The improved effect of co-doping with nano-SiO2 and nano-Al2O3 on the performance of poly(methyl methacrylate-acrylonitrile-ethyl acrylate) based gel polymer electrolyte for lithium ion batteries
Ping Suna,
Youhao Liao*ab,
Xueyi Luoa,
Zihao Lia,
Tingting Chena,
Lidan Xinga and
Weishan Li*ab
aSchool of Chemistry and Environment, South China Normal University, Guangzhou 510631, China. E-mail: liaoyouhao@126.com; liwsh@scnu.edu.cn; Fax: +86-20-39310256; Tel: +86-20-39310256
bEngineering Research Center of MTEES (Ministry of Education), Research Center of BMET (Guangdong Province), Key Laboratory of ETESPG (GHEI), South China Normal University, Guangzhou 510631, China
First published on 16th July 2015
Abstract
In this article, we report a novel gel polymer electrolyte (GPE) for lithium ion batteries, which is prepared using poly(methyl methacrylate-acrylonitrile-ethyl acrylate) (P(MMA-AN-EA)) as a polymer matrix and doping with nano-SiO2 and nano-Al2O3 simultaneously. The influences of the ratio of the two nanoparticles on the pore structure, electrolyte uptake and thermal stability of the resulting membrane, and the ionic conductivity and electrochemical stability of the corresponding GPE are investigated by scanning electron microscopy, mechanical strength, thermogravimetry, electrochemical impedance spectroscopy, linear sweep voltammetry and cyclic voltammetry. The performance of the developed GPE is evaluated in the Li/LiNi0.5Mn1.5O4 half cell by a charge–discharge test for its application in lithium ion batteries. It is found that there exists a synergistic effect between nano-SiO2 and nano-Al2O3. The performances of the resulting membrane and the corresponding GPE are effectively improved by using nano-SiO2 and nano-Al2O3 simultaneously rather than individually. Co-doping 5 wt% nano-SiO2 and 5 wt% nano-Al2O3 provides the membrane with a higher thermal decomposition temperature of 325 °C, and a better electrolyte uptake of 198.1%, the corresponding GPE with an increased ionic conductivity of 2.2 × 10−3 S cm−1 at room temperature and an enhanced oxidative stability up to 5.5 V (vs. Li/Li+), and the LiNi0.5Mn1.5O4 cathode with an improved rate capability of 104.2 mA h g−1 at 2C and an improved capacity retention of 94.8% after 100 cycles. These improved performances result from combining the advantages of both nano-SiO2 and nano-Al2O3, in which the former contributes to the improved ionic conductivity caused by a stronger Lewis-acid property, while the latter to the better thermal and structural stabilities by its stiffness characteristic.
1. Introduction
Secondary lithium ion batteries have been widely used in portable devices for energy storage due to their high energy densities and environmentally friendly effects since their commercialization in the 1990s.1–9 The demand for lithium ion batteries is increasing quickly with the rapid development of electric vehicles and hybrid-electric vehicles (EVs and HEVs).10,11 However, it remains a challenge to avoid the potential dangers in the applications of lithium ion batteries, which are caused by the oxidative decomposition of liquid organic electrolytes, especially when high voltage cathodes are used.12–14Among the diverse electrolytes, gel polymer electrolyte (GPE) is thought to be one of the most effective ways to solve the safety problem and to extend the narrow oxidative potential of liquid organic electrolyte. In GPE, the free movement of the liquid electrolyte is restricted through the swelling of the organic electrolyte into suitable polymer matrices, leading to the improved stability of electrolyte.15–18 However, there are some problems that need to be solved before GPE can be applied in large scale to commercial batteries, such as low ionic conductivity and unsatisfactory rate performance.19–21
Developing a new polymer matrix is proved to be an effective method to enhance the comprehensive performance of the corresponding GPE. Based on our previous research, the characterization of GPE using terpolymers [poly(methyl methacrylate-acrylonitrile-ethyl acrylate), poly(methyl methacrylate-acrylonitrile-vinyl acetate), poly(acrylonitrile-methyl methacrylate-styrene)] is preferable when compared with that of bi-polymers in the form of poly(methyl methacrylate-acrylonitrile), poly(methyl-methacrylate-vinyl acetate), poly(acrylonitrile-vinyl acetate) and poly(butyl meth-acrylate-styrene).22–28
Doping the polymer matrix with the correct amount of inorganic nanoparticles is another effective strategy to improve the performance of GPEs.29–32 Inorganic nanoparticles, on one hand, provide transportation paths for lithium ions due to their Lewis-acid properties, contributing to the increased ionic conductivity. On the other hand, they provide GPEs with enhanced mechanical strength and dimensional stability because of the better thermal and structural stability of inorganic oxides than polymers. Among the inorganic nanoparticles that have been reported in GPEs, nano-Al2O3 and nano-SiO2 are believed to be most effective for the performance improvement of GPEs.33,34 Our groups have chosen these two kinds of nanoparticles as additives in the GPE system. Positive results, such as better electrochemical stability, higher ionic conductivity, good compatibility with a lithium anode and excellent cycle stability, are exhibited in previous reports. Interestingly, it has been found that the GPEs with 10 wt% nanoparticles exhibit the best performances. The involved GPEs include nano-SiO2 doped poly(butyl methacrylate-styrene) GPE,26 poly(methyl methacrylate-acrylonitrile-vinyl acetate) (P(MMA-AN-VAc))35 and poly(methyl methacrylate-co-butyl acrylate)36 based GPEs, and nano-Al2O3 doped poly(methyl methacrylate-vinyl acetate)-co-poly(ethylene glycol) diacrylate,37 poly(ethylene oxide)–poly(vinylidene fluoride-hexafluoropropylene)38 and poly(acrylonitrile-co-methyl methacrylate)39 based GPEs. Recently, we have compared the contributions of nano-SiO2 and nano-Al2O3 to the performance improvement in P(MMA-AN-VAc) based GPE and found that the SiO2 doped GPE has better compatibility with the anode than Al2O3 doped GPE, which can be attributed to the stronger Lewis-acid property of SiO2, while the stiffness characteristic of Al2O3 leads to better thermal stability.40
However, there is less research on the influence of the co-doping inorganic nanoparticles in the GPE system. Synergistic effects have been reported for co-doped cathodes with metallic oxides, such as the electrochemical characterization improvement of a LiNi0.5Mn1.5O4 cathode by co-doping with Co and Cr.41 Here, synergistic effect implies that the performance of the co-doped Co and Cr sample is significantly enhanced compared with that of the samples with individual doping of Co or Cr. Thus, we expect that the performance of the GPE will be improved more effectively by doping nano-SiO2 and nano-Al2O3 simultaneously rather than individually and that a synergistic effect may happen, if the performance of co-doped sample is better than that of the individual one. Accordingly we developed a novel GPE using poly(methyl methacrylate-acrylonitrile-ethyl acrylate) (P(MMA-AN-EA)) as the polymer matrix and doping with nano-SiO2 and nano-Al2O3 simultaneously. The influences of the ratio of the two nanoparticles on the pore structure, electrolyte uptake and thermal stability of the resulting membrane, and the ionic conductivity of the corresponding GPE were understood in this paper. The performance of the developed GPE for its application in lithium ion batteries was evaluated with LiNi0.5Mn1.5O4, a representative high-voltage cathode.
2. Experimental
2.1 Preparation
Polymer poly(methyl methacrylate-acrylonitrile-ethyl acrylate) (P(MMA-AN-EA)) with a monomer mass ratio of MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AN
AN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 4
EA = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was synthesized by emulsion polymerization. This ratio was chosen based on the formation convenience and the performance reliability of the membrane in our previous report.22 The total content of nanoparticles in the polymer was kept to be 10 wt%. The synthesized polymer P(MMA-AN-EA) and 10 wt% nanoparticles were dissolved in dimethylformamide (DMF) at 80 °C for 1 hour to form a slurry containing 3 wt% polymer and nanoparticles. The mass ratios of nano-SiO2 (Aladdin, 99.5%, average particle size 30 nm) and nano-Al2O3 (Aladdin, 99.9%, α-Al2O3 with average particle size of 30 nm) was 10
1 was synthesized by emulsion polymerization. This ratio was chosen based on the formation convenience and the performance reliability of the membrane in our previous report.22 The total content of nanoparticles in the polymer was kept to be 10 wt%. The synthesized polymer P(MMA-AN-EA) and 10 wt% nanoparticles were dissolved in dimethylformamide (DMF) at 80 °C for 1 hour to form a slurry containing 3 wt% polymer and nanoparticles. The mass ratios of nano-SiO2 (Aladdin, 99.5%, average particle size 30 nm) and nano-Al2O3 (Aladdin, 99.9%, α-Al2O3 with average particle size of 30 nm) was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 7.5
0, 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 5
2.5, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 2.5
5, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5, and 0
7.5, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the resulting samples were marked as MS10, MS7.5, MS5, MS2.5 and MS0, respectively. For comparison, a sample without nanoparticles was also obtained and labeled as M0.
10, the resulting samples were marked as MS10, MS7.5, MS5, MS2.5 and MS0, respectively. For comparison, a sample without nanoparticles was also obtained and labeled as M0.
The resulting viscous slurry was cast with a doctor blade onto both sides of a polyethylene (PE) separator, and then transferred into deionized water for 2 hours to induce phase inversion. The resulting membrane was washed with running water, dried in vacuum at 60 °C for 24 hours and finally the porous membrane with average thickness of 80 μm was obtained. In order to prepare the GPE, the membrane was immersed in a liquid electrolyte, 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1/1, v/v, battery grade, Samsung Cheil Industry, Korea) for 0.5 hours in an argon-filled glove box (MBRAUN).
A cathode electrode was fabricated by mixing the active material of LiNi0.5Mn1.5O4, a conductive agent of carbon black and a binder of polyvinylidene fluoride (PVDF) in the weight ratios of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. N-Methylpyrrolidone (NMP) was used as a solvent to prepare the electrode slurry. A CR2025 type coin cell in the structure of Li/GPE/LiNi0.5Mn1.5O4 was assembled in an argon-filled glove box to evaluate the cathode performance when the nanoparticle co-doped GPE was used.
10. N-Methylpyrrolidone (NMP) was used as a solvent to prepare the electrode slurry. A CR2025 type coin cell in the structure of Li/GPE/LiNi0.5Mn1.5O4 was assembled in an argon-filled glove box to evaluate the cathode performance when the nanoparticle co-doped GPE was used.
2.2 Characterization
The morphology of the developed membranes was examined with a scanning electron microscope (SEM, JEOL, JSM-6510, Japan). The mechanical property of the membranes was determined by a microcomputer control electron universal testing machine (model CMT6104). The thermal stability of the membranes was measured with a thermogravimetric analyzer (TGA, Perkin-Elmer TGA7) under N2 atmosphere from room temperature to 600 °C at a heating rate of 10 °C min−1. The membranes (diameter Φ = 18 mm) were immersed into the liquid electrolyte for half an hour and then the excess electrolyte on the surface was removed by pressing lightly between two sheets of filter paper with a weight of 50 g on the top of the upper filter paper. The electrolyte uptake (A) of the membranes was calculated according to eqn (1),
 | (1) |
The GPE was sandwiched between two parallel stainless steel (SS) discs (diameter Φ = 16.2 mm) in order to characterize the ionic conductivity by electrochemical impedance spectroscopy (EIS) on an electrochemical instrument (Metrohm Autolab PGSTAT302N, the Netherlands) using an alternating current signal with a potential amplitude of 10 mV and frequencies from 100 kHz to 1 Hz. The ionic conductivity was calculated from the bulk electrolyte resistance (R) based on eqn (2),
 | (2) |
The activation energy (Ea) of the GPE for the lithium ion transfer was obtained from eqn (3),
 | (3) |
EIS was carried out to characterize the interfacial stability between the GPE and the Li metal electrode. A Li/GPE/Li type of symmetrical structure was fabricated by sandwiching the GPE between two lithium electrodes and measured through an alternating current signal with a potential amplitude of 5 mV and frequencies from 500 kHz to 0.03 Hz. The electrochemical oxidation stability of the GPE was determined in the cell type of Li/GPE/SS by linear sweep voltammetry (LSV) using the scanning rate of 1 mV s−1 on a Metrohm Autolab (PGSTAT302N, the Netherlands). The Li electrode was used as the reference and the counter electrodes, while the SS was used as the working electrode. Cyclic voltammetry (CV) was used to determine the reversible deposition and dissolution of lithium ions on the developed GPE. The measurement was carried out on an electrochemical workstation (Solartron Analytical 1470E, England) and scanned at a rate of 1 mV s−1 with the coin cell structure of SS/GPE/Li in the voltage range of −0.5 V to 5 V. The SS was applied as a working electrode and the lithium as the reference and the counter electrodes.
The coin cells comprising Li/GPE/LiNi0.5Mn1.5O4 were tested on a charge–discharge instrument (Land CT2001A, Wuhan Land Electronic Co. Ltd, China) between 3.5 V and 5.0 V at room temperature. The coin cells were cycled at 0.1C for 3 cycles in the same cyclic voltage range to activate the cathode before the cyclic stability test, while for the rate ability evaluation they were cycled at 0.05C for 5 cycles. The theoretical capacity of the LiNi0.5Mn1.5O4 cathode calculated for one Li in unit cell is 146.7 mA h g−1.
3. Results and discussion
3.1 Morphology of membranes
Fig. 1 shows the SEM images of the PE-supported P(MMA-AN-EA) membranes without and with different mass ratios of nano-SiO2 and nano-Al2O3. The M0 membrane without nanoparticles has poor structure with pores that are dispersed non-uniformly on the surface. Nanoparticles seem to induce the formation of porous membranes. More pores can be observed on the surface of the membrane and the interconnected structure is also present for all of the samples after doping with different species. Based on our previous studies,35,38–40 the membrane doped with 10 wt% nanoparticles exhibited comprehensive performance, higher levels led to agglomeration, resulting from the naturally branched structure of the nanoparticles.42 The MS10 membrane, doped with nano-SiO2, has a large number of pores on an uneven surface and an interconnected structure can be observed under the surface. After introducing 2.5 wt% nano-Al2O3 as the secondary phase into the membrane, the MS7.5 sample keeps the interconnected and porous structure with the uniform pore size, which should be ascribed to the different properties of electric charge for nano-SiO2 and nano-Al2O3. Due to the repulsive interaction between nano-SiO2 and nano-Al2O3, the nanoparticles disperse uniformly in the MS7.5 membrane. Decreasing the content of nano-SiO2 to equal the amount of nano-Al2O3, the MS5 membrane shows the best uniform structure in the form of suitable pore size with average diameter of about 0.5 μm. For the MS2.5 membrane, with 2.5 wt% nano-SiO2, the pore number descends and the pore structure tends to become compact with reduced pore diameter, which is not beneficial for holding the liquid electrolyte effectively. Among the doped membranes, the membrane with the most compact pore structure and the lowest pore number is the MS0 membrane, with 10 wt% nano-Al2O3 only. Comparing MS10 with MS0, it can be found that the porous structure of the membrane is also affected by the species of nanoparticles. Doping nano-SiO2 in the polymer matrix seems to result in pores with bigger diameters, which is in accord with our previous studies that doping nano-SiO2 into P(MMA-AN-VAc) based membranes exhibits better performance than that of nano-Al2O3. On the other hand, there is a synergistic effect of co-doping nanoparticles in the improvement of the porous structure in the co-doped membrane, especially for the MS5 membrane, which presents the best porous structure.3.2 Thermal stability
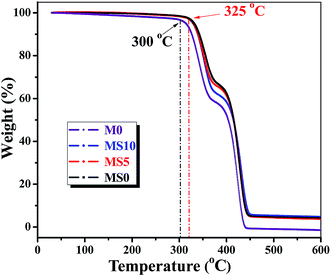
Thermal stability of the membrane is one of the key factors that determines the safety of lithium ion batteries. Fig. 2 presents the TG curves of P(MMA-AN-EA) based membranes with different content of nanoparticles. Here, the decomposition temperature is defined as the temperature at which more than 3 wt% of the original weight has been lost. It can be seen from Fig. 2 that the membranes doped with nanoparticles have higher decomposition temperatures than the membrane without nanoparticles, although the shape of TG curves is similar. The P(MMA-AN-EA) based membrane is thermally stable up to 300 °C, while a much higher decomposition temperature of 325 °C can be observed for all the membranes doped by the different content of nano-SiO2 and nano-Al2O3. Furthermore, the short plateaus between 375–400 °C are observed for the four kinds of membrane, caused by the thermal decomposition of the supporter-polyethylene membrane.43,44 These observations indicate that doping with nanoparticles does improve the thermal stability of the membrane. The bond in the polymer membrane is strengthened by adding nanoparticles that contain the active –Si–O bond or –Al–O bond, which reacts with the polymer chains to form complex bonds. During the heating procedure, the polymer membrane is decomposed by breaking down the inside bonds of the polymer chains, such as –C–H, –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N. The improved bond strength that is modified by the –Si–O or –Al–O groups in the chains subsequently enhances the thermal decomposition temperature of membrane. On the other hand, the melting points of the nanoparticles are much higher than that of the polymer, which also contributes to the improvement in the thermal stability of the membranes. In particular, the membrane containing nano-Al2O3 only, has the best thermal toleration, suggesting that the nano-Al2O3 contributes to the thermal stability by its inherent stiffness characteristic.
N. The improved bond strength that is modified by the –Si–O or –Al–O groups in the chains subsequently enhances the thermal decomposition temperature of membrane. On the other hand, the melting points of the nanoparticles are much higher than that of the polymer, which also contributes to the improvement in the thermal stability of the membranes. In particular, the membrane containing nano-Al2O3 only, has the best thermal toleration, suggesting that the nano-Al2O3 contributes to the thermal stability by its inherent stiffness characteristic.
3.3 Mechanical strength
Mechanical property is an important factor to determine the fabricated character of commercial lithium ion batteries, which is measured by the elongation strength of the membrane. Fig. 3 shows the mechanical strength of the MS0, MS5 and MS10 membranes. The fracture strength of the MS5 membrane (46.9 MPa) is higher than that of the MS0 and the MS10. Thus, the developed membranes are sufficient for application in the practical usage of lithium ion batteries.3.4 Electrolyte uptake
Fig. 4 shows the wettability of the PE-supported P(MMA-AN-EA) membranes with different mass ratios of nano-SiO2 and nano-Al2O3 in the same process time of 15 seconds after applying a drop of liquid electrolyte in an argon-filled glove box. For the PE membrane as support, a dew bead can be observed clearly. However, the liquid electrolyte is quickly spread out, although there are not many differences among the prepared membranes, suggesting that the wettability of the polymer membranes is significantly improved by coating the PE support with the polymer and nanoparticles. This improved wettability results from the better affinity of polymer to the polar carbonated electrolyte because of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups in the polymer chains. Similar findings have been reported for other copolymer membranes.43,44
N groups in the polymer chains. Similar findings have been reported for other copolymer membranes.43,44
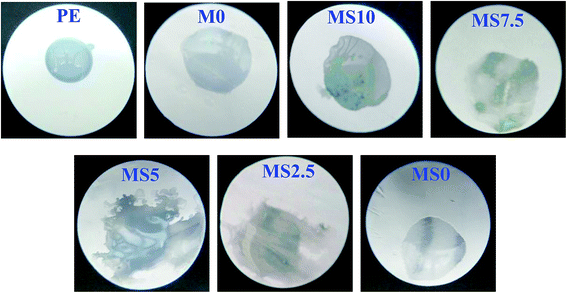 | ||
| Fig. 4 Wettability of the PE separator and PE-supported P(MMA-AN-EA) membranes with different content of nanoparticles. | ||
Fig. 5 presents the dependence of the electrolyte uptake ability on the different membranes. It can be seen from Fig. 5 that the electrolyte uptake of membrane is closely related to its pore structure. The electrolyte uptake of the M0 membrane has the lowest value (102.2%). The uptake ability for all the membranes is enhanced by doping with the proper amount of nanoparticles. As also presented in Fig. 5, the electrolyte uptake of the membrane doped with nano-SiO2 is higher than that of the membrane doped with nano-Al2O3 only, indicating that nano-SiO2 contributes more positively to the electrolyte uptake caused by its stronger Lewis-acid properties. Furthermore, the uptake ability of the co-doped membranes is also further enhanced. Exhilaratingly, the MS5 membrane has the highest electrolyte uptake (198.1%), ascribed to its interconnected structure, its uniform pores and appropriate size, which are beneficial to retain the liquid electrolyte effectively.
3.5 Ionic conductivity
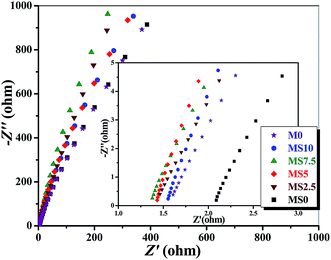
Fig. 6 presents the Nyquist plots of the P(MMA-AN-EA)-based GPEs with different mass ratios of nanoparticles at room temperature. According to eqn (2), the calculated ionic conductivity for the developed GPEs is also presented in Fig. 6. It can be found that the ionic conductivity is almost proportional to the electrolyte uptake that is affected by the pore structure of the membrane. The ionic conductivity of the GPEs with different nanoparticles is always higher than that of the M0 GPE. The huge surface area of nanoparticles helps to form a better pore structure that stores liquid electrolyte effectively, providing more routes for ionic transportation. Besides, the nanoparticles play a positive role in the migration of the lithium ions by forming cross-linked centers, and the tendency of polymer chain reorganization for the doped GPE is also lower compared with the non-doped one, leading to the enhancement of the ionic conductivity by improving the structural stiffness after structure modification.39 Moreover, the ionic conductivity for the GPE doped with nano-SiO2 only has a larger value than that doped by nano-Al2O3 alone. According to the Lewis acid–base theory, the Lewis acid strength of nano-SiO2 is stronger than nano-Al2O3. The competition to act as a Lewis acid between the nanoparticles and Li-ions forces the LiFP6 salt to dissociate more Li-ions, while the stronger Lewis acid, nano-SiO2, promotes the dissociated rate, leading to the enhancement of ionic conductivity. The largest ionic conductivity of 2.2 × 10−3 S cm−1 is of the MS5 GPE, and is higher than that of the M0 GPE, whose value is 1.2 × 10−3 S cm−1 at room temperature. It should be noted that the ionic conductivity of the MS5 is still higher than the MS10 or MS0, caused by the synergistic effect of co-doping with nanoparticles, which is related to the appropriate porous structure and the higher uptake of the liquid electrolyte.From the discussion above, the optimal MS5 GPE is used to further investigate the synergistic effect of co-doping nano-SiO2 and nano-Al2O3. For comparison, the MS10, MS0 and M0 are also further investigated.
In order to clearly understand the conductive mechanism of the GPEs, the ionic conductivity is investigated under different temperatures. From Fig. 7, it can be verified that the ionic conductivity of GPEs with different nanoparticles has the same tendency on the reciprocal temperature, which increases linearly with the absolute temperature. This conductive behavior obviously follows the Arrhenius law. Adding nanoparticles into the polymer does not change the conductive mechanism of the GPE. Based on eqn (3), the value of the activation energy (Ea) can be calculated by the slope of the best-fit lines in the temperature range of 348 K to 298 K. As shown in Table 1 the doped GPEs have much lower Ea than the M0 GPE, indicating that the rate of Li-ion transportation speeds up after doping. The Ea of MS10 GPE is lower than that of the MS0 GPE, which should be ascribed to the stronger interaction between nano-SiO2 and the polymer matrix that facilitates the diffusion and migration of lithium ions. As also presented in Table 1, the lowest Ea is observed for the MS5 GPE, whose value is 9.6 kJ mol−1. Lower Ea means a lower barrier for the diffusion and migration of lithium ions, resulting from temporary transition points produced by the nanoparticles. The co-dopants increase these effective connected points.
| M0 | MS10 | MS5 | MS0 | |
|---|---|---|---|---|
| Ea (kJ mol−1) | 23.2 | 11.0 | 9.6 | 11.5 |
3.6 Electrochemical stability
Irreversible oxidative decomposition of the liquid electrolyte takes place over the potential of 4.4 V (vs. Li/Li+) using the commercial LiCoO2 as cathode, when the outside circuit of the battery suffers from an overcharged condition, which is deleterious to the battery and even causes safety hazards. Thus, it is especially important to understand the oxidative stability of developed electrolyte to avoid overcharging the battery. Fig. 8 presents the decomposition potentials for the different electrolytes. The obtained result for the PE membrane saturated with the liquid electrolyte indicates that the oxidative decomposition happens at the potential of about 4.4 V (vs. Li/Li+), which is ascribed to the low oxidative stability of the solvent components in the organic liquid electrolyte. Much higher oxidative potentials can be observed for the GPEs. The M0 GPE is electrochemically stable up to about 4.9 V. After adding nanoparticles, the oxidative stability is significantly improved for the GPEs with current onset in the anodic region beginning at 5.5 V although different species are doped. Notably, the decomposition potential of MS5 GPE is as high as 5.6 V. | ||
| Fig. 8 Linear sweep voltammograms of various GPEs on stainless steel with the scanning rate of 1 mV s−1. | ||
The interaction between the P(MMA-AN-EA) polymer and liquid electrolyte restricts the free movement of the liquid component by a gelatinization process. By adding the SiO2 and Al2O3 nanoparticles individually to the GPE, the Si–O bonds or Al–O bonds as the connected points, contribute to build a stronger polymer network structure by the formation of Si–O–C or Al–O–C covalent bonds, and the interaction between the polymer and liquid electrolyte is strengthened. The complementary function of the Si–O bonds and Al–O bonds which makes the interaction between the polymer and liquid electrolyte become even stronger, is achieved through the Si–O bonds and Al–O bonds forming a super network structure, while inconsistent electronic charge between SiO2 and Al2O3 promotes the quality and effectively connects points between the polymer and liquid electrolyte. Thus, the best oxidative stability of MS5 GPE is presented by the synergistic effect of co-doping.
Fig. 9 shows the cyclic voltammogram (CV) of various GPEs for the Li/GPEs/SS cell scanned in the voltage range of −0.5 V to 5 V. The four samples have a similar trend, in which there is no obvious current response between 1.0 V and 5.0 V and strong redox peaks arise from the decomposition and precipitation of lithium ions in the voltage range of −0.5 V to 1.0 V. The peaks in the first five cycles are almost overlapping, indicating the reversible process for the deposition and dissolution of lithium ions. The CV measurements are also in accordance with the results of the linear sweep voltammograms; that the developed GPEs are electrochemically stable up to 5 V, and are good candidates for potential application in the 5 V high voltage cathodes.
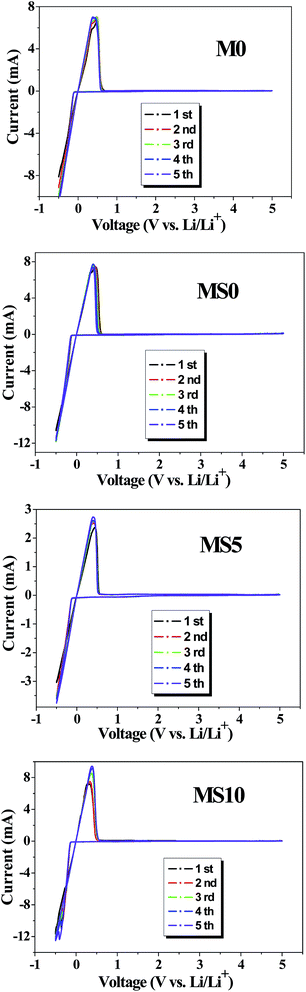 | ||
| Fig. 9 Cyclic voltammograms of various GPEs for the Li/GPEs/SS cell in the voltage range of −0.5 to 5 V, scanning rate: 1 mV s−1. | ||
3.7 Compatibility with a lithium anode
The compatibility of the GPE with a lithium anode was estimated by the interfacial behavior of a Li/GPE/Li coin cell. Fig. 10(a) shows the dependence of interfacial resistance of the diverse GPEs on the storage time in the open circuit. Taking MS5 GPE as an example to illustrate the electrochemical impedance spectroscopy (EIS), as shown in Fig. 10(b), the Nyquist plots are presented in a depressed semicircular arc in the high frequency range and a short line in the low frequency range, while the diameter of depressed semicircle is considered as the interfacial resistance between the GPE and the lithium electrode. It can be found from Fig. 10(a) that the interfacial resistance of the M0 GPE increases quickly after 15 days, however, much lower increased magnitude can be observed for the GPEs doped by nanoparticles. The MS10 GPE shows lower increased magnitude than the MS0 GPE. The surface of nanoparticle contains hydroxyl groups, which can further react with a small quantity of H2O in the liquid electrolyte through forming hydrogen bonds, then the encapsulated trace H2O becomes less likely to react with the lithium metal, leading to stabilization of the interface between the GPE and the lithium metal. Nano-SiO2 has a stronger complexation ability for absorbing small amounts of H2O to inhibit the major side reactions, contributing to the smaller interfacial resistance. | ||
| Fig. 10 (a) The dependence of interfacial resistance of Li/GPEs on the storage time, and (b) the detail electrochemical impedance spectra for MS5 GPE. | ||
MS5 GPE has the lowest increased magnitude, with the resistance value increasing from 51.9 Ω cm2 on the first day to 63.1 Ω cm2 after 15 days, indicating that the interfacial stability of the GPE can be further improved by co-doping with nanoparticles. Because of the incongruous electric charge properties, the different functions of nanoparticles will compete to react with the impurities in the liquid electrolyte, the unwanted side reaction is inhibited effectively by those nanoparticles with large specific surface area, which subsequently reduces the resistance.
3.8 Cathode performance
Fig. 11 presents the rate capability of the LiNi0.5Mn1.5O4 cathode with various electrolytes at different C-rates at room temperature. The cathode is charged at the current of 0.1C and discharged at 0.1C, 0.2C, 0.5C, 1C, 2C for 10 cycles each, and then back to 0.1C to estimate the recovery ability of the rate capacity. As indicated in Fig. 11, the capacity of cathode decreases with increasing the discharge rate for all electrolytes tested. The cathode exhibits better rate performance using a doped GPE, and the difference is magnified at a higher rate. For the MS5 GPE, the discharge capacity delivers 136.4 mA h g−1 at 0.1C rate in the 10th cycle, and the capacity is 135.4 mA h g−1 at 0.2C in the 20th cycle, while the value is reduced to 130.2 mA h g−1 at 0.5C in the 30th cycle. The capacity further dropped to 104.2 mA h g−1 at 2C rate in the 50th cycle, which has 76.4% capacity retention at 0.1C. Finally its capacity fully returns to 133.1 mA h g−1 in the following 51st cycle at the small current of 0.1C, suggesting that the cathode can be reversibly cycled under 0.1C. A similar trend is also observed for the other GPEs and the least capacity at different rates is assigned to the M0 GPE. It can be also noted that the capacity of MS10 at 2C is even a little higher than the MS5 GPE, due to the bigger diameter of pores in this membrane which is convenient for the fast transfer of Li-ion.Fig. 12 presents the cyclic stability of the LiNi0.5Mn1.5O4 cathode using various electrolytes in the voltage range of 3.5 V–5.0 V under 0.2C rate at room temperature. Although the initial capacity of the LiNi0.5Mn1.5O4 cathode is similar for the four kinds of electrolytes, the fading rate is different after cycling. The steadily declining trend is observed for the cathode using the developed GPEs. After 100 cycles, the capacity retention is 94.8% for the MS5, and 89.2% for the M0. The gelation of the liquid electrolyte suppresses the decomposition of the organic electrolyte at the potential over 4.4 V, and doped nanoparticles strengthen this gelation effect. However, the cyclability of the LiNi0.5Mn1.5O4 cell is not only related to the electrolyte, but also determined by the cathode itself. The reason for the capacity fading of the LiNi0.5Mn1.5O4 cell is that a small amount of the extracted Li-ions cannot insert reversibly into the Li1−xNi0.5Mn1.5O4 cathode during cycling.
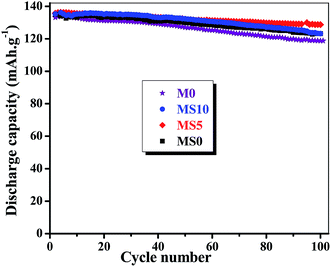 | ||
| Fig. 12 Cyclic stability of LiNi0.5Mn1.5O4 in various electrolytes under 0.2C rate in the voltage range of 3.5 V and 5.0 V at room temperature. | ||
The uniform and interconnected pore structure is beneficial to the cyclability, especially at higher rates, which results from the induction effect of the doped nanoparticles. After co-doping, the GPE shows good ionic conductivity, compatibility with the lithium anode and higher anti-oxidative ability, which in turn play a positive role on the cathode performance, so that current can disperse uniformly and partial polarization is largely avoided, and finally, the irreversible reaction of the electrolyte or cathode is restricted. Thus, the synergistic effect of co-doping with nano-SiO2 and nano-Al2O3 is obvious and strongly affects the performance of the membrane and the corresponding GPE. The results presented in this study indicate that the MS5 membrane can be a promising candidate as membrane for a safer Li-ion battery, and that the MS5 GPE is good alternative for use in high-voltage lithium ion batteries.
4. Conclusions
The synergistic effect of co-doping with nano-SiO2 and nano-Al2O3 on the performance of a P(MMA-AN-EA) based membrane and corresponding GPE is presented in this paper. The best performance is observed for the MS5 membrane and corresponding GPE that contains 5 wt% nano-SiO2 and 5 wt% nano-Al2O3 because of their synergistic effect. Here, nano-SiO2 contributes to the improved ionic conductivity caused by strong Lewis-acid properties, while nano-Al2O3 contributes to better thermal and structural stabilities by its stiffness characteristic. The MS5 membrane exhibits an interconnected structure with suitable pore size, which is beneficial to take up the liquid electrolyte effectively, leading to the enhancement in the ionic conductivity. Subsequently, the synergistic effect of co-doping with nano-SiO2 and nano-Al2O3 should stimulate the development of better performing inorganic ceramic membranes doped with different functional nanoparticles.Acknowledgements
The authors are grateful for the financial support from the joint project of the National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province (Grant No. U1401248), the National Natural Science Foundation (Grant No. 21403076), Natural Science Foundation of Guangdong Province (Grant No. 2014A030310324), the key project of Science and Technology in Guangdong Province (Grant No. 2012A090300012 and 2013B090800013), and the scientific research project of Department of Education of Guangdong Province (Grant No. 2013CXZDA013).References
- L. Wang, H.-J. Zhu, W. Zhai, F. Cai, X.-M. Liu and H. Yang, Study of a novel gel electrolyte based on poly-(methoxy/hexadecyl-poly(ethylene glycol) methacrylate) co-polymer plasticized with 1-butyl-3-methylimidazolium tetrafluoroborate, RSC Adv., 2014, 4, 36357–36365 RSC.
- Y. Zhu, S. Xiao, Y. Shi, Y. Yang, Y. Hou and Y. Wu, A composite gel polymer electrolyte with high performance based on poly(vinylidene fluoride) and polyborate for lithium ion batteries, Adv. Energy Mater., 2014, 4, 1–9 Search PubMed.
- T. F. Yi, Y. Xie, L. J. Jiang, J. Shu, C.-B. Yue, A.-N. Zhou and M.-F. Ye, Advanced electrochemical properties of Mo-doped Li4Ti5O12 anode material for power lithium ion battery, RSC Adv., 2012, 2, 3541–3547 RSC.
- J. L. Shi, L. F. Fang, H. Li, H. Zhang, B. K. Zhu and L. P. Zhu, Improved thermal and electrochemical performances of PMMA modified PE separator skeleton prepared via dopamine-initiated ATRP for lithium ion batteries, J. Membr. Sci., 2013, 437, 160–168 CrossRef CAS PubMed.
- S. Dalavi, M. Xu, L. Zhou, B. Ravdel and B. L. Lucht, Nonflammable electrolytes for lithium-ion batteries containing dimethyl methylphosphonate, J. Electrochem. Soc., 2010, 157, A1113–A1120 CrossRef CAS PubMed.
- P. Zhu, Y. Wu, M. V. Reddy, A. Sreekumaran Nair, B. V. R. Chowdari and S. Ramakrishna, Long term cycling studies of electrospun TiO2 nanostructures and their composites with MWCNTs for rechargeable Li-ion batteries, RSC Adv., 2012, 2, 531–537 RSC.
- R. Miao, J. Yang, X. Feng, H. Jia, J. Wang and Y. Nuli, Novel dual-salts electrolyte solution for dendrite-free lithium-metal based rechargeable batteries with high cycle reversibility, J. Power Sources, 2014, 271, 291–297 CrossRef CAS PubMed.
- Q. Lu, J. Fang, J. Yang, G. Yan, S. Liu and J. Wang, A novel solid composite polymer electrolyte based on poly(ethylene oxide) segmented polysulfone copolymers for rechargeable lithium batteries, J. Membr. Sci., 2013, 425–426, 105–112 CrossRef CAS PubMed.
- Y. Wang, L. Xing, X. Tang, X. Li, W. Li, B. Li, W. Huang, H. Zhou and X. Li, Oxidative stability and reaction mechanism of lithium bis(oxalate)borate as a cathode film-forming additive for lithium ion batteries, RSC Adv., 2014, 4, 33301–33306 RSC.
- H. B. Lin, H. B. Rong, W. Z. Huang, Y. H. Liao, L. D. Xing, M. Q. Xu, X. P. Li and W. S. Li, Triple-shelled Mn2O3 hollow nanocubes: force-induced synthesis and excellent performance as the anode in lithium-ion batteries, J. Mater. Chem. A, 2014, 2, 14189–14194 CAS.
- H. B. Lin, Y. M. Zhang, H. B. Rong, S. W. Mai, J. N. Hu, Y. H. Liao, L. D. Xing, M. Q. Xu, X. P. Li and W. S. Li, Crystallographic facet- and size-controllable synthesis of spinel LiNi0.5Mn1.5O4 with excellent cyclic stability as cathode of high voltage lithium ion battery, J. Mater. Chem. A, 2014, 2, 11987–11995 CAS.
- D. Y. Zhou, G. Z. Wang, W. S. Li, G. L. Li, C. L. Tan, M. M. Rao and Y. H. Liao, Preparation and performances of porous polyacrylonitrile–methyl methacrylate membrane for lithium-ion batteries, J. Power Sources, 2008, 184, 477–480 CrossRef CAS PubMed.
- Z. H. Li, P. Zhang, H. P. Zhang, Y. P. Wu and X. D. Zhou, A lotus root-like porous nanocomposite polymer electrolyte, Electrochem. Commun., 2008, 10, 791–794 CrossRef CAS PubMed.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Challenges in the development of advancedLi-ion batteries: a review, Energy Environ. Sci., 2011, 4, 3243–3262 CAS.
- N. T. Kalyana Sundaram, O. T. Muhammed Musthafa, K. S. Lokesh and A. Subramania, Effect of porosity on PVdF-co-HFP–PMMA-based electrolyte, Mater. Chem. Phys., 2008, 110, 11–16 CrossRef PubMed.
- G. Vijayakumar, S. N. Karthick, A. R. Sathiya Priya, S. Ramalingam and A. Subramania, Effect of nanoscale CeO2 on PVDF-HFP-based nanocomposite porous polymer electrolytes for Li-ion batteries, J. Solid State Electrochem., 2008, 12, 1135–1141 CrossRef CAS.
- Z. H. Li, H. P. Zhang, P. Zhang, G. C. Li, Y. P. Wu and X. D. Zhou, Effects of the porous structure on conductivity of nanocomposite polymer electrolyte for lithium ion batteries, J. Membr. Sci., 2008, 322, 416–422 CrossRef CAS PubMed.
- P. Raghavan, X. Zhao, J. K. Kim, J. Manuel, G. S. Chauhan, J. H. Ahn and C. Nah, Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers, Electrochim. Acta, 2008, 54, 228–234 CrossRef CAS PubMed.
- A. Manuel Stephan and K. S. Nahm, Review on composite polymer electrolytes for lithium batteries, Polymer, 2006, 47, 5952–5964 CrossRef PubMed.
- A. Manuel Stephan, Review on gel polymer electrolytes for lithium batteries, Eur. Polym. J., 2006, 42, 21–42 CrossRef CAS PubMed.
- J. Y. Song, Y. Y. Wang and C. C. Wan, Review of gel-type polymer electrolytes for lithium-ion batteries, J. Power Sources, 1999, 77, 183–197 CrossRef CAS.
- P. Sun, Y. Liao, H. Xie, T. Chen, M. Rao and W. Li, Poly(methyl methacrylate-acrylonitrile-ethyl acrylate) terpolymer based gel electrolyte for LiNi0.5Mn1.5O4 cathode of high voltage lithium ion battery, J. Power Sources, 2014, 269, 299–307 CrossRef CAS PubMed.
- M. M. Rao, J. S. Liu, W. S. Li, Y. Liang and D. Y. Zhou, Preparation and performance analysis of PE supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery applications, J. Membr. Sci., 2008, 322, 314–319 CrossRef CAS PubMed.
- L. Lu, X. X. Zuo, M. Q. Xu, J. S. Liu and W. S. Li, Study on the preparation and performances of PMMA-Vac polymer electrolyte for lithium ion battery use, Acta Chim. Sin., 2007, 65, 475–480 CAS.
- X. P. Li, M. M. Rao, Y. H. Liao, W. S. Li and M. Q. Xu, Non-woven fabric supported poly(acrylonitrile-vinyl acetate) gel electrolyte for lithium ion battery use, J. Appl. Electrochem., 2010, 40, 2185–2191 CrossRef CAS.
- Y. H. Liao, M. M. Rao, W. S. Li, L. T. Yang, B. K. Zhu, R. Xu and C. H. Fu, Fumed silica-doped poly(butyl methacrylate-styrene)-based gel polymer electrolyte for lithium ion battery, J. Membr. Sci., 2010, 352, 95–99 CrossRef CAS PubMed.
- Y. H. Liao, D. Y. Zhou, M. M. Rao, W. S. Li, Z. P. Cai, Y. Liang and C. L. Tan, Self-supported poly(methyl methacrylate-acrylonitrile-vinyl acetate) based gel electrolyte for lithium ion battery, J. Power Sources, 2009, 189, 139–144 CrossRef CAS PubMed.
- L. Chen, M. M. Rao, W. S. Li, M. Q. Xu, Y. H. Liao, C. L. Tan and J. Yi, Performance improvement of polyethylene-supported poly(acrylonitrile-methyl methacrylate-styrene) electrolyte by using urea as foaming agent, Acta Phys.-Chim. Sin., 2011, 27, 1689–1694 CAS.
- P. P. Prosini, P. Villano and M. Carewska, A novel intrinsically porous separator for self-standing lithium-ion batteries, Electrochim. Acta, 2002, 48, 227–233 CrossRef CAS.
- H. S. Kim, K. S. Kum, W. Cho, B. W. Cho and H. W. Rhee, Electrochemical and physical properties of composite polymer electrolyte of poly(methyl methacrylate) and poly(ethylene glycol diacrylate), J. Power Sources, 2003, 124, 221–224 CrossRef CAS.
- J. H. Park, W. Park, J. H. Kim, D. Ryoo, H. S. Kim, Y. U. Jeong, D. W. Kim and S. Y. Lee, Close-packed poly(methyl methacrylate) nanoparticle arrays-coated polyethylene separators for high-power lithium-ion polymer batteries, J. Power Sources, 2011, 196, 7035–7038 CrossRef CAS PubMed.
- P. Zhang, H. P. Zhang, G. C. Li, Z. H. Li and Y. P. Wu, A novel process to prepare porous membranes comprising SnO2 nanoparticles and P(MMA-AN) as polymer electrolyte, Electrochem. Commun., 2008, 10, 1052–1055 CrossRef CAS PubMed.
- Z. Li, G. Su, D. Gao, X. Wang and X. Li, Effect of Al2O3 nanoparticles on the electrochemical characteristics of P(VDF-HFP)-based polymer electrolyte, Electrochim. Acta, 2004, 49, 4633–4639 CrossRef CAS PubMed.
- W. Li, Y. Xing, X. Xing, Y. Li, G. Yang and L. Xu, PVDF-based composite microporous gel polymer electrolytes containing a novel single ionic conductor SiO2 (Li+), Electrochim. Acta, 2013, 112, 183–190 CrossRef CAS PubMed.
- Y. Liao, M. Rao, W. Li, C. Tan, J. Yi and L. Chen, Improvement in ionic conductivity of self-supported P(MMA-AN-VAc) gel electrolyte by fumed silica for lithium ion batteries, Electrochim. Acta, 2009, 54, 6396–6402 CrossRef CAS PubMed.
- H. Xie, Y. Liao, P. Sun, T. Chen, M. Rao and W. Li, Investigation on polyethylene-supported and nano-SiO2 doped poly(methyl methacrylate-co-butyl acrylate) based gel polymer electrolyte for high voltage lithium ion battery, Electrochim. Acta, 2014, 127, 327–333 CrossRef CAS PubMed.
- Y. H. Liao, X. P. Li, C. H. Fu, R. Xu, M. M. Rao, L. Zhou, S. J. Hu and W. S. Li, Performance improvement of polyethylene-supported poly(methyl methacrylate-vinyl acetate)-co-poly(ethylene glycol) diacrylate based gel polymer electrolyte by doping nano-Al2O3, J. Power Sources, 2011, 196, 6723–6728 CrossRef CAS PubMed.
- Y. H. Liao, X. P. Li, C. H. Fu, R. Xu, L. Zhou, C. L. Tan, S. J. Hu and W. S. Li, Polypropylene-supported and nano-Al2O3 doped poly(ethylene oxide)–poly(vinylidene fluoride-hexafluoropropylene)-based gel electrolyte for lithium ion batteries, J. Power Sources, 2011, 196, 2115–2121 CrossRef CAS PubMed.
- M. M. Rao, J. S. Liu, W. S. Li, Y. H. Liao, Y. Liang and L. Z. Zhao, Polyethylene-supported poly(acrylonitrile-co-methylmethacrylate)/nano-Al2O3 microporous composite polymer electrolyte for lithium ion battery, J. Solid State Electrochem., 2010, 191, 255–261 CrossRef.
- Y. Liao, C. Sun, S. Hu and W. Li, Anti-thermal shrinkage nanoparticles/polymer and ionic liquid based gel polymer electrolyte for lithium ion battery, Electrochim. Acta, 2013, 89, 461–468 CrossRef CAS PubMed.
- D. R. Chen, B. Z. Li, Y. H. Liao, H. W. Lan, H. B. Lin, L. D. Xing, Y. T. Wang and W. S. Li, Improved electrochemical performance of LiNi0.5Mn1.5O4 as cathode of lithium ion battery by Co and Cr co-doping, J. Solid State Electrochem., 2014, 7, 2027–2033 CrossRef.
- M. Yanilmaz, Y. Lu, M. Dirican, K. Fu and X. Zhang, Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques, J. Membr. Sci., 2014, 456, 57–65 CrossRef CAS PubMed.
- J. A. Conesa, A. Marcilla, R. Font and J. A. Caballero, Thermogravimetric studies on the thermal decomposition of polyethylene, J. Anal. Appl. Pyrolysis, 1996, 36, 1–15 CrossRef CAS.
- Q. Lu, J. Fang, J. Yang, R. Miao, J. Wang and Y. Nuli, Novel cross-linked copolymer gel electrolyte supported by hydrophilic polytetrafluoroethylene for rechargeable lithium batteries, J. Membr. Sci., 2014, 449, 176–183 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |