 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development and multi-use applications of dengue NS1 monoclonal antibody for early diagnosis
E. Kathiresana,
R. Paramasivanb,
V. Thenmozhib,
Aparup Dasb,
K. J. Dhananjeyanb,
S. Gowri Sankarb,
S. Victor Jerald Leob,
S. Rathnaprabac and
S. John Vennison *a
*a
aDepartment of Biotechnology, Anna University, BIT Campus, Tiruchirappalli 620 024, India. E-mail: johnvennison36@gmail.com
bCentre for Research in Medical Entomology (CRME), Indian Council of Medical Research, Chinna Chokkikulam, Madurai 625 002, India
cDepartment of Animal Biotechnology, Madras Veterinary College, Tamil Nadu Veterinary and Animal Sciences University, Chennai, 600007, India
First published on 12th January 2017
Abstract
Swift and early diagnosis of dengue is important for case management and epidemiological purpose. We developed a dengue NS1 antigen specific capture enzyme-linked immunosorbent assay (ELISA) for early diagnosis using a panel of monoclonal antibodies (mAbs), which are generated against monomeric non-structural (NS1) antigen expressed in E. coli. The mAbs were tested for its usefulness in dengue diagnosis during dengue outbreak in Tamil Nadu, India and compared with routinely used methods like RT-PCR, MAC-ELISA and commercially available NS1 antigen detection kit. The results of the comparative analysis suggest that the raised mAb may be used for the routine screening of suspected sera and also for the detection of dengue virus antigen in field collected vectors (mosquitoes).
Introduction
Dengue is a mosquito-borne viral infection caused by closely related four antigenically distinct virus serotypes (DEN-1, DEN-2, DEN-3 and DEN-4) which are classified under the genus Flavivirus, family Flaviviridae. The majority (∼75%) of infections in humans are asymptomatic or with mild symptoms and may leads to dengue fever (DF) or more severe form known as dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). Due to non-availability of vaccines or drugs, early diagnosis of dengue infection is important to differentiate between dengue and other diseases with similar clinical symptoms, such as malaria, yellow fever, Japanese encephalitis and chikungunya.1,2The dengue virus nonstructural NS1 protein is a ∼47 kDa glycoprotein which is produced during viral replication and it has been identified as an important antigen in dengue virus infection.3 NS1 protein is produced by all flaviviruses and is secreted from infected mammalian cells during early phases of infection.
NS1 glycoprotein can be detected by an enzyme linked immunosorbent assay (ELISA) using a NS1 glycoprotein specific monoclonal antibody (mAb) in dengue affected human sera.4–8 Moreover, this test will provide rapid results, hence it has more advantage on endemic regions.9–13 Sensitivity of commercially available kits varies depending on the severity of the cases and the endemic regions, also varies for the four dengue virus serotypes depending on the geographical region.14–16
The virus is transmitted to humans mainly by biting of infected Aedes aegypti and Ae. albopictus. In the absence of vaccine or antiviral treatment epidemiological investigation as well as virus monitoring in mosquitoes is indispensable for implementing effective control of disease spread by reducing mosquito population. Some of the commercially available NS1 antigen based kits, which are intended for human serum analysis, has also been reported to detect dengue antigen in field caught mosquitoes. However, those kits were not evaluated with respect to the sensitivity and stability for the field collected mosquito pools.1,17 NS1 antigen-capture ELISA is more advantages like, sensitive, ease of performing in testing, cost-effective and non-requirement of sophisticated equipment.
In this present study, monoclonal antibodies have been produced against recombinant dengue NS1 antigen. The isotypes of the monoclonal antibodies were determined and specificity and sensitivity of antigen capture ELISA for the detection of dengue infection were determined compared with the commercially available dengue diagnosis kit during on suspected dengue outbreak. The mAbs were utilized in both ELISA and IFA assay to analyse the virus presence in the field collected Ae. aegypti and Ae. albopictus, also in the virus propagated Toxorhynchites splendens larvae for laboratory dengue virus analysis (Toxo-IFA).
Materials and methods
All experiments were performed in compliance with the relevant laws and institutional guidelines. The study was approved by the ethical committee of the Anna University. Animals were maintained in accordance with the guidelines of the National Institute of Nutrition, Hyderabad, India, and approved by the Institutional Ethical Committee (IEC) of Anna University.Anti-dengue NS1 mouse monoclonal hybridomas
Dengue-2 virus recombinant nonstructural NS1 protein has been expressed in E. coli and purified without any in vitro post translational processes. The production and purification of NS1 protein has been described by us already.18 Myeloma Sp2/0 cells were obtained from NCCS – Pune, India. Four 6–8 week old female BALB/c mice were immunized 4 times intraperitoneally with 50 μg of monomeric NS1 antigen. Complete Freund's adjuvant (Sigma-Aldrich, USA) was used in dose 1 and the incomplete Freund's adjuvant (Sigma-Aldrich, USA) was used in dose 2 and 3. Final dose was immunized with 50 μg of NS1 antigen using PBS. The immune response were analyzed using indirect ELISA and serum titers were estimated.19 The splenocytes were fused with myeloma Sp2/0 cell line at a ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using 50% (w/v) polyethylene glycol (PEG).20
1 using 50% (w/v) polyethylene glycol (PEG).20
Screening and purification of mAbs
Hybridoma supernatants were screened for the presence of mAbs against dengue NS1 antigen by indirect ELISA. 100 μL of purified dengue NS1 protein (10 μg mL−1) in PBS was used for coating 96 well plates and incubated overnight at 4 °C. The wells were blocked with 2% BSA, for 2 h at 37 °C. After three washes with PBS containing 0.05% Tween 20 (PBS-T) the wells were incubated with 100 μL supernatant from hybridoma clone for 2 h at 37 °C. The wells were washed three times with PBS and the antibodies were detected using goat anti mouse IgG conjugated with horseradish peroxidase as secondary antibody at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilutions for 1 h incubation at 37 °C.21 The plate was again washed three times with PBST and 100 μL of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added to the wells. After 15 min of color development, optical density was measured at 450 nm. Positive hybridoma cells were cloned by limiting dilution. The isotypes of the mAbs were determined with the mouse monoclonal antibody isotyping kit (Thermo Scientific, USA) according to manufacturer's instructions. The mAbs from hybridoma culture supernatants were purified using Protein-G Sepharose column (Sigma-Aldrich, USA). The affinity-purified mAbs were dialyzed against PBS pH 7.2 for 16 h. Protein estimation was done by Bradford assay.22
000 dilutions for 1 h incubation at 37 °C.21 The plate was again washed three times with PBST and 100 μL of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added to the wells. After 15 min of color development, optical density was measured at 450 nm. Positive hybridoma cells were cloned by limiting dilution. The isotypes of the mAbs were determined with the mouse monoclonal antibody isotyping kit (Thermo Scientific, USA) according to manufacturer's instructions. The mAbs from hybridoma culture supernatants were purified using Protein-G Sepharose column (Sigma-Aldrich, USA). The affinity-purified mAbs were dialyzed against PBS pH 7.2 for 16 h. Protein estimation was done by Bradford assay.22
Toxo-IFA screening
Dengue serotype 1 (P-23086) virus was obtained from NIV, Pune, India. Tx. splendens larvae were immobilized on ice for few minutes and dengue 1 virus was inoculated intracerebrally with different dilutions.23,24 During rearing period, Tx. splendens larvae were fed with larvae of other species of mosquitoes maintained in the colony and larvae inoculated with sterile BAPS (bovine albumin phosphate buffered saline PH 7.4) were used as controls. Virus inoculated and normal mosquito larvae were grown at 28 ± 1 °C and relative humidity of 80%.After rearing period, head squashes of virus infected and normal larvae were made on the Teflon coated microscopic slides. Slides were air dried and fixed with chilled acetone for 5 minutes. Monoclonal antibody (15 μl diluted in PBS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)/cell culture fluid (20 μl) was added on the smear and incubated in a humid chamber at 37 °C for 30 minutes, followed by 4 times wash with 1× PBS (20 μl); 15 μl of 1
5)/cell culture fluid (20 μl) was added on the smear and incubated in a humid chamber at 37 °C for 30 minutes, followed by 4 times wash with 1× PBS (20 μl); 15 μl of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution of goat anti-mouse IgG FITC conjugate (Sigma-Aldrich) was added, incubated for 30 minutes and washed with 1× PBS for 4 times. The smears were mounted in 10% glycerol and examined under fluorescent microscope. Commercially available dengue virus specific monoclonal antibodies (Abcam, USA) were used as positive control.
100 dilution of goat anti-mouse IgG FITC conjugate (Sigma-Aldrich) was added, incubated for 30 minutes and washed with 1× PBS for 4 times. The smears were mounted in 10% glycerol and examined under fluorescent microscope. Commercially available dengue virus specific monoclonal antibodies (Abcam, USA) were used as positive control.
Cross reactivity and specificity of mAbs
Monoclonal antibodies were coupled with horseradish peroxidase (HRP) according to modified periodate method.25 Cross reactivity and specificity of generated mAbs were analyzed by the ELISA and western blot against all four dengue serotypes and also against Japanese encephalitis (JE), West Nile and chikungunya viruses.NS1 antigen capture ELISA on mosquito pools
Microtitration plates were coated overnight with 100 μl per well of mAb at 4 °C. Wells were washed with PBST buffer (PBS, 0.05% Tween 20) and 100 μl per well of blocking buffer (2% BSA) was added and incubated at room temperature for 2 h. Mosquito homogenate samples were added to wells (100 μl per well) and incubated for 2 h at room temperature. The wells were washed again and incubated for 2 h at 37 °C with 100 μl per well of HRP conjugated NS1 mAbs. After three further washes with PBST, 100 μL of (TMB) substrate was added to the wells, allowed for color development and optical density was measured at 450 nm.26RNA extraction, RT-PCR and MAC-ELISA
A total of 30 suspected serum specimens were collected between days 0 and 7 (acute-phase) during on suspected dengue epidemic in Tamil Nadu, India, in 2015. For cross-reactivity analysis, Japanese encephalitis (JE), West Nile virus and chikungunya serum samples (each 5, total numbers = 15) were analysed. Clinical samples were analyzed using HRP conjugated mAbs and commercially available Panbio Dengue Early ELISA kit (Panbio Diagnostics, Brisbane, Australia) and results were compared. The cut-off value has been calculated using the average absorbance of confirmed negative samples and with their standard deviation. The RNA was extracted from 100 μL of serum sample using QIAamp viral RNA mini kit (Qiagen). All the serum samples were subjected to MAC-ELISA and RT-PCR and serotypes were analysed using specific primers.27 Selected samples were subjected to real time RT-PCR.28Results
Production of anti-dengue NS1 mAbs
Seven positive hybridoma clones were successfully sub-cloned, and three clones (D5, E4 and E7) were selected for further studies based on their reactivity against dengue NS1 protein. All three anti-dengue NS1 mAbs (D5, E4 and E7) had IgG1 type heavy chain and kappa light chain (κ).Toxo-IFA screening
In addition to the usual ELISA screening process, we have also screened hybridoma clones using Toxo-IFA system. It is used on the initial screening of hybridoma clones and also on the final confirmation of monoclonal antibody reactivity. This screening method is simple and it has more advantage for screening the reactivity of monoclonal antibodies. The results were also corroborated with the other screening process (Fig. 1A–C). Positivity of virus presence was identified based on the intensity of the fluorescence and no fluorescence were observed on negative control. With the note of this, mAbs D5, E4, and E7 can also be utilized to detect the presence of antigen on IFA assays (unpublished data).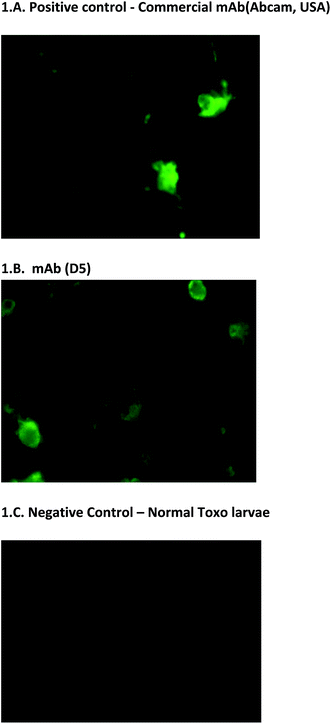 | ||
| Fig. 1 Toxo-IFA screening. (A & B) Fluorescence indicates the presence of dengue virus. (C) No fluorescence were observed on negative control. | ||
Purification of mAbs
The purification of mouse mAbs of the IgG1 and the preparation of immune affinity columns were carried out according to the manufacturer's instructions (Sigma-Aldrich, USA). After the purification, antibody concentration for the clones D5, E4 and E7 were 0.598 mg mL−1, 0.694 mg mL−1 and 0.612 mg mL−1 respectively.Specificity of mAbs
The results showed strong reactivity among all dengue serotypes and no reactivity for JE, West Nile virus and chikungunya. Virus antigen and sera samples were used to analyse the cross reactivity. Specificity of all the three purified mAbs (three clones - D5, E4 and E7) were confirmed.NS1 antigen capture ELISA
The NS1 antigen capture ELISA is similar to a standard antigen capture ELISA. The detection limit of antigen concentration was 0.0625 μg mL−1 and higher sensitivity was observed at 1 μg mL−1. The mAbs were also able to identify the NS1 antigen presence on the field collected mosquitoes.Comparative analysis of different diagnostic methods
In order to evaluate the efficiency of the mAbs for detection of NS1 antigen in clinical samples, NS1 capturing ELISA has been utilized. The results showed that out of 30 samples, 15 samples were positive on Panbio kit and 19 were positive on mAbs D5, E4 & E7. However, the samples were further confirmed by RT-PCR and MAC-ELISA and it was found that, out of 30 samples, only 20 samples were positive either on RT-PCR or MAC-ELISA. With reference to the RT-PCR and MAC-ELISA results, out of 20 positive samples, Panbio kit was able to detect 15 samples and mAbs D5, E4 & E7 were able to detect 19 samples and the specificity was found to be 100% for both, since it showed no false-positive results (Table 1). The detection sensitivity of D5, E4 & E7 was consistent from the samples collected from the onset of illness (0–7 days) (Fig. 2-results shown here for the clone D5 only) and no cross-reactivity was observed with JE, WNV and CHIKV samples. Serotypes were determined for all the positive samples using RT-PCR and for RT-PCR negative samples (sample ID: NG248, NG246, NG256 & NG268) real time RT-PCR was used to determine the serotypes.27,28| Sl no | Sample ID | Days from the onset of illness | RT-PCR | MAC-ELISA | Panbio | D5 mAb | Serotype |
|---|---|---|---|---|---|---|---|
| a Serotype confirmed by the real time RT-PCR. | |||||||
| 1 | NG209 | 0 | + | − | + | + | DEN1 |
| 2 | NG217 | 0 | + | − | + | + | DEN4 |
| 3 | NG251 | 0 | + | − | + | + | DEN2 |
| 4 | NG215 | 1 | + | − | + | + | DEN3 |
| 5 | NG261 | 1 | + | − | − | + | DEN1 |
| 6 | NG227 | 2 | + | + | + | + | DEN1 |
| 7 | NG248a | 2 | − | + | + | + | DEN3 |
| 8 | NG265 | 2 | + | + | − | + | DEN3 |
| 9 | NG206 | 3 | + | + | + | + | DEN2 |
| 10 | NG243 | 3 | + | + | + | + | DEN3 |
| 11 | NG246a | 3 | − | + | − | + | DEN1 |
| 12 | NG262 | 3 | + | + | + | + | DEN1 |
| 13 | NG200 | 4 | + | + | + | + | DEN2 |
| 14 | NG222 | 5 | + | + | + | + | DEN2 |
| 15 | NG240 | 5 | + | + | + | + | DEN1 |
| 16 | NG242 | 6 | + | + | + | + | DEN1 |
| 17 | NG210 | 7 | + | + | − | + | DEN3 |
| 18 | NG256a | 7 | − | + | + | + | DEN2 |
| 19 | NG268a | 7 | − | + | − | − | DEN4 |
| 20 | NG275 | 7 | + | + | + | + | DEN2 |
| Total | 16 | 15 | 15 | 19 | |||
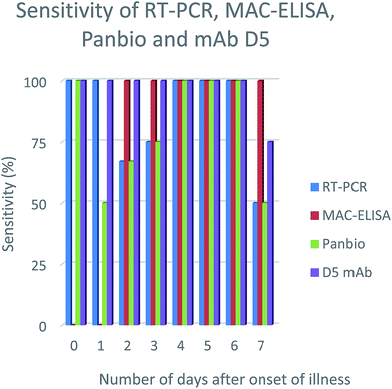 | ||
| Fig. 2 NS1 antigen detection sensitivity percentage using different diagnosis methods on number of days after onset of illness. | ||
Discussion
Monoclonal antibodies have become inevitable components in diagnostic field. It is promising on disease diagnostic field.29–33 The present work focuses on the determination of possible use of developing an antigen capture assay for early diagnosis of acute dengue virus infections. Three monoclonal antibodies producing clones (D5, E4 & E7) have been raised against the dengue virus recombinant NS1 antigen.NS1 antigen capture ELISA has distinct diagnosing potentials than any other diagnosis process. Several studies have been reported using the NS1 antigen detection method for the early diagnosis of dengue virus infection, however still the sensitivity and competency of NS1 antigen based diagnosis need more evaluation.34,35 The sensitivity of our mAbs (D5, E4 & E7) were up to 0.0625 μg mL−1 on NS1 antigen detection.
The mAbs showed no cross reactivity with other closely related members of the genus Flavivirus (Japanese encephalitis (JE), West Nile virus) and chikungunya virus. In addition, the detecting sensitivity of dengue serotypes is almost similar. While comparing with Panbio kit, the results were better (Panbio 75% and mAbs D5, E4 & E7 95%). It is found that results varied with Panbio and the mAbs (D5, E4 & E7) for the clinical samples NG210, NG265. To analyze the possible reason behind this, we serotyped all the samples and found that both NG210 and NG265 were serotype 3. This is consistent with earlier observations reporting Panbio kit has low sensitivity on detecting dengue 3 serotype.36–38
Till date, diagnostic kits intended for the detection of WNV and Saint Louis encephalitis virus (SLEV) are available commercially. No such kits are available for DENV detection in mosquitoes. A handful of researchers have shown that commercially available dengue NS1 antigen detection kit intended for human serum can also be useful for antigen detection in mosquitoes (Dengue NS1 Ag Strip, Bio-Rad France, Panbio Dengue Early ELISA; Panbio, Australia).39 Though the Platelia Dengue NS1 Ag kit has reported 98% sensitivity for detecting DENV in mosquito pools, the application of the test in field conditions still in doubt since the sensitivity was assessed in experimentally infected mosquitoes in lab.40 Panbio kit also reported with better detection sensitivity on infected mosquitoes.41 Our mAbs showed 100% sensitivity when analyzed with field collected mosquitoes (data not shown). The ability of mAbs were also checked in detecting NS1 antigen in dengue virus infected Tx. splendens larvae using Toxo-IFA system.
Conclusions
In this study, we have developed a panel of anti-NS1 mAb secreting hybridoma clones and validated the use of mAbs (D5, E4 & E7) as a diagnostic tool for dengue diagnosis during outbreak investigation. The mAbs were successfully utilized in ELISA and immunofluorescence assay to analyse the virus presence in field caught Ae. aegypti, Ae. albopictus and in virus propagated Tx. splendens larvae (Toxo-IFA).Acknowledgements
This work was supported by the Department of Science Technology (SERB) from the Government of India, Grant No. SR/SO/HS-0188/2012. The authors would like to thank Mr R. Sathish Babu, Mr T. Balaji (Centre for Research in Medical Entomology (CRME), Indian Council of Medical Research) and Ms. S. Saranya (Madras Veterinary College, Tamil Nadu Veterinary and Animal Sciences University) for their technical support.References
- H. Palanivel, S. Nair, A. Subramaniyan, P. V. J. Ratnam and R. Kanungo, Indian J. Pathol. Bacteriol., 2015, 58(3), 328–331 Search PubMed.
- S. Gowri Sankar, K. J. Dhananjeyan, R. Paramasivan, V. Thenmozhi and B. K. Tyagi, Clin. Microbiol. Infect., 2012, 18(1), E8–E10 CrossRef CAS PubMed.
- P. R. Young, P. A. Hilditch, C. Bletchly and W. Halloran, J. Clin. Microbiol., 2000, 38, 1053–1057 CAS.
- S. Alcon, A. Talarmin, M. Debruyne, A. Falconar, V. Deubel and M. Flamand, J. Clin. Microbiol., 2002, 40, 376–381 CrossRef CAS PubMed.
- C. H. Huang, L. L. Kuo, K. D. Yang, P. S. Lin, P. L. Lu, C. C. Lin, K. Chang, T. C. Chen, W. R. Lin, C. Y. Lin, Y. H. Chen and H. S. Wu, J. Microbiol., Immunol. Infect., 2013, 4, 358–365 CrossRef PubMed.
- F. M. Kassim, M. N. Izati, T. A. TgRogayah, Y. M. Apandi and Z. Saat, Southeast Asian J. Trop. Med. Public Health, 2011, 42(3), 562–569 CAS.
- C. Puttikhunt, T. Prommool, N. U-thainual, P. Ong-ajchaowlerd, K. Yoosook, C. Tawilert, T. Duangchinda, A. Jairangsri, N. Tangthawornchaikul, P. Malasit and w. Kasinrerk, J. Clin. Virol., 2011, 50, 314–319 CrossRef CAS PubMed.
- S. Datta and C. Wattal, Indian J. Med. Microbiol., 2010, 28, 107–110 CrossRef CAS PubMed.
- A. K. Falconar, Clin. Vaccine Immunol., 2008, 15, 549–561 CrossRef CAS PubMed.
- A. K. Falconar, Virol. J., 2013, 10, 126 CrossRef CAS PubMed.
- P. Koraka, C. P. Burghoorn-Maas, A. Falconar, T. E. Setiati, K. Djamiatun, J. Groen and A. D. J. Osterhaus, J. Clin. Microbiol., 2003, 41, 4154–4159 CrossRef CAS PubMed.
- R. Lima Mda, R. M. Nogueira, A. M. Filippis, P. C. Nunes, C. C. Sousa, M. H. Silva and F. B. Santos, J. Virol. Methods., 2014, 204, 105–108 CrossRef PubMed.
- M. L. Moi, T. Omatsu, S. Tajima, C. K. Lim, A. Kotaki, M. Ikeda, F. Harada, M. Ito, M. Saijo, I. Kurane and T. Takasaki, J. Trav. Med., 2013, 20(3), 185–193 CrossRef PubMed.
- J. Barniol, R. Gaczkowsk, E. V. Barbato, R. V. da Cunha, D. Salgado, E. Martinez, C. S. Segarra, E. B. Pleites Sandoval, A. Mishra, I. S. Laksono, C. S. Lum, J. G. Martínez, A. Núnez, A. Balsameda, I. Allende, G. Ramírez, E. Dimaano, K. Thomacheck, N. A. Akbar, E. E. Ooi, E. Villegas, T. T. Hien, J. Farrar, O. Horstick, A. Kroeger and T. Jaenisch, BMC Infect. Dis., 2011, 11, 106 CrossRef PubMed.
- J. S. Castleberry and C. R. Mahon, Clin. Lab. Sci., 2003, 16, 34–38 Search PubMed.
- S. Chaterji, J. C. Allen Jr, A. Chow, Y. Leo and E. Ooi, Am. J. Trop. Med. Hyg., 2011, 84(2), 224–228 CrossRef PubMed.
- D. Y. Chao, Y. J. Liu, W. F. Shen, W. C. Tu, J. U. Galula and H. C. Wu, Vector Borne and Zoonotic Diseases, 2015, 52, 134–141 Search PubMed.
- S. Gowri Sankar, K. J. Dhanajeyan, R. Paramasivan, V. Thenmozhi, B. K. Tyagi and S. John Vennison, BioMed Res. Int., 2013, 8, 343195 Search PubMed.
- X. X. Ding, B. DI and X. Y. Che, Xibao Yu Fenzi Mianyixue Zazhi, 2011, 27(7), 777–779 CAS.
- G. Kohler and C. Milstein, Nature, 1975, 256, 495–497 CrossRef CAS PubMed.
- P. Perrin, Laboratory techniques in rabies, 4th edn, World Health Organization, Geneva, 1996, pp. 433–444 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- D. T. Mourya, Trans. R. Soc. Trop. Med. Hyg., 1990, 84, 580 CrossRef CAS PubMed.
- S. Norazizah and A. Sazaly, J. Entomol., 2006, 3(1), 89–94 CrossRef.
- G. B. Wisdom, Methods in molecular biology immunochemical protocols, 3 edn, Humana Press Inc., 2005, pp. 127–130 Search PubMed.
- R. Rajendran, A. Gajanaana and P. Samuel, Annual Report, 1992-93, Centre for Research in Medical Entomology, Madurai, Tamil nadu, 1992, pp. 4–11 Search PubMed.
- R. S. Lanciotti, C. H. Calisher, D. J. Gubler, G. J. Chang and A. V. Vorndam, J. Clin. Microbiol., 1992, 30, 545–551 CAS.
- B. W. Johnson, B. J. Russell and R. S. Lanciotti, J. Clin. Microbiol., 2005, 43(10), 4977–4983 CrossRef CAS PubMed.
- C. Zanluca, G. Mazzarotto, J. Bordignon and C. Santos, PLoS One, 2014, 9(11), e110620 Search PubMed.
- Y. Tang, C. Chiu, C. Lin, C. Huang, Y. Chen, R. Destura, D. Chao and H. Wu, Int. J. Mol. Sci., 2015, 16(11), 27850–27864 CrossRef CAS PubMed.
- A. Ganguly, R. B. Malabadi, P. K. Bhatnagar, X. Tang, D. Das, R. Loebenberg, M. R. Suresh and H. H. Sunwoo, J. Viro. Methods., 2015, 220, 5–12 CrossRef CAS PubMed.
- S. H. Kim, Y. N. Kim, T. T. Truong, N. T. Thu Thuy, Q. Mai le and Y. S. Jang, Biochem. Biophys. Res. Commun., 2016, 473, 894–898 CrossRef CAS PubMed.
- L. Jihoo, K. Hak-Yong, C. Chom-Kyu and S. Hyun-Ok, Diagn. Microbiol. Infect. Dis., 2015, 82, 128–134 CrossRef PubMed.
- T. Gelanew, B. K. Poole-Smith and E. Hunsperger, J. Virol. Methods., 2015, 222, 214–223 CrossRef CAS PubMed.
- A. Fatima, H. Wang, K. Kang, L. Xia, Y. Wang, W. Ye, J. Wang and X. Wang, PLoS One, 2014, 9(4), e95263 Search PubMed.
- L. L. Hermann, B. Thaisomboonsuk, Y. Poolpanichupatam, R. G. Jarman, S. Kalayanarooj, A. Nisalak, I. K. Yoon and S. Fernandez, PLoS Neglected Trop. Dis., 2014, 8(10), e3193 Search PubMed.
- M. Lima, R. Nogueira, H. Schatzmayr and F. Santos, PLoS Neglected Trop. Dis., 2010, 4(7), e738 Search PubMed.
- M. Lima, R. Nogueira, H. Schatzmayr, A. M. Filippis and F. Santos, Clin. Vaccine Immunol., 2011, 18(6), 1031–1033 CrossRef CAS PubMed.
- N. Voge, I. Sánchez-Vargas, C. Blair, L. Eisen and B. Beaty, Am. J. Trop. Med. Hyg., 2013, 88(2), 260–266 CrossRef PubMed.
- T. Cheong-Huat, W. Pei-Sze Jeslyn, L. Mei-Zhi, V. Indra and N. Lee-Ching, Vector-Borne and Zoonotic Diseases, 2011, 11(6), 789–792 CrossRef PubMed.
- D. A. Muller, F. D. Frentiu, A. Rojas, L. A. Moreira, S. L. O'Neill and P. R. Young, J. Virol. Methods, 2012, 183, 90–93 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |
