yielding C–H⋯π contacts.
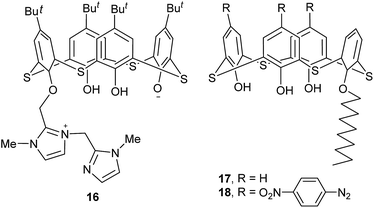
Molecule 16 (ref. 33) (Fig. 12) is a derivative of thiacalix[4]arene containing two imidazole rings linked by a methylene bridge. It shows a pinched cone conformation with 1− charge at O3 and 1+ charge at N2. Two opposite phenolic rings are splayed out with θ values of 139.8 and 125.9°, while the other benzene rings are pinched with θ values of 68.9 and 137.3°. Similar to 13, the thiacalix[4]arene core is very distorted with the middle phenolic ring bent outwards the cavity, resulting from H-bonds between O− and two adjacent OH groups (Fig. 13a). In addition, the asymmetric unit comprises one thiacalix[4]arene molecule, one methanol and two waters of crystallization, in which H-bonds between them are further to stabilize the conformer.
Thiacalix[4]arene derivatives 17 and 18 (Fig. 12),34,35 with one decyloxy group at the lower rim, give a similarly flattened cone conformation with θ ranges of 104.7–147.0° and 92.9–148.7° (Table 1), respectively. Although either conformer is governed by three intramolecular H-bonds, the H-bonding patterns are different: the former created in a sequential way from one OH group to the ether O atom (Fig. 13b), while the latter yielded in such a way that one ether O atom forms two H-bonds with its vicinal OH groups (Fig. 13c).
Lhoták et al. synthesized a phenoxanthiin-containing thia-calix[4]arene 19 (Fig. 14).36 It takes a cone conformer, where the S atom of phenoxanthiin ring makes a radical departure from the other S bridges. Such a structure is supported by an intramolecular circular array of H-bonds between the three OH groups. This bonding array is further strengthened by H-bonds between the S bridges and the adjacent OH groups. In addition, it crystallizes with two CH2Cl2 molecules, one of which is situated directly inside the calix cavity, with Cl atom located above the centre of one phenolic ring with a distance of 3.31 Å, indicating the presence of Cl⋯π interaction.
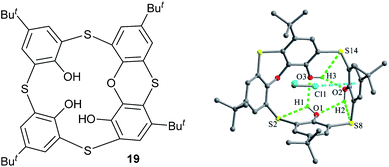 | ||
| Fig. 14 Chemical and crystal structures of compound 19, showing intramolecular H-bonding array and intermolecular Cl⋯π interaction. | ||
2.3 O-Disubstituted thiacalix[4]arenes
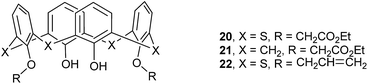
Most disubstituted derivatives of thiacalix[4]arene could be easily obtained in their 1,3-diethers with two free OH groups. Usually, they show a pinched cone conformation with a couple of opposite benzene rings tilting to the same orientation.Compounds 20 and 21 (Fig. 15),37 diacetate derivatives of thiacalix[4]arene and calix[4]arene respectively, have distinct conformational behavior owing to the different H-bonding patterns. In the crystal structure of 20 (Fig. 16a), the distorted cone conformer, with different θ values of 86.5, 147.9, 62.2 and 154.2°, is governed by two intramolecular H-bonds from both OH groups to the same ether O atom. This asymmetrical arrangement of H-bonds decreases the overall symmetry. However, in the case of 21 (Fig. 16b), two free OH groups interact with the different ether O atoms, producing almost symmetrical intramolecular H-bonds and giving a nearly C2-symmetric cone conformation with θ values of 112.1, 123.5, 115.5 and 131.1°. This difference could be ascribed to the size of the thiacalix[4]arene cavity larger (15%) than that of the calix[4]arene.16
Thiacalix[4]arene diallylether 22 (ref. 38) (Fig. 15) adopts a pinched cone conformation with θ values of 71.2, 146.2, 102.4 and 147.3°, in which one ether chain is bent towards the cavity. This conformer is fixed by the same intramolecular H-bonds as in 20 (Fig. 16c). In the packing, an infinite chain of 22 with alternating orientations is formed by the π–π stacking between phenolic rings (Fig. 17).
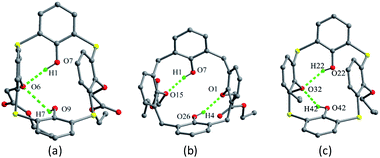
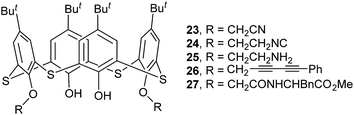
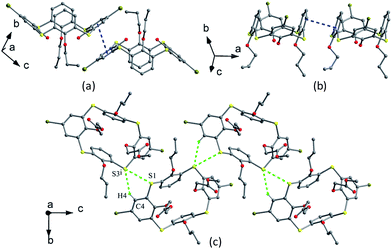
Thiacalix[4]arene derivatives 23–26 (Fig. 18) all take a pinched cone conformation with similar θ values from 68.3 to 140.1° (Table 1). The two opposing aromatic rings attaching ether arm are almost parallel to each other, while two phenolic rings are tilted outwards and nearly perpendicular. The conformer of 23 (ref. 39) is fixed by the H-bonds as mentioned above, in which two opposite aromatic rings with OCH2CN moiety tilt parallel to the same direction (Fig. 19a). Moreover, intramolecular O–H⋯S H-bonds yielded between the OH groups and S bridges are the factor for stabilizing the conformation.
Thiacalix[4]arene diisocyanide derivative 24 (ref. 40) exists two molecules A and B in its crystal structure. In A (Fig. 19b), both OCH2CH2N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C arms point vertically to two sides, while in B (Fig. 19c), both OCH2CH2N
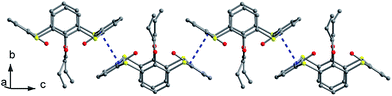
C arms point vertically to two sides, while in B (Fig. 19c), both OCH2CH2N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C arms point parallel to one side owing to the formation of an intramolecular C–H⋯C H-bond between the two arms. Similarly, intramolecular O–H⋯O H-bonds were also found between the OH groups and ether O atoms. In the packing (Fig. 20), an asymmetric dimer of molecule 24 is formed by an intermolecular C90–H90⋯C46 interaction between vicinal molecules A and B. Then, each dimer is connected in turn to produce a 1-D ABAB type of chain by C22–H22⋯O8 H-bonds. Moreover, this chain is further fixed by intermolecular O4⋯S5 and C21–H21⋯π contacts.
C arms point parallel to one side owing to the formation of an intramolecular C–H⋯C H-bond between the two arms. Similarly, intramolecular O–H⋯O H-bonds were also found between the OH groups and ether O atoms. In the packing (Fig. 20), an asymmetric dimer of molecule 24 is formed by an intermolecular C90–H90⋯C46 interaction between vicinal molecules A and B. Then, each dimer is connected in turn to produce a 1-D ABAB type of chain by C22–H22⋯O8 H-bonds. Moreover, this chain is further fixed by intermolecular O4⋯S5 and C21–H21⋯π contacts.
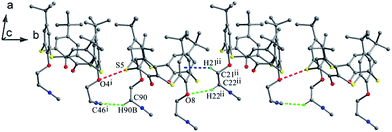 | ||
| Fig. 20 A 1-D chain of 24, showing the C–H⋯C (green), C–H⋯O (green), C–H⋯π (blue) and O⋯S (red) interactions. | ||
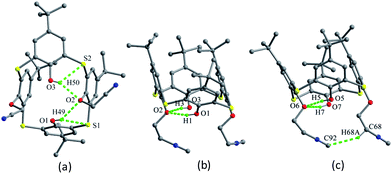
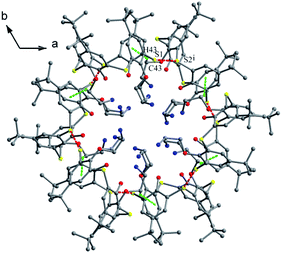
The conformer of 25 (Fig. 21a),40 with two OCH2CH2NH2 arms, is also fixed by the intramolecular O–H⋯O H-bonds from both OH groups to the same ether O atom. Interestingly, one aminoethyl group was disordered over two orientations, where three fifths of an intramolecular N2–H2⋯O3 H-bond further governs the pinched cone conformation, while two fifths of an intermolecular O1W–H1D⋯O3 H-bond occupies the site of N2–H2⋯O3 when the aminoethyl unit is disordered over the other position. In the packing, six molecules of 25 combine in a sequential manner to construct a circular hexamer by co-operative intermolecular C43–H43⋯π and S⋯S contacts (Fig. 22). Although compound 26 (ref. 41) possesses two longer chains, it still displays the similar conformational behavior (Fig. 21b), where two OH groups take part in H-bonds with the same ether O atom.
Thiacalix[4]arene derivative 27 (ref. 42) (Fig. 18) contains two ether arms with amide and ester groups at 1,3-position and can be applied for selective binding Hg2+ ion in acetonitrile solution. In the solid state, there are two molecules A and B in the asymmetric unit, both showing a cone conformation with near C2 symmetry (Table 1). Differently, two OH groups as a donor form O–H⋯O H-bonds not with the same ether O atom but with two O atoms (Fig. 21c). Moreover, each OH group also creates O–H⋯O and N–H⋯O H-bonds with the ester carbonyl O and NH atoms of the other ether strand, as well as O–H⋯S H-bond with the S bridge. The donor and acceptor capability of the OH groups with the ester carbonyl O and amide H formulates the orientation of the flexible arms upwards to the calix core direction. In addition, the terminal methyl H of the ester group is involved in C–H⋯O interaction with the amide O of the opposite arm to orient the phenyl rings outwards from the calix unit. Thus the two tethered arms undergo an unusual crossover based on these unique interactions in the crystal structure.
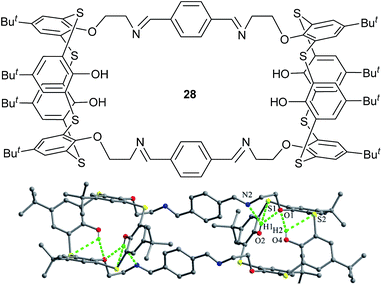
Kumar et al. reported thiacalix[4]tube 28 (Fig. 23),43 which is composed of two thiacalix[4]arene units and two diimine linkages. In the crystal structure, both thiacalix[4]arene cores adopt the same pinched cone conformation and no difference in their θ values (Table 1). In either thiacalix[4]arene core, both OH groups are titled into the cavity and create two O–H⋯O H-bonds with the same ether O atom. In addition, other three H-bonds were found between the OH groups and the imine N atom, as well as two S atoms. This thiacalix[4]tube receptor can quantitatively extract Ag+ ion from aqueous into organic phase under neutral conditions.
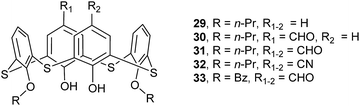
Compounds 29–33 (Fig. 24),44,45 a family of thiacalix[4]arene derivatives modified at its lower and upper rims, all give a cone conformation with similar θ ranges except for 33 (Table 1). These molecules show the same type of intramolecular O–H⋯O and O–H⋯S H-bonds as in 23 discussed above, but they demonstrate diverse assemblies in the solid state. Both 29 and 30 (with one CHO group) (Fig. 25a) are assembled into a kind of head-to-head dimers via interdigitation of aromatic rings with π–π and C–H⋯π contacts, while 31 (with two CHO groups) is associated into a different head-to-head dimer through π–π contact and weak C6–H6⋯O5 and C7–H7⋯O5 H-bonds (Fig. 25b). This could be ascribed to the introduction of the second CHO group. In comparison, molecule 32 (with two CN groups) displays a remarkable difference in the assemblies (Fig. 25c). A unique off-set kind of dimer is formed via Cl⋯Cl and Cl⋯π contacts. In such a dimer, two CHCl3 molecules linked by Cl⋯Cl contact, serve as a bridge to join two vicinal thiacalix[4]arene molecules by Cl⋯π contacts, with one Cl atom of the CHCl3 molecule lodged into the π-basic host cavity.
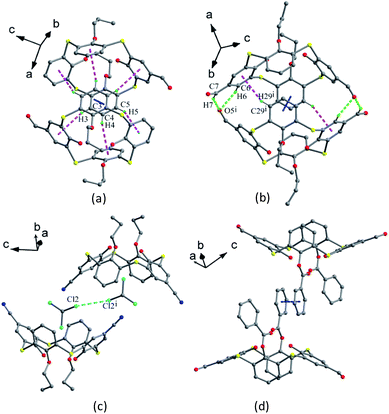 | ||
| Fig. 25 Dimers of 30 (a), 31 (b), 32 (c) and 33 (d), showing diverse intermolecular interactions involving π–π (blue), C–H⋯π (pink), C–H⋯O (green) and Cl⋯Cl (cyan) contacts. | ||
Amongst these compounds, 33 gives the biggest θ range (Table 1) owing to the replacement of n-propyl moiety with bigger benzoyl group. In the packing, it does not generate a head-to-head dimer but yield a tail-to-tail one by interaction between benzoyl groups with π–π stacking (Fig. 25d).
In addition, they all produce a similar type of honeycomb channel architectures. For instance, six molecules of 30 are packed in a back-to-back fashion, creating a disc-like hexamer via weak C7–H7B⋯S2, C13–H13⋯π and C7–H7A⋯π contacts (Fig. 26), while a disordered CHCl3 molecule locates in the centre of such a hexamer with C–H⋯Cl contacts. Then, these hexamers aggregate into a channel through C–H⋯S and S⋯π interactions. And neighbouring channels connect to form a honeycomb architecture by inter-channel π–π and C–H⋯π contacts.
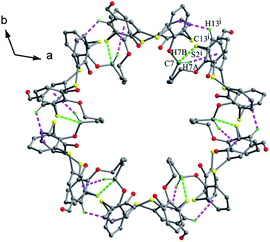 | ||
| Fig. 26 A disc-like hexamer of 30, showing intermolecular C–H⋯S (green) and C–H⋯π (pink) interactions. | ||
In contrast, few of 1,2-disubstituted thiacalix[4]arenes have been obtained in a cone conformation up to now, thus their crystal structures are rarely reported. An only known example is 1,2-bistriflate thiacalix[4]arene derivative 34 (ref. 46) (Fig. 27). In the crystal structure, there exist two molecules A and B in its unit cell. Both molecules adopt a pinched cone conformation and show different θ values (Table 1). As shown in Fig. 27, such a pinched cone conformer is stabilized by an intramolecular O–H⋯O H-bond between the two OH groups and O⋯S contacts between the triflate groups and S bridges. Additionally, in the packing, intermolecular F⋯F and O⋯S contacts are generated and further fix its crystal structure.
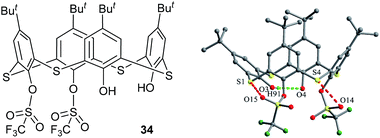 | ||
| Fig. 27 Chemical and crystal structures of compound 34, showing intramolecular H-bond and O⋯S contacts. | ||
2.4 O-Tri/tetrasubstituted thiacalix[4]arenes
Thiacalix[4]arene tridiethylthiophosphate 35 (ref. 47) (Fig. 28) is a sulphur-enriched compound and can efficiently extract Ag+ ion from water into dichloromethane. In the solid state, it shows a markedly distorted cone conformation with four θ angles in great difference (Table 1). In such a conformer, the sole OH group takes part in the formation of H-bonds with O8 and S4 atoms (Fig. 29a).Tetraphosphorylated thiacalix[4]arenes 36 and 37 (ref. 48) (Fig. 28) are good ligands that can selectively extract Pd2+ ion from automotive catalyst residue solution. In the solid state, they adopt a cone conformation with θ ranges of 78.7–123.8° and 65.2–136.7° (Table 1), respectively. Intramolecular S⋯O interactions between S bridges and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units were found to stabilize the conformers of either molecule (Fig. 29b and c). In addition, for 37, its conformer is further governed by intramolecular C–H⋯π interactions. In the packing (Fig. 30), 1-D chains of them are formed with C–H⋯π interactions between aromatic rings and ethyl H atoms of vicinal molecules. In addition, their P
O units were found to stabilize the conformers of either molecule (Fig. 29b and c). In addition, for 37, its conformer is further governed by intramolecular C–H⋯π interactions. In the packing (Fig. 30), 1-D chains of them are formed with C–H⋯π interactions between aromatic rings and ethyl H atoms of vicinal molecules. In addition, their P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups also participate in making their three-dimensional supramolecular assemblies via H-bonds.
O groups also participate in making their three-dimensional supramolecular assemblies via H-bonds.
Thiacalix[4]arene derivative 38 (ref. 49) (Fig. 28) possesses four amide-bridged pyridine arms and takes a cone conformation with a small θ range of 106.0–116.9° (Table 1). All amide O atoms cannot act as an acceptor to form intramolecular N–H⋯O H-bonds as they orient outwards the cavity due to their electron repulsion. However, all NH groups can serve as a donor to create N–H⋯O H-bonds with the phenolic O atom of the same arm, and generate three N–H⋯S H-bonds with the S bridges (Fig. 29d). The O atoms of some amide groups play an important role in the formation of intermolecular H-bonds with H2O molecules in the packing. This molecule shows high affinity towards Ag+ ion in solution owing to the introduction of pyridine function.
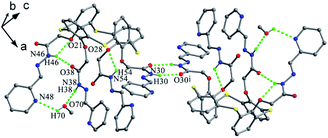
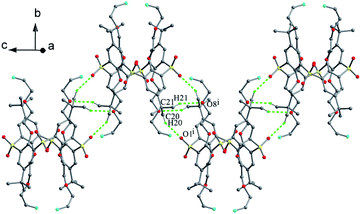
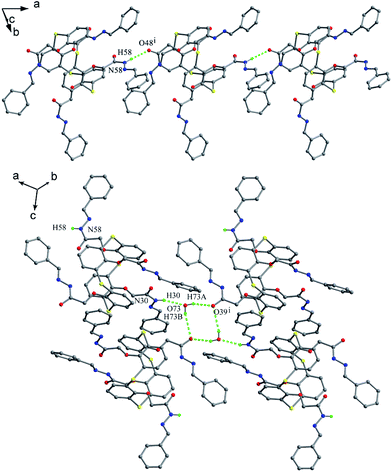
Podyachev et al. reported thiacalix[4]arene derivative 39 (Fig. 28),50 having four identical hydrazone-bridged pyridine arms at the lower rim. The thiacalix[4]arene core displays a cone conformation with a large θ range of 67.5–146.5° (Table 1), where two opposite phenolic rings inclined towards the cavity, and the other two point outwards. Compared with 38, only two NH groups can yield similar N–H⋯O bonds with the ether O atom of the same arm (Fig. 31). The other two NH groups can produce the intermolecular N–H⋯O bonds with adjacent host and guest molecules, respectively. In addition, there is an intramolecular N46–H46⋯O38 H-bond between two amide groups of the vicinal arms. In the packing, a centrosymmetric dimer is also built by two N30–H30⋯O30 H-bonds.
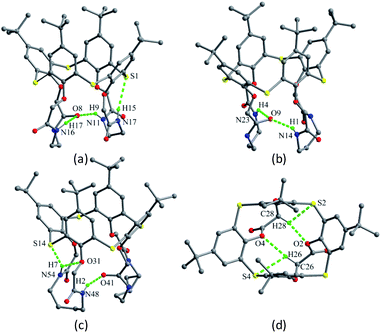
Doubly bridged thiacalix[4]arenes 40–42 (Fig. 32),51 obtained by the aminolysis of thiacalix[4]arene tetraacetate with various α,ω-diamines, generate cage-like structures possessing well preorganised array of –NH–CO– binding sites in the lower rim. X-ray analysis indicates that they all take a cone conformation and possess a very similar configuration with θ ranges of 92.6–131.7°, 90.2–132.8° and 90.3–132.5° (Table 1), respectively. One pair of opposing aromatic rings points outwards the cavity, while the other pair tilts inwards. For three molecules, there is one strong intramolecular N–H⋯O H-bond existed between two crown moieties, resulting in one amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group distorting into the cavity. Moreover, there is one intra-crown H-bond formed between two amide groups in 40 (Fig. 33a) and 41 (Fig. 33b), but between one amide group and one ether O atom in 42 (Fig. 33c). Additionally, an intramolecular N–H⋯S H-bond was found in the crystal structures of 40 and 42. All these H-bonds together govern the cone conformers.
O group distorting into the cavity. Moreover, there is one intra-crown H-bond formed between two amide groups in 40 (Fig. 33a) and 41 (Fig. 33b), but between one amide group and one ether O atom in 42 (Fig. 33c). Additionally, an intramolecular N–H⋯S H-bond was found in the crystal structures of 40 and 42. All these H-bonds together govern the cone conformers.
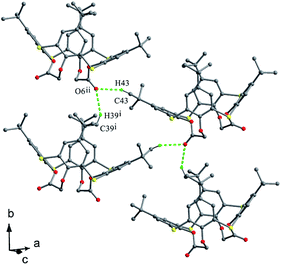
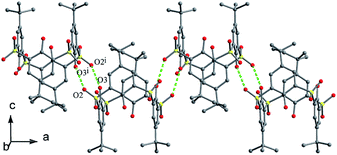
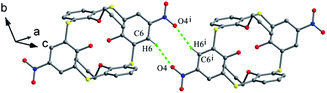
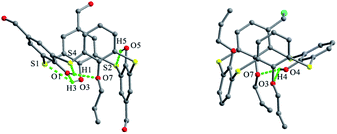
In the crystal structure of 43 (Fig. 33d),52 a dilactone derivative of 1, although the presence of two lactone bridges induces the cone conformer distorted, the whole structure still has approximate C2 symmetry with θ values of 87.4, 140.8, 91.2 and 142.8°. So two opposing aromatic rings are almost coplanar, while the other two are tilted outwards making lactone bridges bent towards the cavity. In such a conformation, two kinds of intramolecular H-bonds exist between lactone methylene H28, H26 atoms and vicinal O2, O4 atoms with contribution of bridging S atoms. In the packing (Fig. 34), only carbonyl O6 participates in the soft intermolecular H-bonds with two H atoms of t-butyl groups from two molecules, creating an infinite network of molecules.
In the crystal structures of dibromothiacalix[4]arenes 44 and 45 (Fig. 35),53,54 the thiacalix[4]arene units give different pinched cone conformations with large θ ranges of 75.0–143.5° and 65.6–153.9° (Table 1), respectively. For 44 (Fig. 36a), two brominated benzene rings are bent outwards the cavity with a long Br⋯Br distance of 13.17 Å. However, the other two benzene rings, one of which is bent towards the cavity, are almost parallel to each other with a dihedral angle of 2.99°. One of the ether O atoms makes two H-bonds with both OH groups. Whereas, for 45 (Fig. 36b), two brominated aromatic rings also incline outwards the cavity with a slight longer Br⋯Br distance of 13.82 Å. Remarkably, the other two aromatic rings are not parallel and both tilt inwards with a large dihedral angle of 33.4°. This may be ascribed to the destruction of the two H-bonds and the influence of the bulky CH2CO2Me moieties.
In the packing of 44, two different molecular chains, one with alternating (Fig. 37a) and the other with tail-to-tail orientations (Fig. 37b), are formed by intermolecular offset-face-to-face π–π stackings of the aromatic rings. However, in the case of 45 (Fig. 37c), an infinite zigzag 1-D chain is formed by intermolecular C–H⋯S H-bonds and S⋯S contacts between the adjacent molecules. Finally, such chains are associated into bilayers by a combination of interchain C–H⋯O H-bonds.
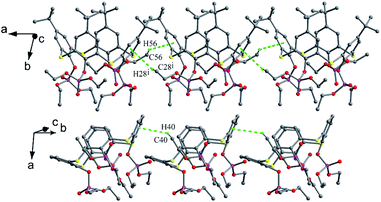
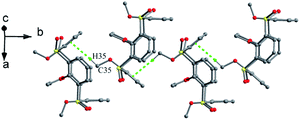
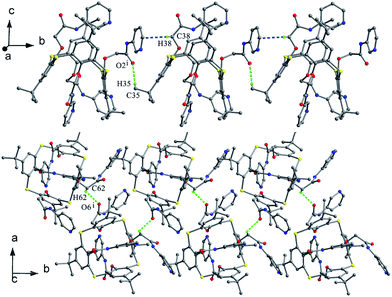
Lhoták et al. reported that tetrasulfinyl thiacalix[4]arene 46 (Fig. 35),55 synthesized by selective oxidation of tetramethoxy thiacalix[4]arene, adopts a nearly C2 symmetric cone conformation with θ values of 106.7, 134.9, 110.9 and 132.0°. Such structure is influenced by intermolecular C35–H35⋯π contacts between the OMe group and its linked aromatic ring of vicinal molecules (Fig. 38). Therefore, an infinite chain is produced and the OMe unit of one molecule is immersed in the cavity of the other one in the packing.
Briefly, only few of thiacalix[4]arenes show a perfect cone conformation possessing the same θ values, while the majority of them give a pinched or distorted cone conformation having different angles. In general, thiacalix[4]arene molecules with four OH or/and NH2 groups at the lower rim exhibit C4 or near C2-symmetry and form an intramolecular annular H-bonding array. For most O-mono- and di-substituted thiacalix[4]arenes, they are favored to take a pinched cone conformation as their free OH groups create asymmetrically intramolecular H-bonds in a sequential pattern from one OH group to the ether O atom or in such a way that one ether O atom forms two H-bonds with its vicinal OH groups. However, for O-tetrasubstituted thiacalix[4]arenes, they usually exhibit different cone conformers as their substitutes are changed. In the assemble, versatile interactions involving H-bonds, C–H⋯π, π–π and hetero atom contacts play a key role in constructing the supramolecular assemblies and fixing the conformations.
3. 1,3-Alternate structures of thiacalix[4]arenes
Thiacalix[4]arenes with various functional groups are very popular because of their highly binding affinity. Especially, these groups in 1,3-alternate orientations markedly enhance their binding properties. Thiacalix[4]arene derivatives are in a 1,3-alternate conformation rather than a cone one based on the chemical modification conditions and the substituents attached. The most such compounds are homo- or hetero-tetrasubstituted thiacalix[4]arene derivatives at the lower rim, and some S-oxidized derivatives.3.1 S-Oxydic thiacalix[4]arenes
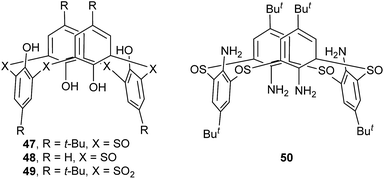
The conformation of some thiacalix[4]arenes may be changed upon oxidation of their S bridges. Tetrasulfinyl thiacalix[4]arenes 47 and 48 (Fig. 39) could be obtained via selective oxidization of the four S bridges.56,57 In the solid state, both compounds display a similar 1,3-alternate conformation with a narrow θ range of 79.5–94.8° and the same θ value of 73.5° (Table 2), respectively. All O atoms of their S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units are alternately oriented above and below the R plane. This kind of conformers is governed by four alternate O–H⋯O H-bonds between the OH groups and the S
O units are alternately oriented above and below the R plane. This kind of conformers is governed by four alternate O–H⋯O H-bonds between the OH groups and the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units, with average bond distances of 2.67 Å for 47 (Fig. 40a) and 2.65 Å for 48 (Fig. 40b). Interestingly, a 3-D network is formed by intermolecular π–π stacking between all four aromatic rings of 48 (Fig. 41). However, such a network was not observed in the crystal structure of 47, owing to the presence of bulky t-butyl groups.
O units, with average bond distances of 2.67 Å for 47 (Fig. 40a) and 2.65 Å for 48 (Fig. 40b). Interestingly, a 3-D network is formed by intermolecular π–π stacking between all four aromatic rings of 48 (Fig. 41). However, such a network was not observed in the crystal structure of 47, owing to the presence of bulky t-butyl groups.
| Compd. | θ (°) | |||
|---|---|---|---|---|
| a Data obtained by calculation with Diamond Version 3.0. b Data obtained from refs. | ||||
| 47 | 79.5 | 86.6 | 94.8 | 87.6 |
| 48 | 73.5 | 73.5 | 73.5 | 73.5 |
| 49 | 89.5 | 89.5 | 89.5 | 89.5 |
| 50 | 95.5 | 95.5 | 95.5 | 95.5 |
| 51 | 107.1 | 105.4 | 107.1 | 105.4 |
| 52 | 102.1 | 106.6 | 102.1 | 106.6 |
| 53 | 93.6 | 94.9 | 93.6 | 94.9 |
| 54 | 110.1 | 113.1 | 122.9 | 116.9 |
| 55 | 108.3 | 117.8 | 124.7 | 110.1 |
| 56 b | 92.2 | 97.7 | 98.7 | 93.9 |
| 57 | 101.9 | 107.4 | 118.4 | 110.1 |
| 58 | 98.2 | 110.9 | 112.9 | 109.6 |
| 59 | 116.1 | 117.8 | 116.1 | 117.8 |
| 60 | 109.2 | 112.9 | 125.7 | 115.5 |
| 61 | 111.6 | 115.3 | 112.1 | 115.0 |
| 62 | 91.2 | 92.2 | 91.2 | 92.2 |
| 63 | 103.9 | 104.6 | 103.9 | 104.6 |
| 64 | 83.5 | 88.8 | 86.4 | 87.8 |
| 65 | 97.1 | 103.0 | 98.3 | 114.7 |
| 66 | 100.3 | 102.4 | 103.9 | 102.6 |
| 67 | 104.3 | 112.5 | 121.6 | 107.9 |
| 68 | 104.2 | 116.5 | 104.2 | 116.5 |
| 69 | 102.4 | 116.8 | 108.4 | 115.2 |
| 70 | 109.2 | 112.9 | 110.6 | 119.5 |
| 71 | 86.3 | 88.2 | 86.3 | 88.2 |
| 72 | 106.0 | 113.8 | 118.4 | 108.3 |
| 73 | 92.5 | 103.5 | 107.4 | 100.8 |
| 74A | 108.1 | 121.1 | 121.6 | 118.1 |
| 74B | 113.9 | 119.1 | 118.0 | 120.0 |
| 75 | 109.8 | 112.4 | 122.0 | 118.2 |
| 76 | 110.7 | 113.3 | 125.4 | 113.5 |
| 77 | 100.9 | 102.9 | 100.9 | 102.9 |
| 78 | 107.2 | 108.6 | 107.2 | 108.6 |
| 80 | 103.7 | 107.5 | 103.7 | 107.5 |
| 81 | 103.1 | 107.2 | 123.0 | 113.6 |
| 82 | 101.5 | 112.1 | 107.6 | 111.4 |
| 83 | 89.5 | 95.8 | 101.9 | 102.8 |
| 84 | 103.1 | 110.5 | 107.3 | 105.9 |
| 85 | 103.9 | 107.2 | 111.4 | 104.8 |
| 86 | 101.0 | 106.7 | 110.6 | 117.9 |
| 87 | 102.1 | 106.1 | 108.4 | 113.8 |
| 88 | 101.4 | 102.2 | 112.6 | 104.9 |
| 89 | 106.4 | 111.5 | 107.4 | 114.2 |
| 90A | 109.2 | 116.3 | 116.9 | 111.8 |
| 90B | 111.3 | 114.4 | 114.9 | 114.7 |
| 91 | 103.6 | 118.7 | 117.9 | 111.4 |
| 92A | 66.1 | 147.7 | 74.2 | 154.6 |
| 92B | 72.8 | 141.8 | 73.7 | 155.9 |
| 93 | 111.4 | 114.0 | 115.9 | 114.3 |
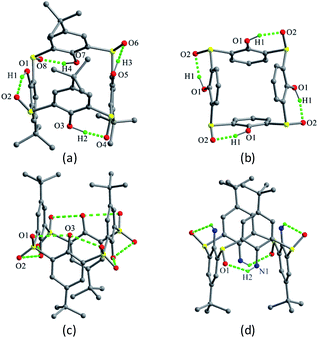 | ||
| Fig. 40 Crystal structures of 47 (a), 48 (b), 49 (c) and 50 (d), showing intramolecular H-bonds. All protons of the OH groups were not found in the crystal of 49. | ||
Using the similar strategy, the corresponding tetrasulfonyl derivative 49 (ref. 58) (Fig. 39) could be prepared and shows a typical 1,3-alternate conformation with the same θ value of 89.5°. Dissimilar to 47 and 48, all sulfonyl O atoms of 49 point outwards the cavity. This conformer can be rationalized by the formation of H-bonds between the OH groups and the SO2 units (Fig. 40c). In the packing, a 3-D network is built by intermolecular H-bonds between the OH and SO2 moieties belonging to adjacent molecules (Fig. 42).
Tetrasulfinyl thiacalix[4]arene analogue 50 (ref. 27) (Fig. 39) also gives a typical 1,3-alternate conformation with the same θ value of 95.5°. Four intramolecular N–H⋯O H-bonds are yielded between the NH2 groups and the sulfinyl O atoms (Fig. 40d). Moreover, a 2-D network is made by intermolecular H-bonds produced in the same way (Fig. 43). It should note that although 48, 49 and 50 all possess four identical θ angles, they increase in order, which may be attributed to the influence of de-t-butyl and the kinds of H-bonds.
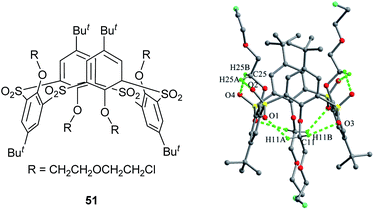
Thiacalix[4]arene 51 (Fig. 44),59 a derivative of 49 with four ether chains ended with Cl atom, also takes a 1,3-alternate conformation and all sulfonyl O atoms point outwards the cavity. In the solid state, although no hard H-bonds are found owing to the etherification of all OH groups, various soft H-bonds involving intra- and intermolecular C–H⋯O govern the conformation. Interestingly, an unusual 24-membered ring is created by a similar H-bonding array consisting of C–H⋯O H-bonds rather than O–H⋯O H-bonds in 49. In this macrocycle, both O atoms of each sulfonyl unit act as H-bond acceptor to H atoms of the two vicinal methylene groups closer phenolic rings. Thus it gives two pairing θ values of 107.1 and 105.4°, larger than that found in 49. In the packing, an infinite zigzag 1-D chain is formed by four intermolecular C–H⋯O H-bonds (Fig. 45).
3.2 O–Homosubstituted thiacalix[4]arenes
O-Homosubstituted thiacalix[4]arenes can be facilely made via incorporating four identical substituents at the lower rim under the controlled reaction conditions.6–9 Compounds 52 and 53 (Fig. 46),60 methyl ether derivatives of thiacalix[4]arenes, show different conformational behaviour in the solid state. Molecule 52 adopts a 1,3-alternate conformer with two pairs of θ angles (Table 2) upon crystallization from CH2Cl2 solvent (Fig. 47a). Square box-shaped cavities of thiacalix[4]arenes are packed parallel to each other and create infinite channels (Fig. 48), of which the inner and outer are filled with H2O and CH2Cl2 molecules, respectively. Whereas, 52 can also take a 1,2-alternate conformation with two pairing θ angles of 96.3 and 112.5° when crystallization from bulky solvents such as Me2CHOH or p-xylene as they cannot accommodate within the channels (Fig. 47b). However, upon crystallization from different solvents, molecule 53 gives a mixture of cone and 1,3-alternate conformers (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) rather than a single one (Fig. 47c). This difference can be ascribed to the loss of t-butyl groups. In the packing (Fig. 48), an infinite channel is constructed with regularly alternating cone and 1,3-alternate conformers via a combination of C–H⋯π and π–π contacts. It should be noted that the methoxy groups are too small to block the conformational interconversion because of the absence of any H-bonds and efficient sterical hindrance.
1) rather than a single one (Fig. 47c). This difference can be ascribed to the loss of t-butyl groups. In the packing (Fig. 48), an infinite channel is constructed with regularly alternating cone and 1,3-alternate conformers via a combination of C–H⋯π and π–π contacts. It should be noted that the methoxy groups are too small to block the conformational interconversion because of the absence of any H-bonds and efficient sterical hindrance.
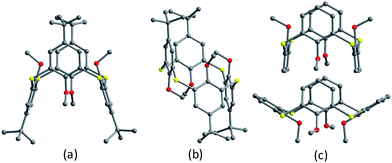 | ||
| Fig. 47 Crystal structures of 52 (a) in 1,3-alternate, (b) in 1,2-alternate, and 53 (c) in cone and 1,3-alternate. | ||
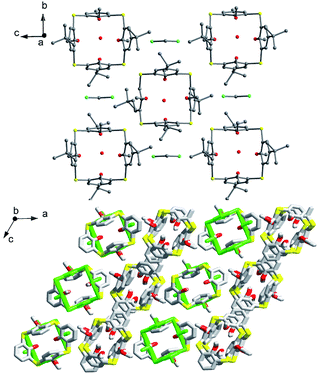 | ||
Fig. 48 Partial packing structures of 52 (top) with CH2Cl2 and H2O, and 53 (bottom) with cone and 1,3-alternate (green) conformers (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Molecule 54 (Fig. 46),61 a thiacalix[4]arene derivative functionalized with four cyanopropyl groups, adopts a 1,3-alternate conformation with four θ angles ranging from 110.1 to 122.9° (Table 2). An intramolecular C–H⋯N H-bond is yielded between CN and OCH2 units in the same arm (Fig. 49a). In the packing, a 2-D network is produced by the combination of intermolecular C–H⋯N and C–H⋯S H-bonds between vicinal molecules (Fig. 50). In addition, the CHCl3 molecules are packed in the crystal by C–H⋯N H-bonds.
Thiacalix[4]arene 55 (Fig. 46),62 bearing four hydrazide arms, gives a 1,3-alternate conformation with four θ values from 108.3 to 124.7° (Table 2). One of the hydrazide arms on each side directs into the cavity and creates an intramolecular N–H⋯O H-bond with the adjacent ether O atom (Fig. 49b). Thus, the H2O molecule locates outside the cavity. In the packing, a centrosymmetric dimer is produced by cooperative N45–H45⋯O30 H-bonds (Fig. 51). Then such dimers associate into molecular layers with H2O bridges linked by O–H⋯O H-bonds.
Compound 56 (Fig. 46),50 a derivative by replacing four pyridyl units of 39 (Fig. 28) with phenyl groups, adopts a 1,3-alternate conformation with four similar θ values (Table 2) rather than a pinched cone conformation in 39. Although both compounds could interchange their conformations owing to removal of t-butyl groups, only 39 keeps a pinched cone conformer due to the hindrance of intermolecular N–H⋯O and O–H⋯N H-bonds between two vicinal arms with a MeOH-bridge. For 56 (Fig. 49c), only two intramolecular N–H⋯O H-bonds are formed in the same arm. In the packing (Fig. 52), an infinite molecular chain is produced by intermolecular N58–H58⋯O48 H-bonds between the NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. These chains are associated in parallel way into a 3-D network bridged by H2O molecules, where four vicinal thiacalix[4]arene molecules are interlinked via hard H-bonds involving two NH and two C
O groups. These chains are associated in parallel way into a 3-D network bridged by H2O molecules, where four vicinal thiacalix[4]arene molecules are interlinked via hard H-bonds involving two NH and two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, as well as a pair of H2O molecules.
O groups, as well as a pair of H2O molecules.
 | ||
| Fig. 52 Partial packing structure of 56, showing an infinite molecular chain (top), and a tetramer linked with two water molecules (bottom). | ||
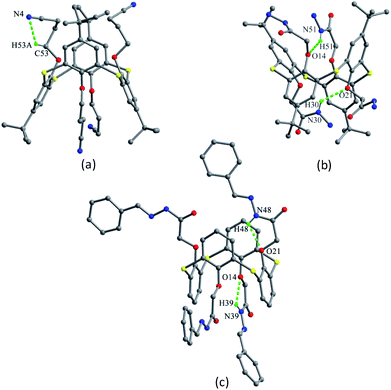
In some 1,3-alternate thiacalix[4]arenes bearing heterocyclic rings, the hetero atoms can play an additional acceptor to yield H-bonds and complex metal ions. Thiacalix[4]arenes 57 and 58 (Fig. 46),63 anchoring isomeric pyridine rings by amide linkage, display a 1,3-alternate conformation. Both molecules can bind Ag+ ion selectively in solution, while the binding efficiency is influenced by the structures of aminopyridyl pendent arms, the former shows a better extraction capacity. In the solid state, it was found that they give the similar thiacalix[4]arene shape with four θ values from 101.9 to 118.4, and 98.2 to 112.9° (Table 2), respectively. However, their whole molecular structures are much more asymmetrical because of the spatial different outspread of the flexible side chains. And the most significant difference between them is the orientation of aminopyridyl side chains, ascribed to the differing position of pyridyl moiety connecting to the NH moiety.
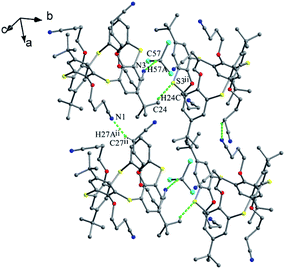
For 57 (Fig. 53), it's favored to form intramolecular hard H-bonds between the N atoms of m-pyridyl rings and the facing NH units of amide groups, while no such hard intermolecular H-bonds were found between the vicinal molecules as all the amide NH groups bury in the cavity of thiacalix[4]arene. Only intermolecular C–H⋯π and C–H⋯O contacts were observed, creating a 1-D chain in a side-by-side mode (Fig. 54). Then a double stranded chain is yielded by inter-chain C62–H62⋯O6 H-bonds between OCH2 units of one strand and carbonyl O atoms of the other strand. Eventually, these double stranded chains are linked by MeOH solvent molecules in the crystal lattice.
 | ||
| Fig. 54 Partial packing structures of 57 showing a 1-D chain (top), and a double stranded chain (bottom). | ||
In comparison, there is no corresponding intramolecular H-bond existed in 58 because of the pyridyl N atom at the p-position rather than the m-position, orienting outwards the cavity (Fig. 53). As a result, the amide N atoms are available for contacts with the pyridyl N atoms of adjacent molecules. As shown in Fig. 55, individual molecules of 58 are in a side-by-side orientation, yielding a 1-D chain by intermolecular N7–H7⋯N4 H-bonds. With the help of H2O molecules, two strips of the resulting 1-D chains are further augmented to a water-bridged double strand.
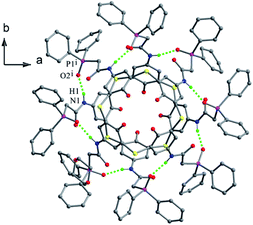
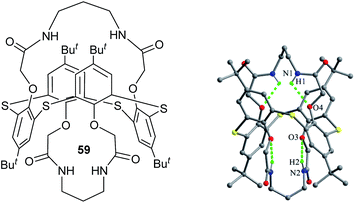
Thiacalix[4]bis-crown analogue 59 (ref. 64) (Fig. 56) takes a 1,3-alternate conformation with four quite similar θ angles (Table 2) and shows two fold rotation symmetry with an cage-like cavity. All four N–H bonds of both amide bridges are oriented into the calix cavity and generate intramolecular H-bonds to all ether O atoms.
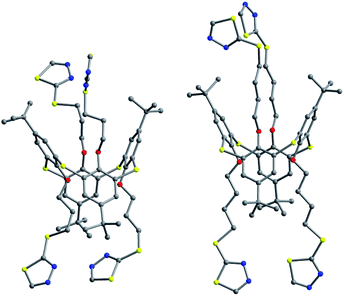
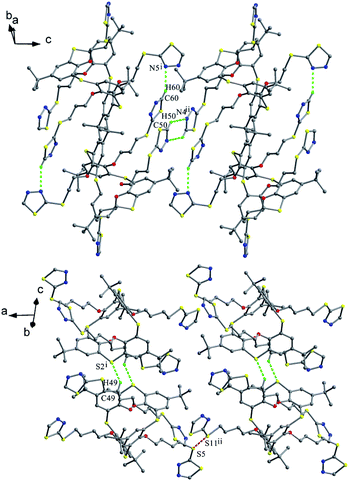
Thiacalix[4]arenes 60 and 61 (Fig. 57),65 with four thiadiazole termini, both give a 1,3-alternate conformation with similar θ angles (Table 2). In the solid state, a couple of opposite thiadiazole rings is nearly parallel to each other, while the other couple is perpendicular mutually (Fig. 58), forming dihedral angles of 10.9, 77.3° for 60 and 25.0, 82.0° for 61. In the packing, molecule 60 creates a 1-D chain by weak C50–H50⋯N4 and C60–H60⋯N5 interactions between two thiadiazole rings of adjacent molecules (Fig. 59). These chains are further extended into a 2-D network by inter-chain S⋯S contacts between S atoms in the thiadiazole rings and 2-positional mercapto S atoms. Eventually, the 2-D networks are assembled into a 3-D supramolecular structure with the lattice water dimers through O–H⋯S H-bonds. In the packing of 61 (Fig. 59), two molecules are connected into a dimer by two intermolecular C–H⋯S contacts between the methylene H atom and the S atom in the thiacalix[4]arene unit. These dimers are further linked into a 1-D chain by S⋯S contacts between the same S atoms linked to the thiadiazole rings of vicinal molecules. Afterwards, these 1-D chains are assembled into a 2-D network with the methanol dimers via C–H⋯N and C–H⋯O H-bonds.
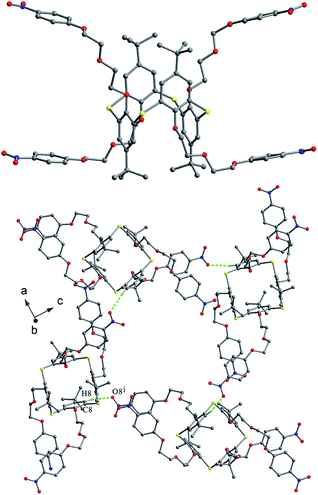
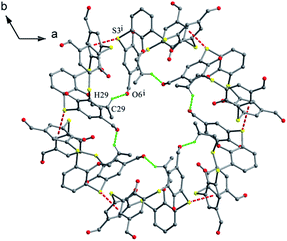
Compound 62 (Fig. 57),66 possessing four polyether arms ended with the 4-NO2C6H4 unit, crystallizes in a typical 1,3-alternate conformation with two pairing θ angles of 91.2 and 92.2°, where the four polyether arms are distorted outboard of the cavity. The terminal benzene rings are nearly parallel to the R plane, with interplanar angles of 14.09 and 9.05. In the packing, a 2-D array composed of the rare cyclic thiacalix[4]arene tetramers with an eighty-membered ring motif (Fig. 60) is formed by a combination of intermolecular C–H⋯O H-bonds. These arrays are further linked into a complex 3-D framework through interlayer C–H⋯S H-bonds and aromatic π–π interactions.
3.3 O-Heterosubstituted thiacalix[4]arenes
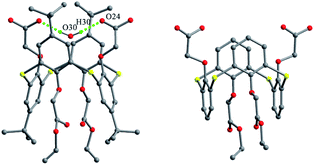
O-Heterosubstituted thiacalix[4]arenes are usually obtained by appending four different substituents at the lower rim via multiple steps. Compounds 63 and 64 (Fig. 61),67 synthesized by selective hydrolysis of tetraester thiacalix[4]arene derivatives, possess both CO2H and CO2Et groups and take a 1,3-alternate conformation with two pairing θ angles of 103.9 and 104.6°, and four similar ones from 83.5 to 88.8°, respectively (Table 2). In the crystal structure of 63 (Fig. 62), one molecule H2O lies in the calix cavity and forms two H-bonds with two CO2H groups. In the packing, a zigzag chain of 63 is apparent by intermolecular O–H⋯O H-bonds, locally creating a four-membered ring motif between the carboxyl OH groups (Fig. 63). However, for 64, a dimeric structure is generated via four intermolecular O–H⋯O H-bonds between CO2H groups, locally yielding two eight-membered ring motifs (Fig. 63).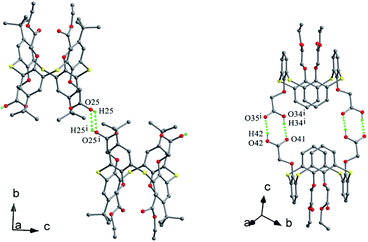 | ||
| Fig. 63 Partial packing structures of 63 (left) and 64 (right), showing different H-bonded ring motifs. | ||
Thiacalix[4]arene derivative 65 (ref. 68) (Fig. 61) contains both amide and ester groups. It has a 1,3-alternate conformation with four θ angles from 97.1 to 114.7° (Table 2). In the solid state, it is clear that the orientations of the carbonyl O atoms of esters are outwardly orientated with respect to the cavity because of the electronic repulsion between O atoms. Interestingly, two intramolecular N–H⋯O H-bonds are formed (Fig. 64a): one is intra-arm type between the NH and ethereal O atom, and the other is inter-arm one between the NH and amide O atom. In solution, 65 can bind K+ ion, while its structural symmetry has not been changed after complexation.
 | ||
| Fig. 64 Crystal structures of 65 (a), 66 (b), 68 (c) and 70 (d), showing the intra- and inter-arm H-bonds. | ||
Thiacalix[4]arene derivatives 66–70 (Fig. 61),15 functionalized with CO2H and/or CONHCONH2 groups, are all in a 1,3-alternate conformation with four similar θ values except for 68 with two pairing θ angles of 104.2 and 116.5° (Table 2). For 66 (Fig. 64b), there is no intramolecular H-bond between the two opposing arms, but two H2O molecules occupy the cavities made by two pairs of facing aromatic rings and create intermolecular H-bonds with the CO2H groups. For the other molecules, there are various intra- and inter-arm hard H-bonds owing to the presence of urea units. In the structures of 68 (Fig. 64c) and 70 (Fig. 64d), for instance, each urea group yields two different intra-arm N–H⋯O H-bonds with O atoms of CH2CO and ether moieties, respectively. Moreover, there are inter-arm O–H⋯O and N–H⋯O H-bonds between two opposing arms. For these molecules, the main difference is the spatial orientations of their arms. When two urea arms locate on the same side, they direct outwards due to the steric repulsion, while there are one urea arm and one CO2H group on the same side, they orientate inwards because of their inter-arm H-bonds.
In the packing of these compounds, the types of H-bonds between vicinal arms play an important role in the assemblies. For instance, two molecules of 67 create a dimer by N4–H4A⋯O3 and N4–H4B⋯O8 H-bonds. These dimers are interlinked to produce a 1-D double-stranded linear chain by the combination of N3–H3⋯O12 and O11–H11⋯O9 H-bonds (Fig. 65). While in the case of 68, a 1-D single-stranded linear chain is formed via N2–H2⋯O2 H-bonds (Fig. 65). Moreover, self-assemble also depends on the spatial orientation of pendant arms. Through the cavity stacking fashion, only 66 can give nanotubes, in which the four pendant arms orient along the basic axis of the molecule. For the others, the inwardly orientated pendant arms obstruct the channel formation owing to protruding into the calix cavity.
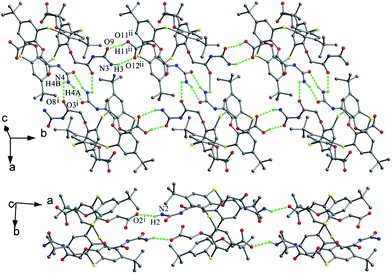 | ||
| Fig. 65 Partial packing structures of 67 (top) showing a 1-D double-stranded chain and 68 (bottom) displaying a 1-D single-stranded chain. | ||
Thiacalix[4]arenes 71 and 72 (Fig. 66),69 modified with thiourea or urea groups, both adopt a 1,3-alternate conformation but have different symmetry. Molecule 71 shows crystallographic C2-symmetry with two pairing θ angles of 86.3 and 88.2°. Two pairs of facing phenolic rings exhibit approximately parallel orientations, forming interplanar angles of 3.7 and 7.4°. However, molecule 72 is asymmetrical with four different θ angles (Table 2). Two couples of opposite phenolic rings are not parallel, creating interplanar angles of 42.1° and 44.7°. In 71 (Fig. 67a), two thiourea groups are far away from each other since two t-butyl groups fill in the space between them, while two urea units of 72 locate closely in a parallel way and thus create an intramolecular H-bond between them as two t-butyl groups are apart away (Fig. 67b). These differences may be attributed to the varied fashions of N,N′-disubstituted thiourea (E,Z) and urea (Z,Z), which distort the macrocycle in different manners, thus yielding diverse spatial orientations of these groups. The other important factor is that the thiourea S atoms have lower H-bond accepting ability than the urea O atoms. In solution, both compounds could be used as potential receptors for anion recognition, but show poor selectivity.
Thiacalix[4]arene 73 (Fig. 66),70 appending two 1,2,3-triazole bridged arms, takes a 1,3-alternate conformation with four θ values from 92.5 to 107.4° (Table 2) (Fig. 67c). In addition, two 1,2,3-triazole rings are nearly parallel to each other with a dihedral angle of 3.0°. In the packing, a 1-D chain is produced by C–H⋯N H-bonds between two 1,2,3-triazole rings of adjacent molecules (Fig. 68).
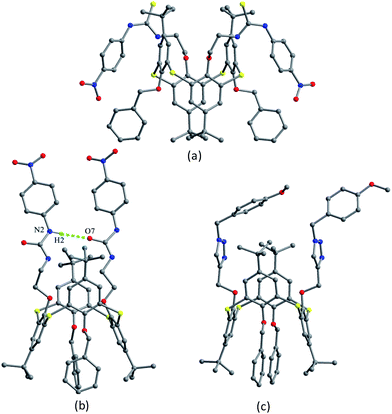
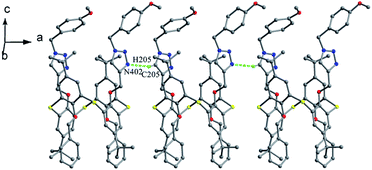
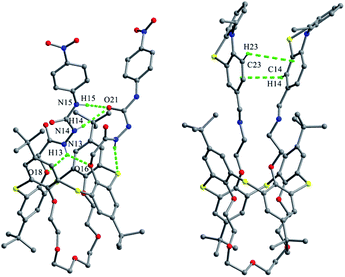
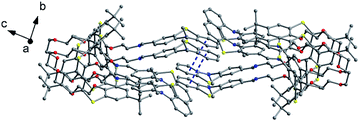
Thiacalix[4]crown derivatives 74 (ref. 71) and 75 (ref. 72) (Fig. 69) both show a 1,3-alternate conformation with four similar θ angles (Table 2). In the unit cell of 74 (Fig. 70), there are two thiacalix[4]arene molecules A and B, in which it was found that all three NH units of one chain act as a donor to form four intermolecular N–H⋯O H-bonds with one urea carbonyl O atom of the other chain and two ether O atoms, and thus the two urea units approach each other in a nearly parallel orientation. In solution, compound 74 can be employed as a ditopic receptor for molecular recognition based on the positive and negative allosteric effects.
In the structure of 75 (Fig. 70), two phenothiazine rings show a butterfly shape and produce a weak intramolecular C–H⋯π contact. In the packing, a molecular dimer is formed by weak π–π stacking contacts between two face-to-face paralleled phenothiazine rings of adjacent molecules (Fig. 71). Compound 75 exhibits a strong fluorescence emission with a large Stokes shift, which can be quenched selectively by Fe3+ and Cr3+ ions in solution.
Thiacalix[4]arene derivative 76 (Fig. 69),73 functionalized with two nitrobenzofurazan (NBD) groups, crystallizes in a 1,3-alternate conformation with similar θ values to 74 and 75. Interestingly, the two NBD rings, one of which faces outward yielding an N–H⋯S H-bond, create a dihedral angle of 135.3° (Fig. 72). Due to the introduction of NBD groups, this compound can be applied as a colorimetric and fluorescence sensor for detection of Ag+ and AcO− in solution.
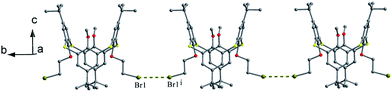
Thiacalix[4]arene derivative 77 (Fig. 69),74 with two OMe and two OCH2CH2Br arms, gives a 1,3-alternate conformation with two pairing θ angles of 100.9 and 102.9° (Fig. 72). In the packing, a 1-D chain based on intermolecular Br⋯Br contacts is generated with an inter-halogen distance of 3.81 Å (Fig. 73), which is less than the sum of the van der Waals radii.
3.4 O/aryl mixed substituted thiacalix[4]arenes
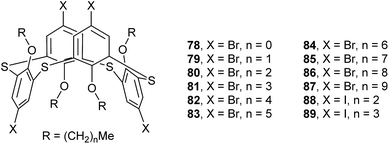
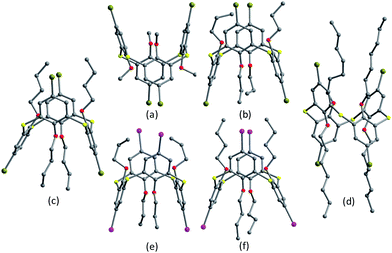
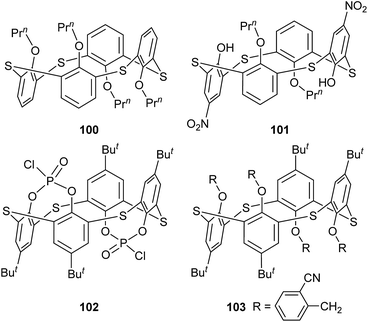
In some cases, chemical modification of thiacalix[4]arenes can be performed at both the lower rim and the calix aryl rings to create new type of O/aryl substituted thiacalix[4]arenes. Hamada et al. have synthesized a series of such a type of thiacalix[4]arenes 78–89 (Fig. 74),75–78 and studied extensively on their halogen interactions, revealing important insights into the formation of molecular assemblies in the solid state.10 These compounds, except for 79 (see 112 in Section 5.2), all take a 1,3-alternate conformation with similar θ values (Table 2), while they exhibit remarkably different assemblies due to the varied length of alkyl chain (methyl to decyl) at the lower rim.Brominated thiacalix[4]arene 78 (Fig. 75a), with the smallest methyl groups, prefers to yield Br⋯π contacts between distinct molecules. In its packing, symmetric expansion of molecules assembles into a layer-supramolecular structure with Br⋯π and C–H⋯π interactions (Fig. 76). In the case of molecule 80 (Fig. 75b), bearing n-propyl groups, a hexameric ring (Fig. 77), with a cyclohexane molecule enclosed, is formed by combining intermolecular C–H⋯S H-bonds, π–π and S⋯π interactions between vicinal molecules. Molecule 81 (Fig. 75c) possesses moderate n-butyl chains and offers a skewed 1,3-alternate conformation with a θ range of 103.1–123.0°, which is favored to create intermolecular Br⋯Br interactions in the crystalline state. Thus an infinite open-network is generated with six trimeric units through triangular Br3 motif halogen–halogen contacts and C35–H35⋯Br4 interactions (Fig. 76). However, such halogen interactions were not observed in the crystal structures of brominated thiacalix[4]arenes 80, 82 and 83.
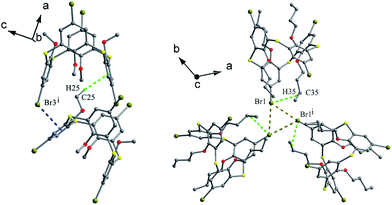 | ||
| Fig. 76 A dimer of 78 (left) built by Br⋯π and C–H⋯π contacts, and a triangular Br3 motif of 81 (right) formed by Br⋯Br and C–H⋯Br interactions. | ||
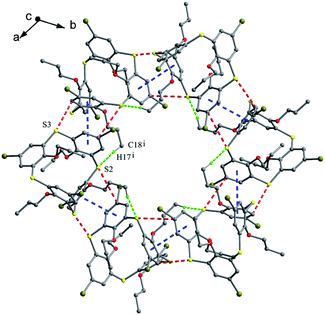 | ||
| Fig. 77 A hexameric assembly of 80, showing intermolecular π–π (blue), S⋯π (red) and C–H⋯S (green) interactions. | ||
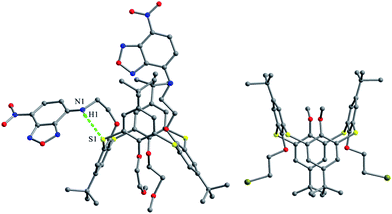
In comparision, brominated thiacalix[4]arene derivatives 84–87, bearing longer chains, are favored to assemble via Br⋯S halogen bonding. Molecule 84 with larger n-heptyl groups (Fig. 75d), for instance, demonstrates Br⋯S contacts as the n-heptyl chain blocks Br atom from interacting with other halogens or aromatic rings (Fig. 78).
Thiacalix[4]arenes 88 (Fig. 75e) and 89 (Fig. 75f) have four I atoms at the upper rim. In the packing of 88, intermolecular I⋯I, S⋯π and C–H⋯I interactions were demonstrated in the supramolecular assembly (Fig. 79), which is produced via preferential I⋯I interactions. However, in the case of 89, a supramolecular assembly is yielded by S⋯I and C–H⋯S bonding, together with weak S⋯π and C–H⋯I interactions (Fig. 79). It should be noted that iodine-containing thiacalix[4]arenes have stronger interactions involving iodine than bromine as the former possesses a stronger polarizing power than the latter.
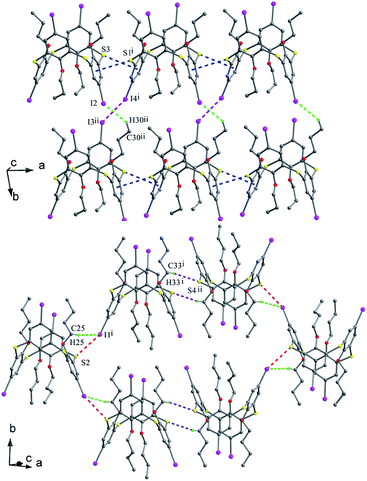 | ||
| Fig. 79 Supramolecular assemblies of 88 (top) built by I⋯I (pink), C–H⋯I (green) and S⋯π (blue) interactions, and 89 (bottom) built by I⋯S (red), C–H⋯I (green) and C–H⋯S (purple) interactions. | ||
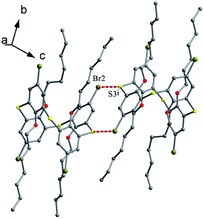
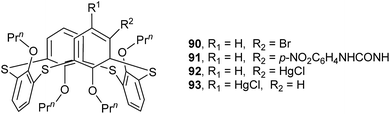
Tetrapropoxythiacalix[4]arene derivatives 90 (ref. 79) and 91 (ref. 80) (Fig. 80), mono-functionalized at the m-position, both exhibit a 1,3-alternate conformation with four similar θ angles (Table 2). In the packing of 90, a unique dimer is yielded through Br⋯π interactions between bromine atoms and phenolic rings of the neighboring molecules (Fig. 81). In the case of 91, two NH units of the ureido group at the m-position create two asymmetric H-bonds with the O atom of THF (Fig. 82). In addition, two p-nitrophenylureido moieties are strictly coplanar to each other, producing a dimer by intermolecular π–π interactions.
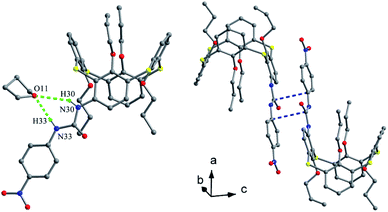 | ||
| Fig. 82 Crystal structure of 91 (left) showing H-bonds with THF molecule, and a dimer of 91 (right) showing π–π interactions. | ||
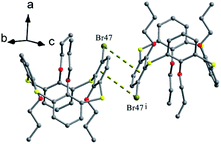
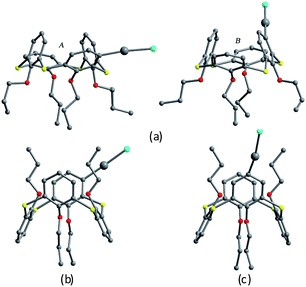
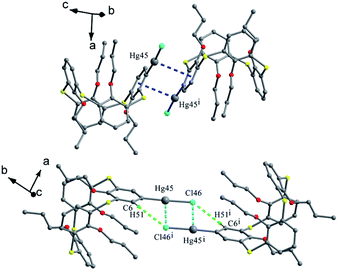
Tetrapropoxythiacalix[4]arenes 92 and 93 (Fig. 80),81m- and p-monomercurated regioisomers, are in a 1,3-alternate or cone conformer. In the asymmetric unit of 92, there are two pinched cone conformers with θ ranges of 66.1–154.6° in A (HgCl unit orienting outsides the cavity) and 72.8–155.9° in B (HgCl unit orienting insides the cavity) (Fig. 83a). In addition, it also gives a 1,3-alternate conformer with a θ range of 111.0–114.6° (Fig. 83b). The aromatic rings bearing the HgCl group at the m-position are parallel to each other and yield a dimer by cation–π interactions (Fig. 84). And the Hg atoms locate exactly above the centroid of the parallel aromatic ring from the vicinal molecules. But for 93, it is only in a 1,3-alternate conformation with a θ range from 111.4 to 115.9° (Fig. 83c). An interesting motif can be found in the crystal packing of 93, where the intermolecular Hg⋯Cl interactions lead to the formation of a dimer (Fig. 84), which is further strengthened by additional C–H⋯Cl H-bonds between the HgCl unit and the aromatic ring.
In summary, the geometry of thiacalix[4]arenes in a 1,3-alternate conformation differs essentially from those in a cone conformer. Most 1,3-alternate thiacalix[4]arenes may be divided into three types based on the θ variants: (1) with the same four θ angles, (2) with two pairs of θ angles, and (3) with four similar θ angles. The 1,3-alternate conformation is also stabilized by the intramolecular H-bonds and weak contacts similar to the cone conformer. In the packing, the most important feature is that the thiacalix[4]arene core and its pendant groups facilitate the formation of more complex supramolecular assemblies with branched intermolecular interactions. Especially, various hetero atoms in these compounds can offer more chances in creating diverse kinds of interactions. Therefore various motifs and beautiful channels are constructed by aggregation of the host molecules.
4. 1,2-Alternate structures of thiacalix[4]arenes
As of now, only few of thiacalix[4]arene derivatives shows a 1,2-alternate conformation, usually with a centrosymmetry as their four phenolic rings locate in two opposite sides.4.1 O-Substituted thiacalix[4]arenes
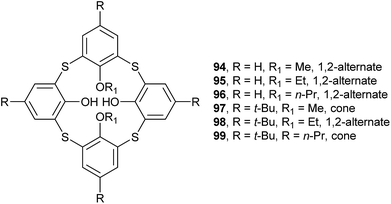
In general, the distally substituted calix[4]arene derivatives prefer a cone conformation in the solid state, while the corresponding thiacalix[4]arene derivatives may adopt either a cone or a 1,2-alternate conformer.Lhoták et al. have studied the conformational behavior of a series of such thiacalix[4]arenes 94–99 (Fig. 85).44,82 In the solid state, 94–96 and 98 take a 1,2-alternate conformation with approximate (94) or complete centrosymmetry (95, 96, and 98) with similar θ ranges (Table 3). This kind of conformers is governed by two H-bonds between OH and alkoxy groups of the adjacent aromatic rings. They create a novel type of molecular channels held together by π–π interactions. For instance, molecule 94 (Fig. 86a),82 a dimethoxy derivative of thiacalix[4]arene, shows a 1,2-alternate conformation fixed mainly by two intramolecular O–H⋯O H-bonds between the OH and OMe groups. Moreover, two intramolecular O–H⋯S H-bonds are yielded between the OH and S moieties. In the packing, a dimer is built by intermolecular π–π interactions between the aromatic rings (Fig. 86b). Further expansion of these dimers generates an infinite molecular channel (Fig. 86c). In addition, the 1,2-alternate conformers of 95 (with ethoxy groups) and 96 (with n-propoxy groups) are further stabilized by intramolecular C–H⋯π interactions between the alkoxy and the neighboring inverted aromatic ring. However, different conformational properties of 97 and 99, with t-butyl at the upper rim, were found in a pinched cone conformation, in which both free OH groups interact with the same ether O atom. Interestingly, molecule 96 also exists in a cone conformer described above (see 29 in Section 2.3), due to the introduction of bulkier n-propyl groups than methyl and ethyl groups in 94 and 95, respectively.
| Compd. | θ (°) | |||
|---|---|---|---|---|
| a Data obtained by calculation with Diamond Version 3.0. | ||||
| 94 | 113.8 | 129.9 | 114.3 | 131.9 |
| 95 | 121.4 | 123.8 | 121.4 | 123.8 |
| 96 | 115.8 | 126.5 | 115.8 | 126.5 |
| 97 | 73.2 | 134.9 | 110.8 | 135.8 |
| 98 | 116.6 | 121.2 | 116.6 | 121.2 |
| 99 | 72.0 | 155.6 | 107.1 | 141.6 |
| 100 | 105.3 | 114.5 | 105.3 | 114.5 |
| 101 | 118.8 | 130.9 | 118.8 | 130.9 |
| 102 | 102.9 | 167.0 | 102.9 | 167.0 |
| 103 | 106.8 | 123.6 | 106.8 | 123.6 |
| 104 | 111.7 | 114.7 | 111.7 | 114.7 |
| 105 | 116.1 | 132.4 | 116.1 | 132.4 |
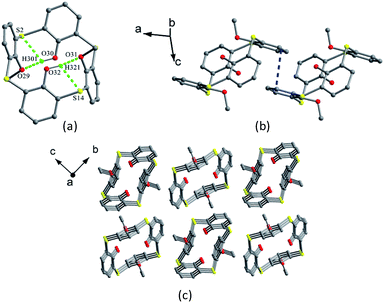 | ||
| Fig. 86 Crystal structure of 94 (a) showing O–H⋯O and O–H⋯S H-bonds, a dimer of 94 (b) built via π–π interactions, and partial packing structure of 94 (c) showing the molecular channels. | ||
Thiacalix[4]arene derivatives 100–103 (Fig. 87), all adopt a centrosymmetric 1,2-alternate conformation with two pairs of θ angles (Table 3), and their opposite inverted aromatic rings are nearly parallel. In the crystal structure of 100 (Fig. 88a),83 bearing four n-propoxy groups at the lower rim, no intramolecular H-bond was observed as in 96 (with two n-propoxy groups), due to the absence of OH groups. However, an infinite channel is still yielded with molecules being packed parallel to each other (Fig. 89).
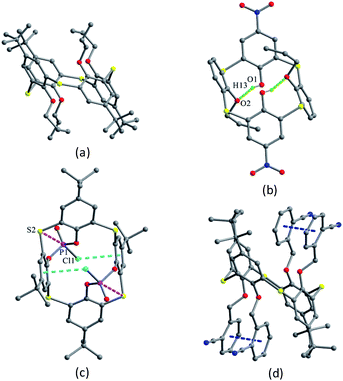 | ||
| Fig. 88 Crystal structures of 100 (a), 101 (b), 102 (c) and 103 (d), showing P⋯S (pink), P–Cl⋯π (cyan), and π⋯π (blue) interactions. | ||
Thiacalix[4]arene derivative 101 (Fig. 88b),84 with two nitro and two n-propoxy groups at the upper and lower rims, repectively, is also stabilized by two intramolecular H-bonds between the OH and the ether O atoms, which is similar to 96. Differently, in the packing, a centrosymmetric dimer is formed by two C–H⋯O H-bonds between the nitro O atom and the phenolic meta-H atom of the vicinal molecules (Fig. 90).
In addition, some other ancillary forces can also stabilize the 1,2-alternate conformer of thiacalix[4]arenes. Molecule 102 (Fig. 88c),85 a phosphorylation derivative of 1, is in a flattened 1,2-alternate conformation. Such a flattened structure is fixed by intramolecular weak P⋯S and P–Cl⋯π interactions, leading one couple of opposite aromatic rings being nearly parallel to the R plane.
In the crystal structure of 103 (Fig. 88d),86 bearing four 2-cyanobenzyloxy units at the lower rim, the two pairs of distal cyanophenyl rings are nearly parallel with the same dihedral angle of 7.3°, owing to the formation of intramolecular π–π interactions between them.
4.2 Aryl-functionalized thiacalix[4]arenes
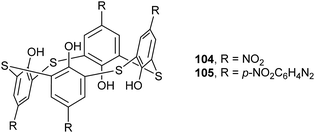
Thiacalix[4]arene derivatives 104 (ref. 87) and 105 (ref. 88) (Fig. 91), modified with four functions at the upper rim, both show a 1,2-alternate conformation with centrosymmetry, indicating that facing inverted phenolic rings are parallel to each other with two pairs of θ values (Table 3). In the solid state, the typical cyclic intramolecular H-bonds disappear, but other H-bonds were observed in the 1,2-alternate conformer. In molecule 104 (Fig. 92), four intramolecular O–H⋯S H-bonds are yielded between the S bridge and its two vicinal OH groups at the same side. Moreover, there are strong intermolecular O14–H14⋯O50 and O13–H13⋯O50 H-bonds with two DMSO guest molecules.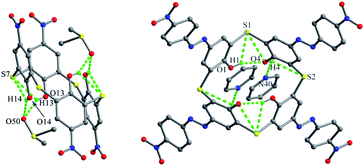 | ||
| Fig. 92 Crystal structures of 104 (left) and 105 (right), showing intra- and intermolecular H-bonds with the solvent molecules. | ||
In the case of 105 (Fig. 92), with four p-nitrophenylazo functions at the upper rim, only two intramolecular O–H⋯O H-bonds govern such a conformation rather than the annular H-bond array, while the other two OH groups create two O–H⋯N H-bonds with the solvent pyridine molecules crystallized in the crystal structure. Moreover, intramolecular O–H⋯S H-bonds and O⋯S contacts were also found.
In brief, identically substituted thiacalix[4]arenes in a 1,2-alternate conformer usually show centrosymmetry with two pairs of θ values. In such an unusual 1,2-alternate conformer, it is hard to create a cycle of intramolecular O–H⋯O H-bonds with four free OH groups, which always exist in the cone conformer. However, the intramolecular O–H⋯O H-bonds are still a key interaction to control the 1,2-alternate conformation. In the packing, the orientations of four phenolic rings at two opposite sides are usually favored to stack parallel to each other and yield the infinite channels.
5. Partial cone structures of thiacalix[4]arenes
In partial cone thiacalix[4]arene derivatives, one of the four phenolic rings is rotated away from the cavity formed by the other three phenolic rings, thus they are all unsymmetrical molecules.5.1 O-Substituted thiacalix[4]arenes
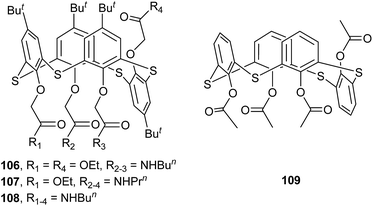
Thiacalix[4]arene derivatives 106–108 possess four ether arms terminated with amide or ester groups at the lower rim (Fig. 93).89 In the solid state, they all take a partial cone conformer, in which the inverted aromatic ring is nearly perpendicular to the R plane with θ angles of 86.6–90.4° (Table 4). In molecule 106 (Fig. 94a), containing two amide and two ester termini, the ester carbonyl group on the inverted aromatic ring is orientated inwards the cavity, while the other one is directed outwards. This conformer is favorable to give an intramolecular N–H⋯O H-bond between the ester carbonyl group and the vicinal amide unit. In the case of 107 (Fig. 94b), although the ester group on the inverted phenolic ring is replaced by an amide group, it adopts a similar conformation and gives the same intramolecular N–H⋯O H-bond as in 106. In the packing, similar infinite chains are produced in 106 (Fig. 95a) and 107 (Fig. 95b) by intermolecular N–H⋯O H-bonds between the amide groups of two vicinal molecules. In addition, such chains in 107 are further linked into supramolecular arrays by inter-chain N–H⋯O H-bonds with the NH groups on the inverted phenolic rings as H-bond donors. However, molecule 108, bearing four amide groups (Fig. 94c), displays a partial cone conformation with the θ range of 80.3–155.0° larger than those in 106 and 107. In such a conformer, all carbonyls of the amide groups are directed away from the cavity, with two intramolecular N–H⋯O H-bonds between the three amide groups at the same side. This H-bonding nature is different from that observed in 106 and 107. In the packing, a zigzag 1-D infinite chain is created by intermolecular N–H⋯O H-bonds (Fig. 95c). In solution, it was observed that only 108 exhibited some affinity towards Na+ and K+ ions.| Compd. | θ (°) | |||
|---|---|---|---|---|
| a Data obtained by calculation with Diamond Version 3.0. | ||||
| 106 | 90.4 | 88.0 | 138.9 | 94.5 |
| 107 | 88.4 | 95.0 | 142.1 | 84.5 |
| 108 | 88.4 | 80.3 | 155.0 | 123.2 |
| 109 | 86.6 | 82.0 | 145.8 | 79.5 |
| 110 | 90.1 | 101.4 | 136.8 | 106.5 |
| 111 | 103.6 | 99.3 | 153.9 | 106.6 |
| 112 | 93.0 | 85.5 | 152.0 | 81.1 |
| 113 | 82.4 | 86.0 | 162.5 | 75.7 |
In the asymmetric unit of tetraacetylated thiacalix[4]arene 109 (Fig. 93),90 there are one thiacalix[4]arene molecule and one CHCl3 solvent molecule. In the packing, a structural motif is formed, in which one thiacalix[4]arene is surrounded by four CHCl3 molecules and vice versa. The CHCl3 molecule is bound by intermolecular C–H⋯O, C–H⋯Cl and Cl⋯O interactions with its adjacent thiacalix[4]arene molecules. In addition, strong intermolecular π–π contacts were found between two parallel phenyl rings, giving rise to a dimer (Fig. 96), which was further fixed by C–H⋯O H-bonds between the carbonyl group and the H atom at the m-position of the opposite phenyl ring.
 | ||
| Fig. 96 Crystal structure of 109 (left), and a dimer of 109 (right) built by C–H⋯O and π–π interactions. | ||
5.2 O/aryl mixed substituted thiacalix[4]arenes
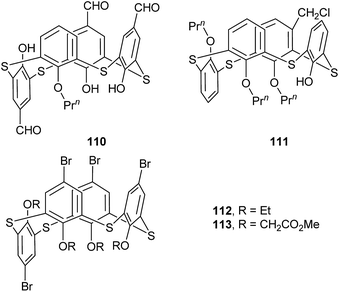
Thiacalix[4]arene derivative 110 (Fig. 97),45 with one n-propoxy at the lower rim and three CHO functions at the upper rim, takes a partial cone conformer, in which the inverted aromatic ring is perpendicular to the R plane with θ value of 90.1° (Table 4). Such a conformer is fixed by intramolecular O–H⋯O and O–H⋯S H-bonds (Fig. 98). In the packing, two molecules of 110 form a head-to-head dimer by π–π stacking between the parallel aromatic rings, two weak C–H⋯O H-bonds and four C–H⋯π interactions (Fig. 99). In addition, six molecules of 110 create a hexameric disc in a back-to-back fashion by S3⋯π and C29–H29⋯O6 interactions (Fig. 100). These discs are further accumulated into a triply helical tube, which is stabilized by C–O⋯π and C–H⋯O interactions.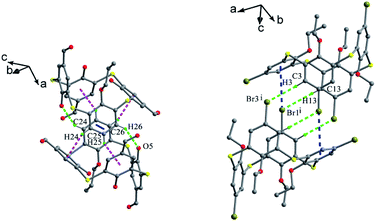 | ||
| Fig. 99 A dimer of 110 (left) built by π–π (blue), C–H⋯O (green) and C–H⋯π (pink) interactions; a dimer of 112 (right) built by Br⋯π (blue) and C–H⋯Br (green) interactions. | ||
Tripropoxythiacalix[4]arene derivative 111 (Fig. 97),91 with a ClCH2 group at the m-position, shows a partial cone conformer with four different θ values (Table 4). Two intramolecular O–H⋯O H-bonds between the OH and two n-propoxy groups at the same side control the strong distortion of the conformer (Fig. 98).
Thiacalix[4]arene derivatives 112 (ref. 78) and 113 (ref. 92) (Fig. 97), functionalized with four Br atoms at the upper rim, both take a partial cone conformation with inverted aromatic ring nearly perpendicular to the plane R in the solid state (Table 4). For compound 112 (Fig. 101), appending four ethyl groups at the lower rim, two facing rings of the three aromatic rings on the same side are flipped slightly inwards, whereas the middle one is flipped considerably outwards. Such a conformer lets a short Br⋯π and two C–H⋯Br interactions contribute to the formation of the self-assembled dimer (Fig. 99). In addition, symmetric expansion of the crystal structure shows S⋯π and C–H⋯S contacts between the dimers.
In the case of 113 (Fig. 101), bearing four ether chains terminated with ester groups, the ring on the opposite of the inverted one is tilted away from the cavity and almost coplanar with the R plane. In the crystal structure, C–H⋯O, C–H⋯S and C–H⋯π interactions fix both the partial cone conformer and the packing. A 1-D chain is created by intermolecular C–H⋯O H-bonds (Fig. 102). These chains further augmented into a 2-D network via ancillary C–Br⋯C interactions.
As shown above, for most of thiacalix[4]arenes in a partial cone conformation, the inverted phenolic ring is almost perpendicular to the R plane with the θ range from 80.3 to 103.6°, while its opposing ring is markedly tilted away from the cavity with the θ range from 136.8 to 162.5° (Table 4). These partial cone conformers tailored with different binding sites are available for the formation of intra- and intermolecular interactions, which are able to stabilize both the partial cone conformers and their supramolecular arrays.
6. Conclusions
In conclusion, we have briefly summarized the development of thiacalix[4]arene derivatives in crystal structures, including the exact description of various conformers, binding patterns and some supramolecular assemblies.In the solid state, thiacalix[4]arenes, in cone, 1,3-alternate, 1,2-alternate and partial cone conformations, show diverse conformational preferences with varied substituents at the lower and/or upper rims, as well as the oxidation of the S bridges. The precise conformations of thiacalix[4]arene cores can be identified with the typical θ angles. In each type of conformers, the θ ranges are very different owing to the features of the substituents. The whole molecular structures are mainly governed by the steric and electronic effects of the substituents belonging to the thiacalix[4]arene molecules and the crystallization conditions.
In the crystal structures of thiacalix[4]arenes, various X–H⋯Y (X = O, N, C; Y = O, N, S, Cl, Br, I) H-bonds, C–H⋯π, π–π, X⋯π (X = S, Cl, Br) and X⋯X (X = S, Cl, Br, I) interactions as well as other contacts between different heteroatoms are found to control the whole molecular conformations and stabilize the supramolecular assemblies. In particular, the introduction of S bridges offers additional binding centres for H-bonds and other non-covalent contacts. Moreover, the solvent molecules play an ancillary role in regulating the conformation and the packing of thiacalix[4]arenes in some cases by two ways: one is guest inclusion occurring in the calix cavity; the other occurs in the holes or channels built by aggregation of the host molecules.
In the supramolecular assemblies, versatile multimeric motifs, chain and channel arrays of thiacalix[4]arenes are created via the interactions described above. In particular, those in cone and 1,3-alternate conformers always display square box-shaped cavities and yield very nice infinite channels, which are extensively influenced with the features of substituents at the lower and/or upper rims. These supramolecular arrays are widely favored to allow inclusion or storage of small molecules with complementary size.
In this review, we have highlighted some of the promising advances of thiacalix[4]arenes in crystallographic researches, but it is clear that there is a long path ahead of to develop the perfect thiacalix[4]arene-based supramolecular assemblies. In the future, more attention should be paid to expand the areas involving design of novel molecules, discovery of new interactions and establishment of the rule in molecular recognition and organic supramolecular assemblies based on the thiacalix[4]arene scaffolds. Understanding well the crystal structures of these scaffolds will provide wide applications in biological molecular recognition, functional organic materials and supramolecular chemistry.
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (No. 21372147).References
- S. Shinkai, Tetrahedron, 1993, 49, 8933–8968 CrossRef CAS.
- C. D. Gutsche, in Calixarenes Revisited, Monographs in Supramolecular Chemistry, ed. J. F. Stoddart, The Royal Society of Chemistry, Cam Cambridge, 1989 Search PubMed.
- H. Kumagai, M. Hasegawa, S. Miyanari, Y. Sugawa, Y. Sato, T. Hori, S. Ueda, H. Kamiyama and S. Miyano, Tetrahedron Lett., 1997, 38, 3971–3972 CrossRef CAS.
- T. Sone, Y. Ohba, K. Moriya, H. Kumada and K. Ito, Tetrahedron, 1997, 53, 10689–10698 CrossRef CAS.
- E. A. Shokova and V. V. Kovalev, Russ. J. Org. Chem., 2003, 39, 1–28 CrossRef CAS.
- N. Iki and S. Miyano, J. Inclusion Phenom. Macrocyclic Chem., 2001, 41, 99–105 CrossRef CAS.
- P. Lhoták, Eur. J. Org. Chem., 2004, 1675–1692 CrossRef.
- N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291–5316 CrossRef CAS.
- R. Kumar, Y. O. Lee, V. Bhalla, M. Kumar and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4824–4870 RSC.
- M. Yamada, M. R. Gandhi, U. M. R. Kunda and F. Hamada, J. Inclusion Phenom. Macrocyclic Chem., 2016, 85, 1–18 CrossRef CAS.
- T. Kajiwara, N. Iki and M. Yamashita, Coord. Chem. Rev., 2007, 251, 1734–1746 CrossRef CAS.
- Y. Bi, S. Du and W. Liao, Coord. Chem. Rev., 2014, 276, 61–72 CrossRef CAS.
- A. Bilyk, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, B. W. Skelton and A. H. White, Inorg. Chem., 2001, 40, 672–686 CrossRef CAS.
- A. Bilyk, J. W. Dunlop, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, G. A. Koutsantonis, B. W. Skelton and A. H. White, Eur. J. Inorg. Chem., 2010, 14, 2089–2105 CrossRef.
- Y. Li, W. Yang, Y. Chen and S. Gong, CrystEngComm, 2011, 13, 259–268 RSC.
- H. Akdas, L. Bringel, E. Graf, M. W. Hosseini, G. Mislin, J. Pansanel, A. D. Cian and J. Fischer, Tetrahedron Lett., 1998, 39, 2311–2314 CrossRef CAS.
- J. Lang, K. Vágnerová, J. Czernek and P. Lhoták, Supramol. Chem., 2006, 18, 371–381 CrossRef CAS.
- N. Iki, C. Kabuto, T. Fukushima, H. Kumagai, H. Takeya, S. Miyanari, T. Miyashi and S. Miyano, Tetrahedron, 2000, 56, 1437–1443 CrossRef CAS.
- N. Morohashi, S. Noji, H. Nakayama, Y. Kudo, S. Tanaka, C. Kabuto and T. Hattori, Org. Lett., 2011, 13, 3292–3295 CrossRef CAS.
- A. Arduini, F. F. Nachtigall, A. Pochini, A. Secchi and F. Ugozzoli, Supramol. Chem., 2000, 12, 273–291 CrossRef CAS.
- J. Hong, C. Yang, Y. Li, G. Yang, C. Jin, Z. Guo and L. Zhu, J. Mol. Struct., 2003, 655, 435–441 CrossRef.
- C. Kabuto, Y. Higuchi, T. Niimi, F. Hamada, N. Iki, N. Morohashi and S. Miyano, J. Inclusion Phenom. Macrocyclic Chem., 2002, 42, 89–98 CrossRef CAS.
- P. Lhoták, T. Šmejkal, I. Stibor, J. Havlíček, M. Tkadlecová and H. Petříčková, Tetrahedron Lett., 2003, 44, 8093–8097 CrossRef.
- O. Kasyan, V. Kalchenko, M. Bolte and V. Böhmer, Chem. Commun., 2006, 18, 1932–1934 RSC.
- N. Morohashi, M. Kojima, A. Suzuki and Y. Ohba, Heterocycl. Commun., 2005, 11, 249–254 CAS.
- H. Katagiri, S. Tanaka, K. Ohkubo, Y. Akahira, N. Morohashi, N. Iki, T. Hattori and S. Miyano, RSC Adv., 2014, 4, 9608–9616 RSC.
- H. Katagiri, N. Iki, T. Hattori, C. Kabuto and S. Miyano, J. Am. Chem. Soc., 2001, 123, 779–780 CrossRef CAS.
- N. Morohashi, H. Katagiri, T. Shimazaki, Y. Kitamoto, S. Tanaka, C. Kabuto, N. Iki, T. Hattori and S. Miyano, Supramol. Chem., 2013, 25, 812–818 CrossRef CAS.
- S. Kharchenko, A. Drapailo, S. Shishkina, O. Shishkin, M. Karavan, I. Smirnov, A. Ryabitskii and V. I. Kalchenko, Supramol. Chem., 2014, 26, 864–872 CrossRef CAS.
- M. Lamouchi, E. Jeanneau, R. Chiriac, D. Ceroni, F. Meganem, A. Brioude, A. W. Coleman and C. Desroches, Tetrahedron Lett., 2012, 53, 2088–2090 CrossRef CAS.
- M. Akkurt, J. P. Jasinski, S. K. Mohamed, O. A. Omran and M. R. Albayati, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2015, 71, o830–o831 CrossRef CAS.
- I. I. Stoikov, D. S. Ibragimova, N. V. Shestakova, D. B. Krivolapov, I. A. Litvinov, I. S. Antipin, A. I. Konovalov and I. Zharov, Supramol. Chem., 2009, 21, 564–571 CrossRef CAS.
- J.-L. Zhao, H. Tomiyasu, X.-L. Ni, X. Zeng, M. R. J. Elsegood, C. Redshaw, S. Rahman, P. E. Georghiou and T. Yamato, New J. Chem., 2014, 38, 6041–6049 RSC.
- O. Kasyan, E. R. Healey, A. Drapailo, M. Zaworotko, S. Cecillon, A. W. Coleman and V. Kalchenko, J. Inclusion Phenom. Macrocyclic Chem., 2007, 58, 127–132 CrossRef CAS.
- O. Kasyan, V. Kalchenko, V. Böhmer and M. Bolte, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o2346–o2348 CrossRef CAS.
- K. Polívková, M. Šimánová, J. Budka, P. Cuřínova, I. Císařová and P. Lhoták, Tetrahedron Lett., 2009, 50, 6347–6350 CrossRef.
- M. Dudič, P. Lhoták, H. Petříčková, I. Stibor, K. Lang and J. Sýkora, Tetrahedron, 2003, 59, 2409–2415 CrossRef.
- O. Kasyan, I. Thondorf, M. Bolte, V. Kalchenko and V. Böhmer, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2006, 62, o289–o294 CrossRef PubMed.
- V. Bhalla, M. Kumar, C. Kabuto, T. Hattori and S. Miyano, Chem. Lett., 2004, 33, 184–185 CrossRef CAS.
- S.-J. Shi, X.-X. Lv, M. Zhao, J.-P. Ma and D.-S. Guo, J. Mol. Struct., 2017, 1127, 81–87 CrossRef CAS.
- V. Burilov, A. Valiyakhmetova, D. Mironova, R. Safiullin, M. Kadirov, K. Ivshin, O. Kataeva, S. Solovieva and I. Antipin, RSC Adv., 2016, 6, 44873–44877 RSC.
- T. Sreeja, V. B. Ganga, L. Praveen and R. L. Varma, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2011, 50, 704–714 Search PubMed.
- V. Bhalla, M. Kumar, H. Katagiri, T. Hattori and S. Miyano, Tetrahedron Lett., 2005, 46, 121–124 CrossRef CAS.
- H. Dvořáková, J. Lang, J. Vlach, J. Sýkora, M. Čajan, M. Himl, M. Pojarová, I. Stibor and P. Lhoták, J. Org. Chem., 2007, 72, 7157–7166 CrossRef.
- W. Wang, W. Yang, R. Guo and S. Gong, CrystEngComm, 2015, 17, 7663–7675 RSC.
- A. Habashneh, C. R. Jablonski, J. Collins and P. E. Georghiou, New J. Chem., 2008, 32, 1590–1596 RSC.
- S. Bouhroum, J. S. Kim, S. W. Lee, P. Thuéry, G. Yap, F. Arnaud-Neu and J. Vicens, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 239–250 CrossRef CAS.
- K. U. M. Rao, T. Kimuro, M. Yamada, Y. Kondo and F. Hamada, Heterocycles, 2015, 91, 989–1000 CrossRef CAS.
- T. Yamato, C. Pérez-Casas, A. Yoshizawa, S. Rahman, M. R. J. Elsegood and C. Redshaw, J. Inclusion Phenom. Macrocyclic Chem., 2009, 63, 301–308 CrossRef CAS.
- S. N. Podyachev, B. M. Gabidullin, V. V. Syakaev, S. N. Sudakova, A. T. Gubaidullin, W. D. Habicher and A. I. Konovalov, J. Mol. Struct., 2011, 1001, 125–133 CrossRef CAS.
- V. Št'astný, I. Stibor, H. Petříčková, J. Sýkora and P. Lhoták, Tetrahedron, 2005, 61, 9990–9995 CrossRef.
- P. Lhoták, M. Dudicč, I. Stibor, H. Petříčková, J. Sýkora and J. Hodačová, Chem. Commun., 2001, 8, 731–732 RSC.
- L.-L. Liu, L.-S. Chen, J.-P. Ma and D.-S. Guo, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, o1110–o1111 CrossRef CAS.
- L.-J. Zhang, L.-L. Liu, Q.-K. Liu and D.-S. Guo, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o1353–o1354 CrossRef CAS.
- P. Lhoták, J. Morávek, T. Šmejkal, I. Stibor and J. Sýkora, Tetrahedron Lett., 2003, 44, 7333–7336 CrossRef.
- G. Mislin, E. Graf, M. W. Hosseini, A. D. Cian and J. Fischer, Tetrahedron Lett., 1999, 40, 1129–1132 CrossRef CAS.
- N. Morohashi, N. Iki, C. Kabuto and S. Miyano, Tetrahedron Lett., 2000, 41, 2933–2937 CrossRef CAS.
- G. Mislin, E. Graf, M. W. Hosseini, A. D. Cian and J. Fischer, Chem. Commun., 1998, 13, 1345–1346 RSC.
- L. Hu, Y. Liu, J.-P. Ma and D.-S. Guo, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o385–o386 CrossRef CAS.
- P. Lhoták, M. Himl, I. Stibor, J. Sýkora, H. Dvořáková, J. Lang and H. Petříčková, Tetrahedron, 2003, 59, 7581–7585 CrossRef.
- L. Liu, K. Huang and C. G. Yan, J. Inclusion Phenom. Macrocyclic Chem., 2010, 66, 349–355 CrossRef CAS.
- V. V. Syakaev, S. N. Podyachev, A. T. Gubaidullin, S. N. Sudakova and A. I. Konovalov, J. Mol. Struct., 2008, 885, 111–121 CrossRef CAS.
- X. Li, S.-L. Gong, W.-P. Yang, Y.-Y. Chen and X.-G. Meng, Tetrahedron, 2008, 64, 6230–6237 CrossRef CAS.
- X. Li, S.-L. Gong, Y.-F. Liu, Q. Zheng and Y.-Y. Chen, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o2097–o2098 CrossRef CAS.
- B.-T. Zhao, Z. Zhou and Z.-N. Yan, J. Chem. Sci., 2009, 121, 1047–1052 CrossRef CAS.
- D.-S. Guo, Z.-P. Liu, J.-P. Ma and R.-Q. Huang, Tetrahedron Lett., 2007, 48, 1221–1224 CrossRef CAS.
- S. N. Podyachev, S. N. Sudakova, B. M. Gabidullin, V. V. Syakaev, A. T. Gubaidullin, W. Dehaen and A. I. Konovalov, Tetrahedron Lett., 2012, 53, 3135–3139 CrossRef CAS.
- T. Yamato, C. Pérez-Casas, S. Rahman, Z. Xi, M. R. J. Elsegood and C. Redshaw, J. Inclusion Phenom. Macrocyclic Chem., 2007, 58, 193–197 CrossRef CAS.
- C. Pérez-Casas, H. Höpfl and A. K. Yatsimirsky, J. Inclusion Phenom. Macrocyclic Chem., 2010, 68, 387–398 CrossRef.
- X.-L. Ni, X. Zeng, D. L. Hughes, C. Redshaw and T. Yamato, Supramol. Chem., 2011, 23, 689–695 CrossRef CAS.
- H. Tomiyasu, J.-L. Zhao, X.-L. Ni, X. Zeng, M. R. J. Elsegood, B. Jones, C. Redshaw, S. J. Teat and T. Yamato, RSC Adv., 2015, 5, 14747–14755 RSC.
- Q. Sun, L. Mu, X. Zeng, J. Zhao, T. Yamato and J. Zhang, Sci. China: Chem., 2015, 58, 539–544 CrossRef CAS.
- Y. Fu, X. Zeng, L. Mu, X.-K. Jiang, M. Deng, J.-X. Zhang and T. Yamato, Sens. Actuators, B, 2012, 164, 69–75 CrossRef CAS.
- B.-T. Zhao, L. Wang and B.-X. Ye, Acta Chim. Sin., 2007, 65, 1663–1669 CAS.
- F. Hamada, M. Yamada, Y. Kondo, S. Ito and U. Akiba, CrystEngComm, 2011, 13, 6920–6922 RSC.
- M. Yamada, Y. Ootashiro, Y. Kondo and F. Hamada, Tetrahedron Lett., 2013, 54, 1510–1514 CrossRef CAS.
- M. Yamada, R. Kanazawa and F. Hamada, CrystEngComm, 2014, 16, 2605–2614 RSC.
- M. Yamada and F. Hamada, Cryst. Growth Des., 2015, 15, 1889–1897 CrossRef CAS.
- J. Lukášek, S. Böhm, H. Dvořáková, V. Eigner and P. Lhoták, Org. Lett., 2014, 16, 5100–5103 CrossRef.
- O. Kundrát, V. Eigner, P. Cuřínová, J. Kroupa and P. Lhoták, Tetrahedron, 2011, 67, 8367–8372 CrossRef.
- F. Botha, S. Böhm, H. Dvořáková, V. Eigner and P. Lhoták, Org. Biomol. Chem., 2014, 12, 5136–5143 RSC.
- P. Lhoták, L. Kaplánek, I. Stibor, J. Lang, H. Dvoráková, R. Hrabal and J. Sýkora, Tetrahedron Lett., 2000, 41, 9339–9344 CrossRef.
- P. Lhoták, M. Himl, I. Stibor and H. Petříčková, Tetrahedron Lett., 2002, 43, 9621–9624 CrossRef.
- J. Kroupa, I. Stibor, M. Pojarová, M. Tkadlecová and P. Lhoták, Tetrahedron, 2008, 64, 10075–10079 CrossRef CAS.
- I. S. Antipin, I. I. Stoikov, A. T. Gubaidullin, I. A. Litvinov, D. Weber, W. D. Habicher and A. I. Konovalov, Tetrahedron Lett., 1999, 40, 8461–8464 CrossRef CAS.
- S. Dong, W. Zhu, D. Yuan and X. Yan, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2002, 58, o376–o377 CrossRef.
- C. Desroches, S. Parola, F. Vocanson, M. Perrin, R. Lamartine, J.-M. Létoffé and J. Bouix, New J. Chem., 2002, 26, 651–655 RSC.
- C. Desroches, S. Parola, F. Vocanson, N. Ehlinger, P. Miele, R. Lamartine, J. Bouix, A. Eriksson, M. Lindgren and C. Lopes, J. Mater. Chem., 2001, 11, 3014–3017 RSC.
- S. P. Singh, A. Chakrabarti, H. M. Chawla and N. Pant, Tetrahedron, 2008, 64, 1983–1997 CrossRef CAS.
- M. Šimánová, H. Dvořáková, I. Stibor, M. Pojarová and P. Lhoták, Tetrahedron Lett., 2008, 49, 1026–1029 CrossRef.
- C. Desroches, V. G. Kessler and S. Parola, Tetrahedron Lett., 2004, 45, 6329–6331 CrossRef CAS.
- W.-N. Xu, J.-M. Yuan, Y. Liu, J.-P. Ma and D.-S. Guo, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2008, 64, o349–o352 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |