Oxidative annulations triggered by a simple Lewis acid: facile synthesis of benzofurans†
Abstract
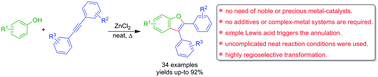
The first example of Lewis acid promoted efficient one-pot synthesis of benzofurans is presented. Unlike previous reports on oxidative annulations between phenols and internal acetylenes, a simple and highly facile Lewis acid mediated protocol is presented. The Lewis acid (ZnCl2) played a crucial role to promote dual (C–H and O–H) bond formation, presumably via a six-membered cyclic transition state. Significantly, a variety of benzofurans were accomplished with symmetrical/unsymmetrical acetylenes.

 Please wait while we load your content...
Please wait while we load your content...