Hydrodynamics and mass transfer study of oil-water micro-emulsion in a three phase external loop airlift reactor
Mostafa Keshavarz Moraveji* and
Jaber Gharib
Department of Chemical Engineering, Amirkabir University of Technology (Tehran Polytechnic), 424 Hafez Avenue, Tehran 15875-4413, Iran. E-mail: moraveji@aut.ac.ir; Tel: +98-9363098063
First published on 7th November 2014
Abstract
In this study, the impact of operating conditions, liquid properties and loading of a solid phase on the hydrodynamics and mass transfer coefficient in an airlift reactor with an external loop was investigated. The gas phase sparged through the reactor was air. Oil-in-water micro emulsions containing diesel, kerosene, heavy naphtha and light naphtha made up the liquid phase with an oil concentration of 5%. The impact of the solid phase was studied with solid loadings of 0, 0.25 and 0.5 wt%. The results showed that light naphtha which had the lowest density, viscosity and surface tension had the highest mass transfer and gas holdup and the lowest liquid circulation velocity. Solid addition decreased the gas holdup and mass transfer while increasing the liquid circulation velocity. This effect was considerable on the parameters above. In addition, a correlation based on dimensionless numbers was gained to predict the Sherwood number. Good agreement was observed between the correlated and experimental values.
1. Introduction
Airlift reactors which play an important role in mass transfer operations in chemical, petrochemical and, in particular, biochemical industries, such as waste water treatment, are among the top class multi-phase contactors.1 This wide application of airlift reactors is due to their advantages including low operation and maintenance cost and high mass transfer rate.2 Airlift reactors consist of two cylindrical vessels (riser and down-comer). Air is sparged through the riser which makes the fluid flow upward in the riser and downward in the down-comer. This leads to continues circulation in the airlift reactor.3Oil-in-water micro-emulsions are one of the main wastes of oil refinery, chemical and petrochemical companies. A study shows that more than 2 billion tons of wastewater is released into the environment by the European countries’ oil refineries.4 This huge amount of potential damage to the environment has motivated researchers to study the treatment of waste water by various airlift reactors. The influence of solid particles is important to be considered due to their existence in chemical and biochemical operations as catalysts and microorganism carriers.5 Several researchers have studied hydrodynamics and mass transfer in three-phase reactors. They found that solid addition caused gas holdup and mass transfer reduction but increased the liquid circulation velocity.
Hatta et al.6 studied the three-phase flow in a vertical pipe and they found that the solid particle motion strongly depended on the gas-phase volumetric flux. Kassab et al.7 developed a theoretical model for predicting the airlift-pump performance in the air–water–solid three-phase flow. The mass flow rate of solid particles increased with decreasing particle size although the submergence ratio increased at the same air flow rate.
Mehrnia et al.8 investigated the hydrodynamics and volumetric mass transfer coefficient in a draft tube airlift bioreactor (DTAB) using water-in-kerosene emulsions in the range of (10–0%) of water-in-oil for a bio-catalytic desulfurization process. They also correlated their results to the viscosity and aeration velocity although the applied variables were valid for the homogenous flow regime zone.
Sun et al.9 developed a hydrodynamics model for the prediction of liquid circulation and gas holdup in the riser of an air–water–silica sands three-phase annulus airlift reactor. They reported that the gas holdup in the riser increased when increasing the superficial gas velocity and decreasing solid concentration although the liquid circulation velocity increased with the solid concentration’s reduction.
Talvy et al.10 used particles with a density lower than water and with a diameter close to the bubbles diameter (with loadings of 20% and 40% v/v). They found that at a superficial gas velocity of 0–2.5 cm s−1, the gas holdup in the three-phase airlift reactor is similar to the two-phase airlift reactor, however, at higher superficial gas velocities, a lower gas holdup may be observed. They also reported that the liquid velocity decreased at various solid particle loadings.
Rujiruttanakul and Pavasant11 examined various types of external loop airlift contactors for their hydrodynamic and mass transfer behavior. The investigation covered a variety of design parameters including the length of connection tubes, height of riser and down-comer, and airlift configurations while maintaining the cross-sectional area ratio between down-comer and riser constant at 0.269. The results demonstrated that the behavior of the external loop airlift could be modified by adjusting the design and operating variables. In general, a faster liquid velocity led to a lower gas holdup and gas–liquid mass transfer rate. Increasing the length of connection tubes and height of riser and down-comer seemed to increase the liquid velocity while decreasing the overall gas holdup (ε) and the overall volumetric mass transfer coefficient. Empirical correlations for the estimation of the system’s behavior were also formulated.
Moraveji et al.12 considered the impact of liquid properties, loading of a solid phase and operating conditions on the hydrodynamics and mass transfer coefficient in some micro-emulsion systems in a three-phase internal loop airlift reactor. The experimental results showed that a micro-emulsion with the lowest density, viscosity and surface tension had maximum mass transfer and gas holdup and minimum liquid circulation velocity. The gas holdup and mass transfer decreased and the liquid circulation velocity increased by solid particle addition. Further enhancing the solid loading had a dramatic effect on the mentioned parameters.
In the another research, Moraveji13 studied the hydrodynamics and mass transfer characteristics in a 15.6 × 10−3 m3 external loop airlift reactor for oil-in-water micro-emulsions with oil-to-water volumetric ratios (φ) ranging from 3% to 7% (by volume). An increase in φ of micro-emulsion systems results in an increment in the gas holdup and a decrease in the volumetric gas–liquid oxygen transfer coefficient and liquid circulation velocity, attributed to the escalation in the viscosity of micro-emulsions.
Also, Moraveji14 investigated the effects of aeration velocity and liquid properties on the pertinent hydrodynamic and mass transfer parameters in a split-cylinder airlift reactor (with and without packing). They found that the packing installation increased the overall gas–liquid volumetric mass transfer coefficient by increasing the flow turbulence and Reynolds number, compared to the unpacked column. The packing increased the gas holdup and decreased the bubble size and liquid circulation velocity.
The aim of this research is to investigate the impact of aeration velocity, liquid properties and solid concentration on the hydrodynamics parameters and volumetric mass transfer coefficient in an external loop airlift reactor. Various micro-emulsion systems were chosen with similar concentrations to wastewaters of petroleum industries. Oil-in-water micro-emulsions with an oil concentration of 5% (v/v) are prepared and polyamide 6 is used as the solid phase with loadings of 0, 0.25 and 0.5 wt%. According to our experimental data, a new correlation was developed and examined for the Sherwood number ((Sh) as a symbol of mass transfer) based on dimensionless numbers (such as Schmidt (Sc), Reynolds (Re) and Bond (Bo) numbers).
2. Experimental
2.1 Materials
The used micro-emulsions were prepared locally from water and petroleum fractions and nonylphenol (NP).15 Various petroleum fractions containing diesel, kerosene, heavy naphtha and light naphtha were purchased from Shazand Oil refinery Company (Iran).Nonylphenol ethoxylate (NPE, C15H24O) with 99.5% purity was purchased from Isfahan Copolymer Company. Nonylphenols are produced by the acid-catalyzed alkylation of phenol with a mixture of nonenes. To make NPEs, manufacturers treat NP with ethylene oxide under basic conditions. The structures of the NPs may vary. The nonyl group can be attached to the phenol ring at various locations which results in different products with different Hydrophilic Lipophilic Balance (HLB) values. An appropriate concentration of NF60 (nonylphenol ethoxylated 6 molar) was gradually added to a mixture of tap water and petroleum for about 20 min until a stable micro-emulsion was obtained. Various solutions of petroleum fractions (3%, 5% and 7% (v/v)) were locally prepared.
The physical properties of the emulsifier, petroleum fractions and micro-emulsion solutions are summarized in Tables 1–3, respectively. The surface tensions were measured using a tensiometer (model: KRUSS GmbH, Germany) and the densities were measured using the Buoyancy method.
| Trade name | Avg EO mole | Appearance | Avg Mw | Water wt% | Density at 20 °C (g cm−3) | pH | HLB |
|---|---|---|---|---|---|---|---|
| NF60 | 6 | Oily liquid | 484 | 0.5 max | 1.045 ± 0 | 5–7 | 10.9 |
| Trade name | SP gravity 15.5/15.5 (°C) | IBP | FBP (°C) | Pour point | H2S | RSH (ppm) |
|---|---|---|---|---|---|---|
| Diesel | 0.8265 | 239 | 111 | 3 | Free | <10 |
| Kerosene | 0.8035 | 161 | 49 | — | Free | <10 |
| Heavy naphtha | 0.7495 | 95 | — | — | Free | <10 |
| Light naphtha | 0.668 | 47 | — | — | Free | <10 |
| Liquid | Oil-in-water volume | Density (kg m−3) | Kinematic viscosity 10−6 (m2 s−1) | Surface tension (mN m−1) |
|---|---|---|---|---|
| Water | — | 998.2 | 0.902 | 72.8 |
| Water–diesel | 5% | 988.351 | 23.00 | 27.044 |
| Water–kerosene | 5% | 987.204 | 3.46 | 25.663 |
| Water–h. naphtha | 5% | 984.512 | 1.92 | 24.356 |
| Water–l. naphtha | 5% | 980.449 | 1.31 | 23.413 |
Polyamide particles with densities of 0.28–0.3 g cm−3 and sizes of 15–20 micron were used as the solid phase.
2.2 Experimental set-up
The applied external airlift reactor is schematically shown in Fig. 1. The reactor consists of a column as the riser which is 1.7 m high and 0.1 m in diameter and a column as the down-comer which is 0.995 m high and 0.07 m in diameter both made of Pyrex glass. The initial liquid height (before sparging with gas) in the reactor was 1.215 m in all runs. Tap water was used as the liquid phase for the three-phase (gas–liquid–solid) system and was filled in from the top of the column. The gas sparger located at the bottom of the riser is a spherical ball which is 0.02 m in diameter and made of sintered ceramic. The volumetric flow rate of air in the riser zone was controlled using a regulating valve and a calibrated rotameter. The superficial gas velocity varied from 0.02 cm s−1 to 1.0 cm s−1 which could be controlled by an air compressor (FAD: 50 l min−1 and power 3 kW).The inverted U-tube manometer with water as the measuring liquid was used for the holdup measurements. All experiments were carried out under ambient conditions (atmospheric pressure and room temperature (25 ± 0.5 °C)).
A dissolved oxygen electrode (WTW Cellox 325) was positioned in the riser zone at the height of 1.18 m of the riser. The probe’s tip was at an angle of 30° to the horizontal for preventing oxygen bubbles sticking to it. The volumetric oxygen transfer coefficient (kLa) was measured using the well-known dynamic gassing-in method and nitrogen was used for de-oxygenating the solution.
A conductivity electrode (Model 740i, WTW, Germany) was used for liquid circulation velocity measurements and was positioned in the riser zone at a depth of 0.2 m from the bottom of the reactor. A U-tube manometer was used to measure the gas holdup. All experiments were carried out under ambient conditions (atmospheric pressure and room temperature (25 ± 0.5 °C)).
2.3 Measurement method
 | (1) |
 | (2) |
By integrating eqn (2), eqn (3) is obtained:
 | (3) |
By plotting  versus t, the slope gives the volumetric mass transfer coefficient. The concentration of dissolved oxygen is measured using a polarographic dissolved oxygen electrode (as shown in Fig. 1). Chisti17 based his experiments on three assumptions involving a constant gas-phase composition, well-mixed liquid phase and negligible dynamic effect of the dissolved oxygen electrode. In addition to these assumptions, we assumed that the temperature was constant during the experiments. Since temperature fluctuations change the saturation dissolved oxygen concentration (C*) the room was equipped with a thermostat, a cooler and an electric heater which kept the room temperature constant (20 °C). Another assumption was that there was no bubble accumulation on the oxygen electrode’s tip surface, because oxygen bubbles sticking to the surface prevent the probe from monitoring the real amount of dissolved oxygen in the reactor. The electrode was positioned at an angle of 30° to the horizontal to eliminate the possibility of bubbles sticking to the surface. The compression from the compressor was assumed constant. Since the air humidity of the room affects the compression behavior, the experiments were done on dry days.
versus t, the slope gives the volumetric mass transfer coefficient. The concentration of dissolved oxygen is measured using a polarographic dissolved oxygen electrode (as shown in Fig. 1). Chisti17 based his experiments on three assumptions involving a constant gas-phase composition, well-mixed liquid phase and negligible dynamic effect of the dissolved oxygen electrode. In addition to these assumptions, we assumed that the temperature was constant during the experiments. Since temperature fluctuations change the saturation dissolved oxygen concentration (C*) the room was equipped with a thermostat, a cooler and an electric heater which kept the room temperature constant (20 °C). Another assumption was that there was no bubble accumulation on the oxygen electrode’s tip surface, because oxygen bubbles sticking to the surface prevent the probe from monitoring the real amount of dissolved oxygen in the reactor. The electrode was positioned at an angle of 30° to the horizontal to eliminate the possibility of bubbles sticking to the surface. The compression from the compressor was assumed constant. Since the air humidity of the room affects the compression behavior, the experiments were done on dry days.
3. Results and discussion
3.1 Gas holdup
Gas holdup is an important factor that affects the operational volume. The effect of adding different solid particle fractions (0, 0.25 and 0.5 wt%) in different oils in water micro-emulsions with a concentration of 5% (v/v), on the gas holdup in the riser is shown in Fig. 2. Also, the obtained results were compared with pure water data.The gas hold-up increased with increasing aeration velocity. This increase is more considerable for the high gas velocities. Schafer et al.18 reported that high values of superficial gas velocity increased the bubble collision frequency which caused more coalescence and the production of larger bubbles. The release of larger bubbles may result in a smaller enhancement of the bubble portion in the fluid and consequently in gas holdup increase.
The rate of increase in the light naphtha based micro-emulsion without solid particles is higher than those in the other fractions. As shown in Table 3, surface tension, kinematic viscosity and density in the light naphtha based micro-emulsion without solid particles are lower than those of the other micro-emulsions.
According to the literature, the minimum required energy for bubbles to break led to an enhancement of the bubble break up rate and reduction of the bubble diameter.19,20 Furthermore, the buoyancy force decreased in the liquid phase for low density solutions and consequently decreased the bubbles’ rise velocity. In addition, the break up rate increased in the low kinematic viscosity solutions.1,21 Therefore, surface tension, density and kinematic viscosity affected the gas holdup.
At a low aeration velocity, the effect of the type of micro-emulsion is not clear although at high air flow rates, the gas holdup (εg) in micro-emulsion solutions increased as follows:
| εg for water < εg for diesel < εg for kerosene < εg for heavy naphtha < εg for light naphtha. |
Furthermore, the solid loading enhancement sharply affects the mentioned parameters. The gas holdup in heavy naphtha decreased about 25.4% and 43.09% with solid loadings of 0.25 wt% and 0.5 wt%, respectively, in comparison with the micro-emulsion without solid particles. This output is supported by some researchers.9 Solid particles increased the micro-emulsion’s apparent viscosity. As the commonly recommended equation by Barnea and Mizrahi22 points out, the apparent slurry viscosity increases almost linearly below a solid volume fraction of 30% and increases exponentially for higher solid volume fractions. Thus, enhancing the micro-emulsion viscosity increases the coalescence rate which leads to reduction of the gas holdup. Although at low gas velocities the effect of volume fraction of solid particles is not clear, at high aeration rates this influence is considerable.
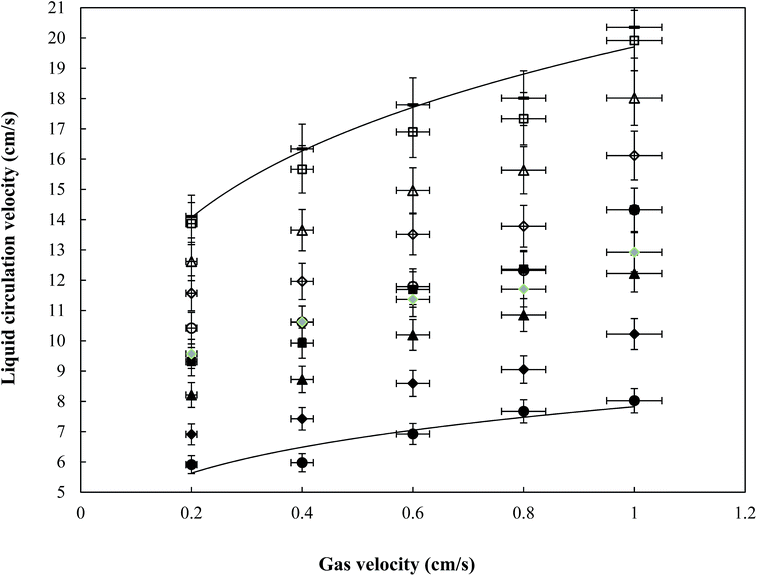
3.2 Liquid circulation velocity
In airlift reactors the density difference (because of the gas holdup difference) between riser and down-comer causes a driving force for liquid circulation.3 Compared to the single phase system, the riser gas holdup in a three-phase reactor is slightly lower (Fig. 3) and, simultaneously, the down-comer gas holdup is somewhat higher in the three-phase system. Adding solid to micro-emulsion solutions therefore leads to a decrease in the driving force and consequently to a decrease in the liquid circulation velocity of the micro-emulsion solutions. The small bubbles in micro-emulsions can import the down-comer when the buoyancy force decreases. Furthermore, some bubbles traverse a part of the reactor bottom. So the transition flow regime is observed in all of the superficial gas velocities in micro-emulsions.The minimum liquid circulation velocity was observed in light naphtha (49% lower than pure water at the highest superficial gas velocity in the riser (Ugr)). The liquid circulation velocity decreased as follows:
| UL for light naphtha < UL for heavy naphtha < UL for kerosene < UL for diesel < UL for water. |
Moreover, the liquid circulation velocity increases with increasing solid particles. This effect is enhanced with increasing solid loading. The liquid velocity in the riser is more affected by the presence of a solid than the gas holdup. The increase in solid loading increases the system turbulence, which leads to a decrease in the rising bubbles’ sizes.
3.3 Gas–liquid mass transfer
Fig. 4 shows the overall volumetric oxygen mass transfer coefficient kLaL versus gas velocity for various micro-emulsions with solid loadings of 0, 0.25 and 0.5 wt% and pure water. In all micro-emulsions kLaL is higher in comparison with that in pure water. This output is supported in the literature.12 The volumetric mass transfer coefficient between gas and liquid depends on two separate parameters involving the mass transfer coefficient (kL) and the active surface (a) between the operation phases. In micro-emulsion solutions, oil and emulsifier make a rigid layer between gas and liquid that reduces the surface renewal rate and, hence, kL decreases. Since the bubble diameter decreases in micro-emulsions (due to a decrease in surface tension in comparison with pure water), the active surface increases. Furthermore, the bubble residence time in the reactor increases when a reduction in the bubbles’ diameters and the buoyancy force occurs. Therefore, the mass transfer between the operating phases is enhanced. These parameters effectively increase the volumetric mass transfer coefficient.This enhancement was more considerable at higher gas velocities. The volumetric oxygen mass transfer coefficient for light naphtha was the highest among all oil-in-water micro-emulsions. The mass transfer in the micro-emulsion solutions increased as follows: water < diesel < kerosene < heavy naphtha < light naphtha.
The viscosity greatly affects the overall mass transfer coefficient. Enhancing the liquid viscosity increases the bubble coalescence rate because of a decrease in the gas liquid interfacial area (a). Therefore, the liquid boundary layer of all bubbles thickens.23 On the other hand, higher viscosity leads to lower diffusivity of the solute. Therefore, the kLa values decrease.
Adding solid particles to micro-emulsion solutions increased the mass transfer coefficient. The increase for heavy naphtha with loadings of 0.25 wt% and 0.5 wt% was 10.05% and 24.7%, respectively. Two facts can be pointed out for this enhancement: the first is the fact that small solid concentrations improve the surface renewal and turbulence in the liquid film, increasing kL and thus kLa; the second is associated with the presence of fine particles in the liquid film at the gas–liquid interface which may hinder the coalescence behaviour of the bubbles, consequently increasing the gas–liquid interfacial area.
3.4 Mass transfer correlations
Liquid bulk properties (such as viscosity, density and surface tension), interfacial properties (such as active surface and mass diffusion coefficient) and body forces (gravitational forces) affect the micro-emulsion solutions’ hydrodynamics and mass transfer characteristics. Therefore, it is possible to develop a correlation between the measured data using dimensionless numbers.An equation based on the Sherwood number (Sh) was developed to correlate the experimental mass transfer data as follows:
| Sh = 0.00408eCsRe1.083Sc0.695Bo0.365 | (4) |
In the above correlation, the Sh number (the ratio of convective to diffusive mass transfer) is related to the Re number (the ratio of inertial forces to viscous forces), Sc number (the ratio of momentum diffusivity (viscosity) to mass diffusivity) and Bo number (the ratio of body forces to surface tension forces).
Since the solid particles’ effect is radical, the term eCs is considered in the correlation. The correlation coefficient of the present fitting (R2) was 0.985 which showed the high quality of the fitting. The correlated data obtained from this work and the literature3,24,25 were compared with the experimental data, as shown in Fig. 5.
 | ||
Fig. 5 Correlated Sherwood number versus the Sherwood number obtained from the literature and the present experimental work. ○: Akita and Yoshida,25 ×: Moraveji et al.,12 ![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) : Asgharpour et al.,24 □: this study. : Asgharpour et al.,24 □: this study. | ||
Table 4 shows the correlations based on the literature and the present correlation. Very good agreement (with a mean error of 8.41%) was observed between our correlation and the experimental data. The mean errors between the experimental data and the literature data were around 45.08%, 36.37% and 25.27% for the correlations by Asgharpour et al.,24 Akita and Yoshida25 and Moraveji et al.,3 respectively.
| Author | Remarks | Correlations |
|---|---|---|
| Asgharpour et al.24 | 0.118 < UG < 2.35 cm s−1 | Sh = 0.15Re2/3Sc1/2Bo2/3 |
| Akita et al.25 | Homogeneous flow | Sh = 0.6Re1/2Sc1/2Bo3/8 |
| Moraveji et al.12 | 0.2 < UG < 1 cm s−1 | Sh = 0.169eCsRe2/3Sc1/2Bo0.426 |
| db < 3.5 mm | ||
| This study | 0.2 < UG < 1 cm s−1 | Sh = 0.00408eCsRe1.083Sc0.695Bo0.365 |
| db < 3.5 mm |
4. Conclusions
In this study, the effect of four oil-in-water micro-emulsions and their concentrations with solid particles loadings of 0, 0.25 and 0.5 wt% on the gas holdup, liquid circulation velocity and volumetric mass transfer coefficient in an external airlift reactor was studied. The micro-emulsions enhanced the break up rate which increased the gas holdup and volumetric mass transfer coefficient while decreasing the liquid circulation velocity in comparison with water. This effect was more considerable at higher concentrations and higher gas velocities. The results also showed that the addition of solid particles to the micro-emulsion solutions reduced the gas holdup and mass transfer coefficient while the liquid circulation velocity increased. According to the experimental data, mass transfer parameters were correlated using dimensionless numbers. A satisfactory agreement between the correlation and experimental data was observed.Nomenclature
| a | [m2 m−3] Gas–liquid interfacial area per unit volume of the liquid |
| Bo | [-] Bond number |
| Cs | [wt%] Solid particles loading |
| CL | [kg m−3] Concentration of dissolved oxygen at any time t |
| C0 | [kg m−3] Initial concentration of dissolved oxygen |
| C* | [kg m−3] Saturation concentration of dissolved oxygen |
| D | [m2 s−1] Gas diffusivity |
| Sh | [-] Sherwood number |
| Sc | [-] Schmidt number |
| Re | [-] Reynolds number |
| kLaL | [s−1] Overall volumetric gas–liquid mass transfer coefficient |
| R | [-] Residual |
| ε | [-] Overall gas holdup |
| ρL | [kg m−3] Density of liquid |
| ρG | [kg m−3] Density of air |
| dhM | [m] The manometer reading |
| dz | [m] The distance between manometric taps |
| UG | [cm s−1] Superficial aeration velocity in the riser zone |
| db | [mm] Bubble diameter |
References
- A. Ferreira, C. Ferreira, J. A. Teixeira and F. Rocha, Temperature and solid properties effects on gas–liquid mass transfer, Chem. Eng. J., 2010, 162, 743–752 CrossRef CAS PubMed
.
- J. H. Yang, J. Yang, H. J. Kim, D. H. Chun, H. Lee and H. Jung, Two regime transitions to pseudo-homogeneous and heterogeneous bubble flow for various liquid viscosities, Chem. Eng. Process., 2010, 49, 1044–1050 CrossRef CAS PubMed
.
- M. K. Moraveji, B. Sajjadi and R. Davarnejad, Gas–liquid hydrodynamics and mass transfer in aqueous alcohol solutions in a split-cylinder airlift reactor, Chem. Eng. Technol., 2011, 34, 465–474 CrossRef CAS
.
- A. S. Jonsson and G. Tragardh, Ultrafiltration applications, Desalination, 1990, 77, 135–179 CrossRef
.
- K. Isaka, S. Yoshie, T. Sumino, Y. Inamori and S. Tsuneda, Nitrification of landfill leachate using immobilized nitrifying bacteria at low temperatures, Biochem. Eng. J., 2007, 37, 49–55 CrossRef CAS PubMed
.
- N. Hatta, H. Fujimoto, M. Isobe and J. Kang, Theoretical analysis of flow characteristics of multiphase mixtures in a vertical pipe, Int. J. Multiphase Flow, 1998, 24, 539–561 CrossRef CAS
.
- S. Z. Kassab, H. A. Kandil, H. A. Warda and W. H. Ahmed, Experimental and analytical investigations of airlift pumps operating in three-phase flow, Chem. Eng. J., 2007, 131, 273–281 CrossRef CAS PubMed
.
- M. R. Mehrnia, J. Towfighi, B. Bonakdarpour and M. M. Akbarnejad, Gas holdup and oxygen transfer in a draft-tube airlift bioreactor with petroleum based liquids, Biochem. Eng. J., 2005, 22, 105–110 CrossRef CAS PubMed
.
- S. Sun, X. Bao, C. Liu, J. Xu and W. Wei, Hydrodynamic model for three-phase annulus airlift reactors, Ind. Eng. Chem. Res., 2005, 44(2), 7550–7558 CrossRef CAS
.
- S. Talvy, A. Cockx and A. Line, Global modeling of a gas–liquid–solid airlift reactor, Chem. Eng. Sci., 2005, 60, 5991–6003 CrossRef CAS PubMed
.
- Y. Rujiruttanakul and P. Pavasant, Influence of configuration on the performance of external loop airlift contactors, Chem. Eng. Res. Des., 2011, 89, 2254–2261 CrossRef CAS PubMed
.
- M. K. Moraveji, M. Jafarkhani, B. Sajjadi and R. Davarnejad, Hydrodynamics and mass transfer of oil–water micro-emulsion in a three phase internal airlift reactor, Fuel, 2012, 97, 197–201 CrossRef PubMed
.
- M. Ebrahimi Fakhari, M. Keshavarz Moraveji and R. Davarnejad, Hydrodynamics and Mass Transfer of Oily Micro-emulsions in An External Loop Airlift Reactor, Chin. J. Chem. Eng., 2014, 22(3), 1–7 Search PubMed
.
- M. Keshavarz Moraveji, E. Mohsenzadeh, M. Ebrahimi Fakhari and R. Davarnejad, Hydrodynamics and Oxygen Mass Transfer Characteristics of Petroleum Based Micro-Emulsions in a Packed Bed Split-Cylinder Airlift Reactor, Braz. J. Chem. Eng., 2013, 30(3), 541–550 CrossRef PubMed
.
- M. K. Moraveji, B. Sajjadi and R. Davarnejad, The Influence of Oil-water Ratio on the Operation of an Airlift Reactor Containing Petroleum-based Micro-emulsion, Pet. Sci. Technol., 2014, 32, 1–7 CrossRef
.
- J. Gharib, M. K. Moraveji, R. Davarnejad and M. E. Malool, Hydrodynamics and mass transfer study of aliphatic alcohols in airlift reactors, Chem. Eng. Res. Des., 2013, 91, 925–932 CrossRef CAS PubMed
.
- M. Y. Chisti, Airlift bioreactors, Elsevier Applied Science, London, UK, 1989 Search PubMed
.
- R. Schafer, C. Marten and G. Eigenberger, Bubble size distributions in a bubble column reactor under industrial conditions, Exp. Therm. Fluid Sci., 2002, 26, 595–604 CrossRef CAS
.
- J. J. Jeng, J. R. Maa and Y. M. Yang, Surface effects and mass transfer in bubble column, Ind. Eng. Chem. Process Des. Dev., 1986, 25, 486–495 Search PubMed
.
- E. Camarasa, C. Vial, S. Poncin, G. Wild, N. Midoux and J. Bouillard, Influence of coalescence behavior of the liquid and of gas sparging on hydrodynamics and bubble characteristics in a bubble column, Chem. Eng. Process., 1999, 38, 329–344 CrossRef CAS
.
- J. J. Heijnen and K. Van’t Riet, Mass transfer, mixing and heat transfer phenomena in low viscosity bubble column reactors, Chem. Eng. J., 1984, 28, B21–B42 CrossRef CAS
.
- E. Barnea and J. Mizrahi, A generalized approach to the fluid dynamics of particulate systems: part 1: general co-relation on for fluidization and sedimentation in solid multi-particle systems, Chem. Eng. J., 1973, 5(2), 171–189 CrossRef CAS
.
- P. Appasani, CFD simulation of the hydrodynamics and inter-phase mass transfer in airlift reactors, in Partial fulfillment of the requirements for the degree of master of applied science, Dalhousie University, 2007 Search PubMed
.
- M. Asgharpour, M. R. Mehrnia and N. Mostoufi, Effect of surface contaminants on oxygen transfer in bubble column reactors, Biochem. Eng. J., 2010, 49, 351–360 CrossRef CAS PubMed
.
- K. Akita and F. Yoshida, Bubble size, interfacial area and liquid-phase mass transfer coefficient in bubble columns, Ind. Eng. Chem. Process Des. Dev., 1974, 13, 84–91 CAS
.
| This journal is © The Royal Society of Chemistry 2014 |