Open Access Article
Open Access ArticleEffects of Osteo-F, a new herbal formula, on osteoporosis via up-regulation of Runx2 and Osterix
Ji Eun Lee†
a,
Mi Hye Kim†a,
Jongki Hongb,
Hyuck Jai Choic,
Jongrak Parkd and
Woong Mo Yang*a
aDepartment of Convergence Korean Medical Science, Graduate School, Kyung Hee University, Seoul 02447, Republic of Korea. E-mail: wmyang@khu.ac.kr; Fax: +82-2-961-2209; Tel: +82-2-961-2209
bCollege of Pharmacy, Kyung Hee University, Seoul, Republic of Korea
cEast-West Medical Research Institute, Kyung Hee University, Seoul, Republic of Korea
dMungyeong Omijavalley Inc., Mungyeong, Republic of Korea
First published on 4th January 2017
Abstract
Osteoporosis is characterized by low bone mass and structural weakness, resulting in a high risk of fracture. The aim of this study is to investigate the ameliorative effects of a herbal formula, Osteo-F, containing Schizandra chinensis, Lycium chinensis and Eucommia ulmoides, on osteoporosis. Female ICR mice were randomly assigned to a sham-operated group (Sham) and five ovariectomized (OVX) groups: OVX with vehicle (OVX), OVX with calcium (Ca), and OVX with 1, 10 and 100 mg per kg per day Osteo-F (OF1, OF10 and OF100). Oral administration of Ca or Osteo-F started 7 weeks after OVX and lasted for 13 weeks. The femurs were collected to analyze the bone mineral content (BMC) and bone mineral density (BMD) and bone histology. Blood was collected to examine serum calcium concentration. In addition, the expressions of Runx2 and Osterix in SaOS-2 osteoblast cells were analyzed to confirm the mechanism on osteoblast differentiation. In the present study, Osteo-F treatment significantly restored the low BMC and BMD in the femur. As shown in histological analysis, hyperplasia of the growth plate in the epiphyseal plate was markedly recovered in Osteo-F-treated bone. In addition, serum calcium concentrations were increased with Osteo-F treatment. In the in vitro study, the expressions of Runx2 and Osterix were significantly increased in Osteo-F-treated SaOS-2 osteoblast cells. These results indicate that oral administration of Osteo-F, a newly developed formula, has ameliorative effects on osteoporosis by increasing osteoblast related markers, such as Runx2 and Osterix.
1 Introduction
Since bone is a major tissue that provides internal skeletal support, debilitating bone diseases, including osteoporosis, osteopetrosis, osteogenesis imperfecta and Paget's disease, are serious dangers during the remaining life time.1 In particular, over 200 million people worldwide suffer from osteoporosis, one of the common skeletal disorders.2,3 Osteoporosis is characterized by low bone mass and bone mineral density (BMD), resulting in an increase of fracture risk.4Although various reasons could induce osteoporosis, estrogen deficiency is known to disrupt the equilibrium of bone remodeling.2 A number of recent studies have demonstrated that estrogen plays roles as a regulator of bone turnover and population of osteoclasts.5 Therefore, osteoporosis is highly distributed in post-menopausal women who have stopped having ability to produce estrogen.6 For that reason, hormone replacement therapy (HRT) is primarily used to treat post-menopausal osteoporosis. However, increase of cancer risk by long-term use of HRT is concern in osteoporotic patients.7 Prescribed bisphosphonate to prevent bone loss in post-menopausal women is reported to induce atrial fibrillation and osteonecrosis of the jaw.8,9 Thus approach to find alternative treatment of osteoporosis with no adverse effects is desirable.
Osteo-F is a newly developed herbal formula consisted of the fruit of Schizandra chinensis, Lycium chinensis and radix of Eucommia ulmoides based on the classic book. According to the Korean traditional literature, S. chinensis, L. chinensis and E. ulmoides have been used for treating bone disease.10 Additionally, studies regarding on their pharmacological relevancies were recently published. In previous study, S. chinensis exhibited ameliorative effect on osteoporosis in ovariectomized (OVX) mice by increasing estrogen receptor-α and -β.11 L. chinensis exerted an inhibition of osteoclast formation.12 The bone remodeling-related factors, Osterix, runt-related transcription factor 2 (Runx2), receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG) were regulated by E. ulmoides in SaOS-2 osteoblast cells.13
Based on previous evidences, we combined a new formula to improve osteoporosis. In this study, the effects of Osteo-F on osteoporosis were determined to analyze the bone mineral content (BMC) and BMD in OVX mice. To understand its mechanism of actions on bone remodeling, the expressions of Runx2 and Osterix in SaOS-2 cells were further investigated.
2 Experimental
2.1 Preparation of Osteo-F
The dried fruit of S. chinensis and L. chinensis, and the radix of E. ulmoides were obtained from Jung-do Herb (Seoul, Korea). Each 200 g of S. chinensis and L. chinensis, and E. ulmoides were extracted twice with 2 L of distilled water, respectively, for 2 h under reflux at 100 °C. Then the extracts were evaporated using BÜCHI Rotavapor R-220 (BÜCHI Labortechnik, Switzerland) and freeze-dried using lyophilizer (Ilshin Biobase, Korea). The final yields of each herb were 35%, 31.8% and 10.1%, respectively. Each voucher specimens (SC-W100, LC-W100 and EU-W100) were deposited at our laboratory. Osteo-F consists of S. chinensis, L. chinensis and E. ulmoides according to the dilution ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. For the treatment, 60 mg S. chinensis, 30 mg L. chinensis and 10 mg E. ulmoides extract powders were mixed and dissolved in 1 mL distilled water (finally prepared 100 mg mL−1 Osteo-F), considered on the final yields of each extract.
1, respectively. For the treatment, 60 mg S. chinensis, 30 mg L. chinensis and 10 mg E. ulmoides extract powders were mixed and dissolved in 1 mL distilled water (finally prepared 100 mg mL−1 Osteo-F), considered on the final yields of each extract.
The quality evaluation of S. chinensis, L. chinensis and E. ulmoides was performed with three reference compounds of schizandrin, betaine and geniposidic acid, respectively, by high-performance liquid chromatography diode array detector (HPLC-DAD) on an Agilent Series 1100 HPLC system. Chromatographic separation was achieved using the Atlantis HILIC silica (4.6 × 150 mm, 5 μm). The eluent for schizandrin, betaine and geniposidic acid consisted of 30 mM ammonium acetate and acetonitrile at a flow rate of 1.0 mL min−1. The detection wavelength was 220 nm at 30 °C. The peaks of schizandrin, betaine and geniposidic acid were synchronized with their standards (Fig. 1). The concentrations of schizandrin, betaine and geniposidic acid were 273.03 μg mL−1 (1.82%), 360.32 μg mL−1 (1.44%) and 19.56 μg mL−1 (0.07%), respectively.
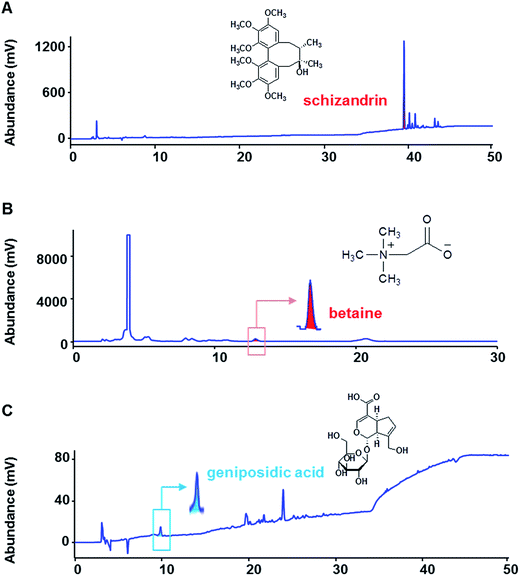 | ||
| Fig. 1 High-performance liquid chromatography (HPLC) analysis of Schizandra chinensis, Lycium chinensis and Eucommia ulmoides. | ||
2.2 Mice
ICR female mice aged 6 weeks were purchased from RAONBIO Inc. (Yongin, Korea). Mice were maintained in an air-conditioned room and a 12 h light-dark cycle with freely available of food and water. All experiments were conducted according to the guidelines of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Committee on Care and Use of Laboratory Animals of the Kyung Hee University (KHUASP(SE)-15-093).2.3 Induction of ovariectomy-induced osteoporosis
After 1 week of adaption, the mice were randomly divided into six groups (n = 7); Sham, OVX, Ca (calcium chloride-treated group), OF1, OF10 and OF100 (Osteo-F-treated groups at the doses of 1, 10 and 100 mg per kg per day, respectively). All mice except Sham group were OVX and seven mice in Sham group were sham-operated. To recover and induce osteoporosis, mice were maintained for 7 weeks. After that, Sham and OVX groups were treated with the vehicle (PBS containing 1% DMSO). 150 mg kg−1 of calcium chloride (Sigma, MO, USA) was orally administrated to Ca group as positive control. And Osteo-F was orally treated five times per week during 6 weeks. The mice were sacrificed after blood collection by cardiac puncture. The both side of femurs were obtained to analyze the BMC, BMD and bone histology.2.4 Bone histology
The right femurs were fixed in 10% neutralized formalin for 18 h and demineralized with 0.1 M ethylene diamine tetraacetic acid aqueous solution for 1 month. After demineralization, femur samples were dehydrated by ethanol and xylene. Paraffin-embedded femur samples were sagittal sectioned into glass slides. The slides were stained with hematoxylin and eosin. Histological changes were monitored under a light microscope using the Leica Application Suite (LAS; Leica Microsystems, Buffalo Grove, IL, USA). Digital images were taken at a magnification of ×40.2.5 Measurement of BMC and BMD
The left femurs were stored in 10% neutralized formalin until use. The levels of BMC and BMD of the left femurs were determined by dual-energy X-ray absorptiometry with an InAlyzer instrument (MEDIKORS, Seoul, Korea).2.6 Serum analysis
Collected blood samples were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min to obtain serum. The serum levels of calcium and magnesium were estimated by using commercial ELISA kit (Adipogen, Shizuoka, Japan) according to the manufacturers' instructions. The optical density was read at 570 nm and 660 nm using a microplate reading instrument (BioTek, PA, USA).
000 rpm for 30 min to obtain serum. The serum levels of calcium and magnesium were estimated by using commercial ELISA kit (Adipogen, Shizuoka, Japan) according to the manufacturers' instructions. The optical density was read at 570 nm and 660 nm using a microplate reading instrument (BioTek, PA, USA).
2.7 Cell culture and proliferation analysis
SaOS-2 cells (human osteoblastic cell line) were maintained in Dulbecco's modified minimal essential medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 1% penicillin and 10% heat-inactivated fetal bovine serum (FBS) at 37 °C in an atmosphere containing 5% CO2 that 95% humidity. The culture medium was changed every 3–4 day.The cells were seeded into 96-well plates at a density of 1 × 106 cells per well. After 24 h, the cells were treated with culture medium containing different concentrations of Osteo-F extract (1, 10 and 100 μg mL−1) for 24 h. Then, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) solution (2 mg mL−1) was added into each well and cultured for 2 h at 37 °C. Afterwards, MTT medium was discarded and DMSO was added. The absorbance at 570 nm was measured. The experiments were performed in triplicate.
2.8 Western blotting analysis
RIPA buffer (50 mM Tris–HCl (pH 7.4), 1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mM NaCl) containing protease inhibitors (Roche, Hoffmann, USA) was used for cell protein extraction. The protein concentration was determined using the Bradford protein assay. 20 μg of protein was denatured with sodium dodecylsulfate buffer, then electrotransferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). Primary antibody (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in 3% bovine serum albumin; Cell Signaling, USA) was then added to the membrane. Secondary antibodies (Santa Cruz, CA, USA) were incubated for 2 h at RT. The membranes were then visualized using an enhanced chemiluminescence detection reagent (Amersham Pharmacia, Piscataway, NJ, USA).
1000 in 3% bovine serum albumin; Cell Signaling, USA) was then added to the membrane. Secondary antibodies (Santa Cruz, CA, USA) were incubated for 2 h at RT. The membranes were then visualized using an enhanced chemiluminescence detection reagent (Amersham Pharmacia, Piscataway, NJ, USA).
2.9 Statistical analysis
Significance was determined by one-way analysis of variance (ANOVA) and Dunnett's multiple comparison tests. In all analyses, p < 0.05 was taken to indicate statistical significance.3 Results and discussion
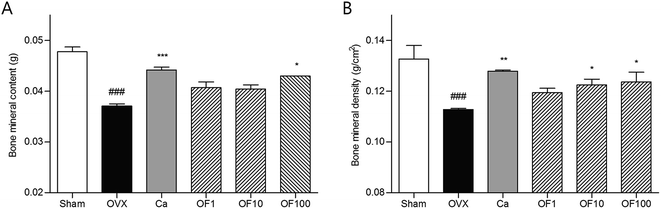
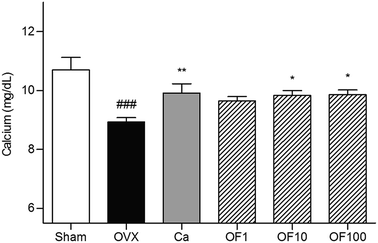
The maintenance of bone homeostasis is controlled by constant replenishment as known as bone remodeling.14 The two equal, opposite and balanced forces of bone formation by osteoblasts and bone resorption by osteoclasts regulate bone equilibrium.15 Interaction of bone-forming cells and bone-resorbing cells occurs on the bone surface during growth of bone.16 For that reason, imbalance of bone metabolism leads to disruption of bone remodeling process.17 Predominant bone resorption by osteoclast activation rather than osteoblast-mediated bone formation in osteoporosis finally leads to loss of bone mass.18 The clinically osteoporotic patient has significant decreased levels of BMD and BMC. BMD is well known as a hallmark of diagnosis for bone structural deterioration. In addition to BMD, BMC is a one of the primary standards to analyze mechanical strength.19 The recovery of bone parameters, BMD and BMC, is strongly associated with loss of bone.20In this study, OVX-induced osteoporotic mice showed decreases of femur BMC and BMD compared to Sham group. Ca treatment significantly increased the both of BMC and BMD. Similar to result from Ca group, there was significant increment of BMC level in 100 mg kg−1 Osteo-F-treated femur. The decreased BMD was markedly recovered in OF10 and OF100 groups (Fig. 3). In addition, the hyperplasia of growth plate was appeared in osteoporosis-like bone in OVX mice. Administration of Ca suppressed the growth plate thickness compared to OVX group. Increases of growth plate thickness were ameliorated by Osteo-F administration, indicating that Osteo-F attenuated hyperplasia of growth plate (Fig. 2). Furthermore, calcium concentration in serum as bone turnover marker was significantly decreased by OVX surgery. However, administration of 1, 10 and 100 mg kg−1 Osteo-F markedly increased the calcium levels in a dose-dependent manner. Especially, the serum of calcium level was nearly recovered to normal range in 100 mg kg−1 Osteo-F-treated group (Fig. 4), while the level of magnesium was not changed by Osteo-F (data not shown). These results suggest that Osteo-F, newly developed formula, is effective on bone formation by increase of serum calcium levels and has anti-osteoporotic effect in OVX mice.
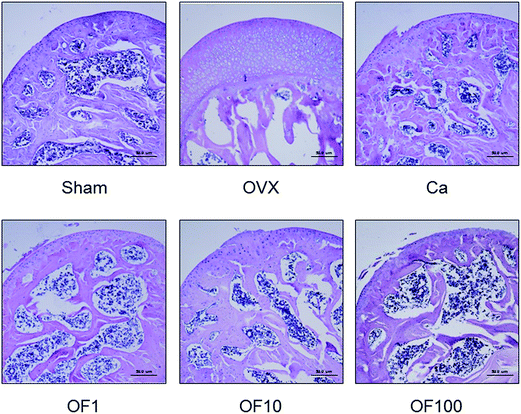 | ||
| Fig. 2 The effects of Osteo-F on the growth plate thickness of femur. The sagittal sections were stained with hematoxylin and eosin (H & E) and the magnification was ×200. | ||
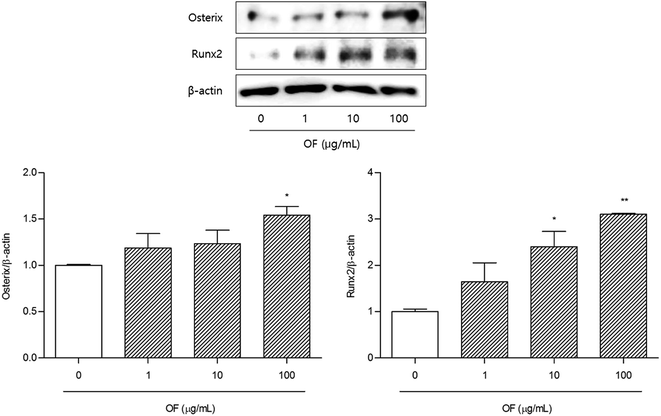
To clarify the mechanisms of Osteo-F on osteoporosis, osteoblast differentiation-related factors such as Runx2 and Osterix were determined in osteoblast like SaOS-2 cells. Two transcriptional factors, Runx2 and Osterix, have been reported to be essential for osteoblast differentiation and maturation from pre-osteoblasts.21 Because bone formation depends on differentiation of osteoblast (bone-forming cells), the expressions of Runx2 and Osterix are regarded as osteogenic transcriptional factors for skeletal development.22,23 The expression level of Runx2 protein was significantly increased by 100 μg mL−1 Osteo-F treatment in SaOS-2 osteoblast cells. Similarly, 10 and 100 μg mL−1 Osteo-F treatment up-regulated the expression of Osterix protein, compared to non-treated cells (Fig. 5). Especially, 100 μg mL−1 Osteo-F treatment significantly increased the both of Osterix and Runx2. All concentrations of Osteo-F exerted equivalent effects on SaOS-2 cell viability. Osteo-F was not cytotoxic to SaOS-2 cell (data not shown). Osteo-F could differentiate from pre-osteoblasts to mature osteoblasts by up-regulating Runx2 and Osterix demonstrating the potential mechanism of action of Osteo-F on bone remodeling, especially osteoblast differentiation.
4 Conclusions
Osteo-F, a newly combined formula, has anti-osteoporotic effects in OVX mice. The levels of BMC and BMD were ameliorated, and histological changes of femur were recovered by Osteo-F treatment. In addition, Osteo-F showed bone remodeling by promoting osteoblast differentiation via up-regulation of Runx2 and Osterix. Taken together, Osteo-F could be one of the alternative materials for the improvement of osteoporosis.Conflict of interest
The authors declare no conflict of interest.Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (PJ011668)” Rural Development Administration, Republic of Korea.References
- T. C. Phan, J. Xu and M. H. Zheng, Histol. Histopathol., 2004, 19, 1325–1344 CAS.
- S. R. Cummings and L. J. Melton, Lancet, 2002, 359, 1761–1767 CrossRef.
- N. Harvey, E. Dennison and C. Cooper, Nat. Rev. Rheumatol., 2010, 6, 99–105 CrossRef PubMed.
- B. L. Clarke and S. Khosla, Radiol. Clin., 2010, 48, 483–495 CrossRef PubMed.
- J. A. Cauley, Steroids, 2015, 99, 11–15 CrossRef CAS PubMed.
- K. Venken, F. Callewaert, S. Boonen and D. Vanderschueren, Osteoporosis Int., 2008, 19, 1517–1525 CrossRef CAS PubMed.
- J. E. Rossouw, G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen and J. Ockene, J. Am. Med. Assoc., 2002, 288, 321–333 CrossRef CAS PubMed.
- T. J. Bunch, J. L. Anderson, H. T. May, J. B. Muhlestein, B. D. Horne, B. G. Crandall, J. P. Weiss, D. L. Lappe, J. S. Osborn and J. D. Day, Am. J. Cardiol., 2009, 103, 824–828 CrossRef CAS PubMed.
- N. Yarom, R. Yahalom, Y. Shoshani, W. Hamed, E. Regev and S. Elad, Osteoporosis Int., 2007, 18, 1363–1370 CrossRef CAS PubMed.
- J. Heo, DONGUIBOGAM Treasured Mirror of Eastern Medicine, BUBIN PUBLISHERS CO, Seoul, Republic of Korea, fourth edn, 2005, pp. 765–766 Search PubMed.
- M. H. Kim, Y. Y. Choi, J. M. Han, H. S. Lee, S. B. Hong, S. G. Lee and W. M. Yang, Food Funct., 2014, 5, 1594–1601 CAS.
- J. Yin, Y. Tezuka, K. Kouda, Q. L. Tran, T. Miyahara, Y. Chen and S. Kadota, Biol. Pharm. Bull., 2004, 27, 583–586 CAS.
- J. E. Lee, M. H. Kim, Y. Y. Choi, H. J. Lee and W. M. Yang, Orient. Pharm. Exp. Med., 2016, 16, 53–57 CrossRef CAS.
- T. Luhmann, O. Germershaus, J. Groll and L. Meinel, J. Controlled Release, 2012, 161, 198–213 CrossRef CAS PubMed.
- M. Mittal and N. Chattopadyay, Indian J. Endocrinol. Metab., 2012, 16, S279–S281 Search PubMed.
- W. Sipos, P. Pietschmann, M. Rauner, K. Kerschan-Schindl and J. Patsch, Wien. Med. Wochenschr., 2009, 159, 230–234 CrossRef PubMed.
- M. L. Brandi, Rheumatology, 2009, 48(suppl. 4), 3–8 Search PubMed.
- G. Karsenty, Genes Dev., 1999, 13, 3037–3051 CrossRef CAS PubMed.
- S. Satpathy, A. Patra and B. Ahirwar, J. Complementary Integr. Med., 2015, 12, 251–266 Search PubMed.
- M. Kleerekoper, Osteoporosis Int., 2006, 17, 1707–1715 CrossRef CAS PubMed.
- T. Komori, J. Cell. Biochem., 2006, 99, 1233–1239 CrossRef CAS PubMed.
- N. Nadiminty, W. Lou, S. O. Lee, F. Mehraein-Ghomi, J. S. Kirk, J. M. Conroy, H. Zhang and A. C. Gao, Clin. Cancer Res., 2006, 12, 1420–1430 CrossRef CAS PubMed.
- X. Zhou, Z. Zhang, J. Q. Feng, V. M. Dusevich, K. Sinha, H. Zhang, B. G. Darnay and B. de Crombrugghe, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 12919–12924 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |