Open Access Article
Open Access ArticleLigand- and copper-free Sonogashira and Heck couplings of (Het)aryl chlorides and bromides catalyzed by palladium nanoparticles supported on in situ generated Al(OH)3†
Xing Li*,
Xiaolei Gong,
Zhipeng Li,
Honghong Chang,
Wenchao Gao and
Wenlong Wei*
and
Wenlong Wei*
Department of Chemistry and Chemical Engineering, Taiyuan University of Technology, 79 West Yingze Street, Taiyuan 030024, People's Republic of China. E-mail: lixing@tyut.edu.cn; weiwenlong@tyut.edu.cn
First published on 12th January 2017
Abstract
The ligand- and copper-free Sonogashira reaction of (Het)aryl halides (Br and Cl) with various terminal alkynes and the Heck coupling of (Het)aryl halides (Br and Cl) with a series of olefins, catalyzed by palladium nanoparticles supported on newly generated Al(OH)3, were developed. The catalyst can be readily recovered and reused 6 times without significant loss of activity and palladium leaching.
The efficiency of heterogeneous catalysis in organic synthesis can be improved by employing nanosized catalysts because of their extremely small size and large surface-to-volume ratio.1 Recently, it has been demonstrated that palladium nanoparticles (PdNPs) as catalysts offer significant potential for a wide range of applications in organic synthesis.2 The surface properties of these PdNPs and their catalytic activity are mainly decided by the nature of catalyst supports and the methods for their preparation. PdNPs are usually prepared by chemical vapor deposition from Pd precursors in the presence of hydrogen gas or by classical methods like impregnation or co-precipitation. To improve their catalytic activity, however, some improved methods for their preparation are required.
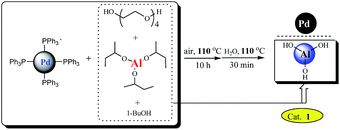
Palladium-catalyzed cross-coupling reactions have become one of the most prominent and powerful methods for the formation of carbon–carbon bonds.3 Among them, the Sonogashira4 and Heck5 reactions have been found useful in the synthesis of a variety of target compounds with applications ranging from natural products and pharmaceuticals to organic functional materials. Significant progress has been achieved by using palladium salts6,7 or homogeneous palladium complexes8,9 as catalysts in the absence of copper co-catalysts. However, these two reactions still suffer from some limitations about the environmental and economical sustainability. In most cases, catalysts failed to be recycled and reused, and phosphorus ligands were also employed. To address these challenges, intense research efforts have been devoted to find suitable heterogeneous Pd catalysts of broad scope, capable of allowing the elimination of copper and phosphorus ligands, as well as affording recovery and reuse of costly palladium catalyst. Although several notable examples of truly green conditions for the Sonogashira10 and Heck11 reactions were reported, the substrate scope is still limited and successful examples for heteroaryl halides remain rare because the corresponding reactions of heteroaryl bromides proved to be more challenging.12,13 From the viewpoint of synthetic cost, developing a generally applicable catalytic system with broader substrate scope has received considerable attention and is highly desirable. Herein, we will report the application of such a palladium nanoparticles catalyst14 supported on Al(OH)3 which was in situ formed (see Scheme 1) in ligand- and copper-free Sonogashira and Heck cross-coupling reactions of (Het)aryl bromides and chlorides. The catalyst has exhibited obviously higher catalytic activity than that prepared by co-precipitation, which demonstrate that the preparation methods of the catalyst exerted an important impact.14d
We first investigated the Sonogashira reaction between 4-methoxybromobenzene (1o) with phenylacetylene (2a) to optimize reaction conditions (Table 1). Screening of common solvents showed that DMSO was the best choice over DMF, and H2O (entry 2 vs. 1 and 3). Bases have a strong effect on the yield, and NaOAc was the best among the bases screened, including K2CO3, K3PO4 and KOAc (entries 7 vs. 2, 4, 5). The reaction did not give 3o at all when KOH was used (entry 6). It was noteworthy that the addition of TBAB improved the result further, providing 3o in 91% yield (entry 8).
| Entry | Solvent | Base | Yieldb (%) |
|---|---|---|---|
| a Reactions were performed with 4-methoxybromobenzene (0.2 mmol) under N2 atmosphere at 120 °C for 40 h.b Isolated yield.c N.D. = Not detected.d Reaction conditions: 4-methoxybromobenzene (0.2 mmol), phenylacetylene (1.5 equiv.), Pd catalyst 1 (8.8 mg, 0.2 mol%), NaOAc (1.5 equiv.), TBAB (0.5 equiv.), DMSO (1.0 mL), N2, 120 °C. | |||
| 1 | DMF | K2CO3 | 21 |
| 2 | DMSO | K2CO3 | 36 |
| 3 | H2O | K2CO3 | Trace |
| 4 | DMSO | K3PO4 | 13 |
| 5 | DMSO | KOAc | 37 |
| 6 | DMSO | KOH | N.D.c |
| 7 | DMSO | NaOAc | 62 |
| 8d | DMSO | NaOAc | 91 |
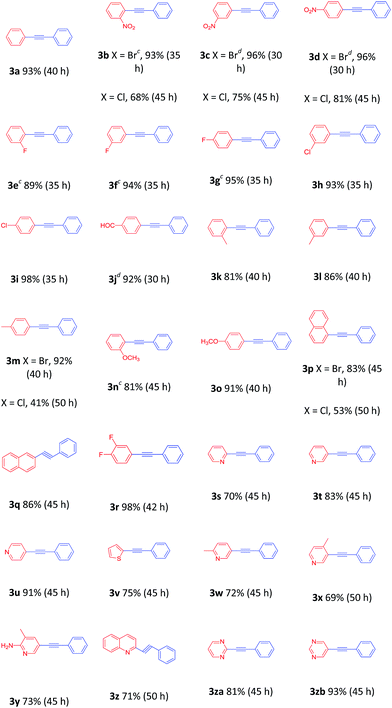
With the optimized protocol in hand, the scope of this catalytic system was next explored. Aryl halides in reaction with 2a were investigated first (Table 2). The reaction worked very well for a range of aryl bromides with various substituents at the phenyl ring, and the products were isolated in good to excellent yields. Aryl bromides with electron-withdrawing substituents at the phenyl ring afforded the desired 1,3-diynes in high yields (3b–3j), whereas aryl bromides bearing electron-donating substituents provided the desired 1,3-diynes in 81–91% yields (3k–3o). The experimental results indicated that α-, and β-bromide substituted naphthalene afforded similarly good yields (3p and 3q). Sterically hindered 1-bromo-3,4-difluorobenzene was also suitable for this transformation (3r). Moreover, the arene ring is not limited to benzene rings. Heteroaryl bromides derived from pyridines, thiophenes, quinolines, and pyrimidines could be converted to the corresponding cross-coupled products in modest to high yields (3s–3zb). NO2-substituted aryl chlorides were also deemed to be suitable cross-coupling partners (3b–3d). Unfortunately, 1-chloronaphthalene and 4-methylchlorobenzene gave only 53% and 41% yields, respectively (3p and 3m).
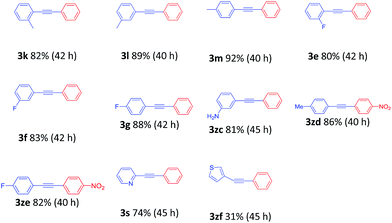
Consequently, the scope of the arylacetylenes was examined in the coupling with bromobenzene. Phenylacetylene bearing electron-donating and electron-withdrawing groups in the benzene ring furnished the products in good to excellent yields, respectively (Table 3, 3e–3m, 3zc). The reactions of 4-ethynyltoluene and 1-ethynyl-4-fluorobenzene with 4-nitrobromobenzene were smoothly carried out to furnish the desired products (Table 3, 3zd and 3ze). In addition, 2-ethynylpyridine and 3-ethynylthiophene, heteroaryl alkynes, were also viable partners, providing 74% and 31% yields, respectively (Table 3, 3s and 3zf).
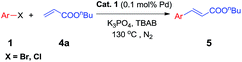
To test the effect of this nano-Pd catalyst in the Heck coupling reaction, the coupling of bromobenzene 1a and butyl acrylate 4a was chosen for initial study. Different solvents were explored in an effort to optimize the yield of 5a. As shown in Table 4, TBAB gave 5a in the highest yield and only poor to moderate yields were obtained with other solvents like dioxane, DMF, NMP, and acetonitrile (Table 4, entry 5 vs. 1–4). Among the bases evaluated, K3PO4 was found to be optimal. Lower yields were provided with K2CO3, TBAA, Et3N and NaOAc (Table 4, entry 5 vs. 6–9). Further optimization clearly indicated that lower catalyst loading and temperature resulted in bad results (Table 4, entries 10–11 vs. 5).
| Entry | Solvent | Base | Cat. 1 (mol% Pd) | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: bromobenzene (0.2 mmol), butyl acrylate (0.26 mmol, 1.3 equiv.), catalyst 1, base (0.2 mmol), solvent, N2 for 19 h.b Isolated yield. | |||||
| 1 | DMF (0.5 mL) | K3PO4 | 0.1 | 130 | 78 |
| 2 | NMP (0.5 mL) | K3PO4 | 0.1 | 130 | 74 |
| 3 | CH3CN (0.5 mL) | K3PO4 | 0.1 | 130 | 61 |
| 4 | Dioxane (0.5 mL) | K3PO4 | 0.1 | 130 | Trace |
| 5 | TBAB (0.3 g) | K3PO4 | 0.1 | 130 | 99 |
| 6 | TBAB (0.3 g) | K2CO3 | 0.1 | 130 | 48 |
| 7 | TBAB (0.3 g) | TBAA | 0.1 | 130 | 86 |
| 8 | TBAB (0.3 g) | Et3N | 0.1 | 130 | 61 |
| 9 | TBAB (0.3 g) | NaOAc | 0.1 | 130 | 79 |
| 10 | TBAB (0.3 g) | K3PO4 | 0.07 | 130 | 82 |
| 11 | TBAB (0.3 g) | K3PO4 | 0.1 | 120 | 89 |
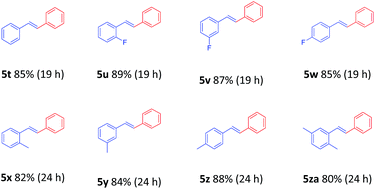
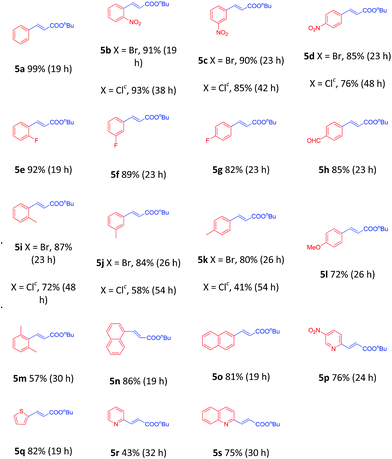
To demonstrate the generality of this nanoparticles pd catalyst 1, our attention was next focused on investigating the substrate scope for Heck cross-coupling using a variety of aryl halides.15 In all of our cases, both electron-rich and electron-poor groups and some heteroaryl rings substituted aryl bromides reacted with butyl acrylate to give the desired products in moderate to high yields, exhibiting a good efficiency (Table 5, 5a–5s). It was found that moderate to good yields could also be obtained when NO2- and Me-substituted chlorobenzenes were used as substrates with longer reaction time and more catalyst loading (Table 5, 5b–5d and 5i–5k). Furthermore, a series of functional groups, including Me-, F-, and diMe-substituted styrenes could smoothly couple with bromobenzene to provide good results (Table 6, 5t–5za).
| a Reaction conditions: (hetero)aryl bromide (0.2 mmol), butyl acrylate (0.26 mmol), catalyst 1 (0.1 mol% Pd), K3PO4 (0.2 mmol), TBAB (0.3 g), N2, 130 °C.b Isolated yield.c Reaction conditions: aryl chloride (0.2 mmol), butyl acrylate (0.26 mmol), catalyst 1 (0.1 mol% Pd), K3PO4 (0.2 mmol), TBAB (0.3 g), DMF (0.2 mL), N2, 130 °C. |
|---|
 |
We further turned our attention to the recovery and reuse of the nano-Pd catalyst 1 through the Sonogashira reaction between 4-methoxybromobenzene (1o) with phenylacetylene (2a) and the results are shown in Table 7. The catalyst could be recovered through membrane filtration and reused in the next reaction. The experimental results showed that the catalytic activity and reaction yield did not obviously decrease after the sixth consecutive cycles. Moreover, the palladium leaching during the recovery process was not obviously observed which was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Table 7, runs 1–6 vs. 0).
| Run | Time (h) | Yieldb (%) | Pd contentc (wt%) |
|---|---|---|---|
| a Reaction conditions: 1o (1.0 equiv.), 2a (1.5 equiv.), Pd catalyst 1 (0.2 mol% Pd), NaOAc (1.5 equiv.), TBAB (0.5 equiv.), DMSO (1.0 mL), N2, 120 °C.b Isolated yield.c The Pd content of the recovered catalyst 1. | |||
| 0 | 40 | 91 | 0.48 |
| 1 | 40 | 91 | 0.48 |
| 2 | 41 | 90 | 0.47 |
| 3 | 42 | 90 | 0.46 |
| 4 | 42 | 90 | 0.46 |
| 5 | 43 | 90 | 0.46 |
| 6 | 45 | 89 | 0.45 |
In conclusion, we have developed efficient, practical and general copper-free Sonogashira and Heck cross-coupling reactions using a nanoparticles pd catalyst supported on in situ generated Al(OH)3. Broad substrate scope, high levels of functional group compatibility especially with heteraryl compounds, and modest to high yields of products are the notable features of the present reactions.
Acknowledgements
We appreciate gratefully the Natural Science Foundation of Shanxi Province (No. 201601D011028 and 20130110094) for financial support.Notes and references
- (a) V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed; (b) D. Wang and D. Astruc, Chem. Rev., 2014, 114, 6949 CrossRef CAS PubMed.
- (a) C. Deraedt and D. Astruc, Acc. Chem. Res., 2014, 47, 494 CrossRef CAS PubMed; (b) A. Bej, K. Ghosh, A. Sarkar and D. W. Knight, RSC Adv., 2016, 6, 11446 RSC; (c) A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181 RSC.
- (a) See ref. 2a; (b) A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973 RSC; (c) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133 CrossRef CAS PubMed; (d) Á. Molnár, Chem. Rev., 2011, 111, 2251 CrossRef PubMed; (e) J. C. C. C. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed; (f) C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027 CrossRef CAS.
- (a) R. Chinchilla and C. Nájera, Chem. Rev., 2007, 107, 874 CrossRef CAS PubMed; (b) T. Ren, Chem. Rev., 2008, 108, 4185 CrossRef CAS PubMed; (c) R. Chinchilla and C. Nájera, Chem. Soc. Rev., 2011, 40, 5084 RSC; (d) H. Doucet and J.-C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834 CrossRef CAS PubMed; (e) C. A. Fleckenstein and H. Plenio, Chem. Soc. Rev., 2010, 39, 694 RSC; (f) N. M. Jenny, M. Mayor and T. R. Eaton, Eur. J. Org. Chem., 2011, 4965 CrossRef CAS.
- For general reviews of Heck coupling, see: (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed; (b) D. M. Cartney and P. J. Guiry, Chem. Soc. Rev., 2011, 40, 5122 RSC; (c) N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef CAS; (d) C. I. M. Santos, J. F. B. Barata, M. Amparo, F. Faustino, C. Lodeiro and M. G. P. M. S. Neves, RSC Adv., 2013, 3, 19219 RSC.
- (a) E. Negishi and L. Anastasia, Chem. Rev., 2003, 103, 1979 CrossRef CAS PubMed; (b) T. Fukuyama, M. Shinmen, S. Nishitani, M. Sato and I. Ryu, Org. Lett., 2002, 4, 1691 CrossRef CAS PubMed; (c) B. Liang, M. Dai, J. Chen and Z. Yang, J. Org. Chem., 2005, 70, 391 CrossRef CAS PubMed; (d) Y. Liang, Y. X. Xie and J. H. Li, J. Org. Chem., 2006, 71, 379 CrossRef CAS PubMed.
- (a) K. Hirotaki and T. Hanamoto, J. Org. Chem., 2011, 76, 8564 CrossRef CAS PubMed; (b) S. Li, Y. Lin, H. Xie, S. Zhang and J. Xu, Org. Lett., 2006, 8, 391 CrossRef CAS PubMed; (c) P. Cyr, S. T. Deng, J. M. Hawkins and K. E. Price, Org. Lett., 2013, 15, 4342 CrossRef CAS PubMed; (d) H. L. Parker, J. Sherwood, A. J. Hunt and J. H. Clark, ACS Sustainable Chem. Eng., 2014, 2, 1739 CrossRef CAS.
- (a) B. de Carné-Carnavalet, A. Archambeau, C. Meyer, J. Cossy, B. Folléas, J.-L. Brayer and J.-P. Demoute, Org. Lett., 2011, 13, 956 CrossRef PubMed; (b) H. Hu, F. Yang and Y. Wu, J. Org. Chem., 2013, 78, 10506 CrossRef CAS PubMed; (c) P. Ehlers, A. Neubauer, S. Lochbrunner, A. Villinger and P. Langer, Org. Lett., 2011, 13, 1618 CrossRef CAS PubMed; (d) D. Mery, K. Heuze and D. Astruc, Chem. Commun., 2003, 1934 RSC; (e) J. Cheng, Y. Sun, F. Wang, M. Guo, J. H. Xu, Y. Pan and Z. Zhang, J. Org. Chem., 2004, 69, 5428 CrossRef CAS PubMed; (f) D. Lee, Y. Kwon and M. Jin, Adv. Synth. Catal., 2011, 353, 3090 CrossRef CAS; (g) K. W. Anderson and S. L. Buchwald, Angew. Chem., Int. Ed., 2005, 44, 6173 CrossRef CAS PubMed.
- (a) C. Wu and J. Zhou, J. Am. Chem. Soc., 2014, 136, 650 CrossRef CAS PubMed; (b) C. Zhang, C. B. Santiago, J. M. Crawford and M. S. Sigman, J. Am. Chem. Soc., 2015, 137, 15668 CrossRef CAS PubMed; (c) M. G. Lauer, M. K. Thompson and K. H. Shaughnessy, J. Org. Chem., 2014, 79, 10837 CrossRef CAS PubMed; (d) M. L. Kantam, P. Srinivas, J. Yadav, P. R. Likhar and S. Bhargava, J. Org. Chem., 2009, 74, 4882 CrossRef CAS PubMed; (e) D.-H. Lee, A. Taher, S. Hossain and M.-J. Jin, Org. Lett., 2011, 13, 5540 CrossRef CAS PubMed; (f) L. Qin, H. Hirao and J. Zhou, Chem. Commun., 2013, 49, 10236 RSC; (g) M. Oberholzer and C. M. Frech, Green Chem., 2013, 15, 1678 RSC; (h) G. R. Rosa and D. S. Rosa, RSC Adv., 2012, 2, 5080 RSC; (i) J. Mo, L. Xu and J. Xiao, J. Am. Chem. Soc., 2005, 127, 751 CrossRef CAS PubMed.
- (a) B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127 CrossRef CAS PubMed; (b) A. Ohtaka, T. Teratani, R. Fujii, K. Ikeshita, T. Kawashima, K. Tatsumi, O. Shimomura and R. Nomura, J. Org. Chem., 2011, 76, 4052 CrossRef CAS PubMed; (c) R. Ciriminna, V. Pandarus, G. Gingras, F. Béland, P. D. Carà and M. Pagliaro, ACS Sustainable Chem. Eng., 2013, 1, 57 CrossRef CAS; (d) L.-M. Tan, Z.-Y. Sem, W.-Y. Chong, X. Liu, W. L. Kwan and C.-L. K. Lee, Org. Lett., 2013, 15, 65 CrossRef CAS PubMed; (e) P. D. Stevens, G. Li, J. Fan, M. Yen and Y. Gao, Chem. Commun., 2005, 4435 RSC; (f) A. Komáromi and Z. Novák, Chem. Commun., 2008, 4968 RSC; (g) M. Jin and D. Lee, Angew. Chem., Int. Ed., 2010, 49, 1119 CrossRef CAS PubMed.
- (a) L. K. Yeung and R. M. Crooks, Nano Lett., 2001, 1, 14 CrossRef CAS; (b) E. H. Rahim, F. S. Kamounah, J. Frederiksen and J. B. Christensen, Nano Lett., 2001, 1, 499 CrossRef CAS; (c) B. H. Lipshutz and B. R. Taft, Org. Lett., 2008, 10, 1329 CrossRef CAS PubMed; (d) R. Bernini, S. Cacchi, G. Fabrizi, G. Forte, F. Petrucci, A. Prastaro, S. Niembro, A. Shafir and A. Vallribera, Green Chem., 2010, 12, 150 RSC; (e) R. Bernini, S. Cacchi, G. Fabrizi, G. Forte, S. Niembro, F. Petrucci, R. Pleixats, A. Prastaro, R. M. Sebastián, R. Soler, M. Tristany and A. Vallribera, Org. Lett., 2008, 10, 561 CrossRef CAS PubMed; (f) A. R. Siamaki, A. E. R. S. Khder, V. Abdelsayed, M. S. El-Shall and B. F. Gupton, J. Catal., 2011, 279, 1 CrossRef CAS.
- (a) C. Torborg, J. Huang, T. Schulz, B. Schäffner, A. Zapf, A. Spannenberg, A. Börner and M. Beller, Chem.–Eur. J., 2009, 15, 1329 CrossRef CAS PubMed; (b) X. Pu, H. Li and T. J. Colacot, J. Org. Chem., 2013, 78, 568 CrossRef CAS PubMed; (c) J. Pschierer and H. Plenio, Org. Lett., 2009, 11, 2551 CrossRef CAS PubMed; (d) H. Remmele, A. Kollhofer and H. Plenio, Organometallics, 2003, 22, 4098 CrossRef CAS.
- (a) D. W. Tay, H. Jong, Y. H. Lim, W. Wu, X. Chew, E. G. Robins and C. W. Johannes, J. Org. Chem., 2015, 80, 4054 CrossRef CAS PubMed; (b) W. Pei, J. Mo and J. Xiao, J. Organomet. Chem., 2005, 690, 3546 CrossRef CAS.
- (a) X. Li, X. Y. Yan, H. H. Chang, L. C. Wang, Y. Zhang, W. W. Chen, Y. W. Li and W. L. Wei, Org. Biomol. Chem., 2012, 10, 495 RSC; (b) X. Li, L. C. Wang, H. H. Chang, C. X. Zhang and W. L. Wei, Appl. Catal., A, 2013, 462, 15 CrossRef; (c) X. Li, W. W. Chen, H. H. Chang, Z. Q. Shao and W. L. Wei, Synthesis, 2014, 46, 1593 CrossRef; (d) X. Li, T. Zhu, Z. Shao, Y. Li, H. Chang, W. Gao, Y. Zhang and W. Wei, Tetrahedron, 2016, 72, 69 CrossRef CAS.
- Extensive screening showed that the optimized reaction conditions were 0.2 mmol (hetero)aryl bromide, 1.3 equiv. of butyl acrylate, catalyst 1 (4.4 mg, 0.1 mol% Pd), 1 equiv. of K3PO4 and TBAB (0.3 g) under N2 atmosphere at 130 °C.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra25416k |
| This journal is © The Royal Society of Chemistry 2017 |