Luminescent porous organic polymer nanotubes for highly selective sensing of H2S†
Lin
Guo‡
a,
Meng
Wang‡
a,
Xiaofei
Zeng
*a and
Dapeng
Cao
 *ab
*ab
aState Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, People's Republic of China. E-mail: caodp@mail.buct.edu.cn; zengxf@mail.buct.edu.cn
bBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, People's Republic of China
First published on 11th October 2017
Abstract
In this study, we synthesize a luminescent porous organic polymer nanotube (PNT-1) through copolymerization of the monomers 1,3,5-tris-(3-bromo-phenyl)-[1,3,5]triazine (TBT) and 2,6-dibromopyridine (2,6-DP) by Ni-catalyzed Yamamoto reaction. Results indicate that PNT-1 exhibits high luminescence intensity, stability and reusability, and it can be used as an efficient luminescent probe for sensitively and selectively sensing H2S. Furthermore, we also use UV-absorption as well as EDS and XPS spectra to further explore the luminescence quenching mechanism of PNT-1 as a luminescent probe for sensing H2S. All the results reveal that the formation of N–S bond between PNT-1 and H2S makes the electron in PNT-1 more stable and therefore leads to the luminescence quenching effect of PNT-1 for sensing H2S. Moreover, we also find that PNT-1 as a luminescent probe is still applicable for sensing H2S in vitro assay system simulated using PBS buffer solution.
1. Introduction
As is well known, hydrogen sulfide (H2S) gas is a toxic gas with a rotten egg smell and is fatally harmful to humans. Recently, H2S as a signal transmission molecule in human body,1–3 same as nitric oxide (NO) and carbon monoxide (CO), has attracted increasing attention. Generally, H2S presents in the form of HS− and S2− to achieve equilibration in biological environments, and it plays an important role in physiological and pathological processes,4–6 such as in nervous, respiratory, cardiovascular, digestive and endocrine systems. It is very crucial to detect H2S in living cells for human health.Currently, several methods have been used to detect H2S, for example, time-resolved laser-induced breakdown spectroscopy,7 electrochemical method,8 gas chromatography,9 and high performance liquid chromatography.10 Although these techniques can detect H2S, they also have some drawbacks, such as high cost and complex operations. Recently, luminescence-based method has emerged owing to its high sensibility and selectivity, short response time and high practicability.11–17 Therefore, it is important to develop luminescent materials-based chemical sensors for detecting H2S, and in fact, Na2S has always been used as an H2S substitute for study in the area of luminescence sensing.18,19
A lot of luminescent materials have been reported to detect H2S, in which Na2S was used as the analyte.20–22 Xiong et al.23 reported a type of Au–Ag core–shell nanoparticles (NPs) for highly sensitive detection of H2S in live cells. Zhang et al.24 reported a UiO-66-(COOH)2 MOF modified with Eu3+ and Cu2+ for sensing H2S. Nagano et al.25 synthesized a novel fluorescence probe HSip1 for sensing H2S. Tropiano et al.26 reported a lanthanide based sensor (Eu complex) for highly sensitive and selective detection of H2S. Yu et al.27 synthesized a carbon-dot-based ratiometric luminescent sensor for the detection of H2S in aqueous media. Liu et al.28 designed a lanthanide coordination polymer for sensing H2S in biological systems. Ma et al.29 reported quinoline group-modified Fe3O4@SiO2 luminescent NPs for detection of Zn2+ and H2S in aqueous solutions. Chen et al.30 reported a luminescent probe constructed by combining coumarin and merocyanine, and it can be used for specific ratiometric sensing of H2S. Although these metal-containing materials can act as luminescent probes for effectively sensing H2S, some disadvantages still exist, such as low solvent thermal stability and the possibility of secondary pollution.
Covalent organic materials (COMs)31–33 are widely studied as luminescent probes because of their excellent luminescence property and high hydrothermal stability. For example, Jiang's group34 reported a luminescent azine-linked covalent organic framework (COF) for the detection of 2,4,6-trinitrophenol explosive. Liu et al.35 reported an azine-linked COF-JLU3 for detecting Cu2+. Recently, porous covalent organic polymer (COP),36 a subclass of COM materials, has also been widely used as a luminescent probe for sensing nitro explosives, small organic molecules and metal ions.37–39 Xiang et al.40 reported three types of luminescent COP materials with clear-cut layer texture structure for sensing nitro explosives and small organic molecules. Guo et al.41 synthesized a series of color-tunable COP materials with spherical structure for sensing PA and Fe3+. Apparently, these COP luminescent probes show excellent sensing properties for the detection of nitro explosives, metal ions and small organic molecules, but they have not been applied in the detection of H2S yet.
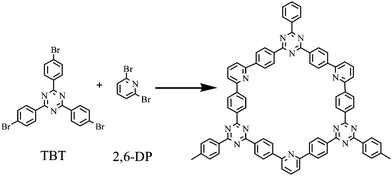
In this work, we use 1,3,5-tris-(3-bromo-phenyl)-[1,3,5]triazine (TBT) and 2,6-dibromopyridine (2,6-DP) as double monomers to synthesize a porous organic polymer nanotube (PNT-1) via Ni-catalyzed Yamamoto reaction, and the synthesis process is presented in Scheme 1. Then, we further investigate its potential luminescence property for sensing H2S. Moreover, we also explore its feasibility as a luminescent probe for detection of H2S in vitro assay system by simulating the human environment. Finally, some discussion is addressed.
2. Experimental
2.1 Synthesis of polymer nanotube (PNT-1)
The synthesized process of PNT-1 was divided into two parts: the reaction process in glove box and the post-treatment process in draught cupboard. First, 1,5-cyclooctadiene (cod, 0.50 mL, 3.96 mmol), bis(1,5-cyclooctadiene)nickel(0) ([Ni(cod)2], 1.125 g, 4.09 mmol), and 2,2′-bipyridyl (0.640 g, 4.09 mmol) were added into dry dimethylformamide (DMF) (65 mL) and stirred until the mixed solution turned purple and completely dissolved. Then, 1,3,5-tris-(3-bromo-phenyl)-[1,3,5]triazine (TBT, 0.122 g, 0.314 mmol) and 2,6-dibromopyridine (2,6-DP, 0.112, 0.471 mmol) were subsequently added to the resulting purple solution and heated at 80 °C overnight under an argon atmosphere. After cooling to room temperature, the reaction vessel was put into the draught cupboard for further post-treatment process. The concentrated HCl was added to the deep purple suspension continuously, and stirred until the solution changed into aqua transparent solution. After filtration, the residue was washed by chloroform (CHCl3, 5 × 15 mL), tetrahydrofuran (THF, 5 × 15 mL) and H2O (5 × 15 mL), respectively, and dried in a vacuum oven. The dried sample was placed in containers and stored in a desiccator.2.2. Characterization of PNT-1 materials
FTIR spectroscopy was performed on a Nicolet 8700 instrument with the wave range of 4000–400 cm−1. Scanning electron microscopy (SEM) images were obtained on a Hitachi S-4700 SEM instrument. EDS measurements were performed on an energy dispersive spectrometer (Oxford INCA Energy 350 ADD), which is an accessory of SEM instrument, using Mn Kα radiation. Thermogravimetric analysis (TGA) data were obtained on a STA449C (NETZSCH) instrument, with a heating rate of 10 °C min−1 under flowing N2. Ultraviolet absorption spectra were recorded using TU-1901 spectrophotometers. N2 adsorption/desorption isotherms at 77 K were measured by a Micromeritics ASAP 2020 system. Pore size distribution data were calculated from the N2 sorption isotherms based on the NLDFT model in the Micromeritics ASAP 2020 software package. The XPS data were recorded with a ThermoFisher ESCALAB 250 X-ray photoelectron spectrometer equipped with a twin anode Mg-Kα X-ray source. The luminescent spectra were measured by Hitachi F-7000 Fluorescence Spectrophotometer with a PMT voltage of 420 V and a scan speed of 120 nm min−1, and the width of the excitation slit is 5 nm and that of emission slit is 10 nm for the PNT-1 material.2.3 Luminescence measurements for sensing anions
The sodium salt analytes used for the luminescent sensing experiments are Na2S, NaF, Na2SO4, NaCl, NaBr, NaI, NaIO3, NaSCN, NaHSO3, Na3PO4, NaNO3, and Na2CO3. The dried powder of PNT-1 was dispersed in methanol (CH3OH, 2 mg/10 mL) solution for ultra-sonication for about an hour, and the resulting PNT-1–CH3OH suspension was used for the subsequent experiment. The photoluminescence (PL) sensing properties of the synthesized PNT-1 material in CH3OH solutions were investigated at room temperature. To explore the luminescence properties of PNT-1 for sensing anions, the fluorescence spectra of PNT-1–CH3OH suspension were recorded by successive addition of aliquots of NaxM (Mx− = S2−, F−, SO42−, Cl−, Br−, I−, IO3−, SCN−, HSO3−, PO43−, NO3−, and CO32−, respectively). The width of the excitation slit is 5 nm and that of the emission slit is 10 nm for PNT-1.3. Results and discussion
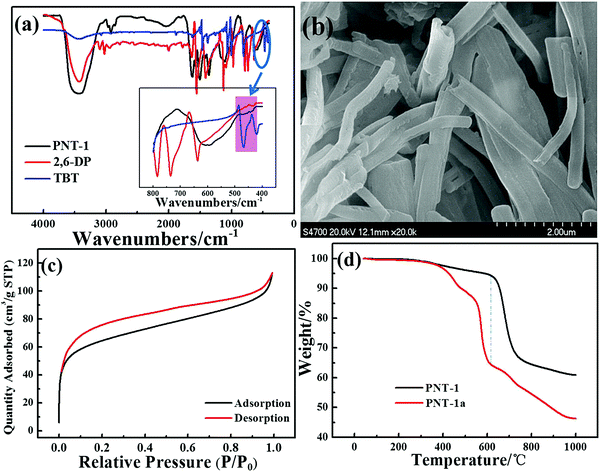
Several basic characterizations have been performed to determine the inherent property of the PNT-1 material. The success of the phenyl–phenyl coupling in PNT-1 can be confirmed by Fourier transform infrared (FTIR) analysis (see Fig. 1a). The characteristic absorption bands for carbon–bromine bonds are highlighted in the pink region (see inset of Fig. 1a), in which the lack of bromine peak in the PNT-1 indicates completion of copolymerization. As shown in Fig. 1b, the SEM image clearly shows the hollow tubular structure of PNT-1. We also further measured N2 adsorption isotherm of PNT-1 at 77 K (Fig. 1c). The pore size distribution calculated by N2 uptake is shown in Fig. S1 (ESI†). The BET specific surface area of PNT-1 is 238.5 m2 g−1 and its pore size has a wide range from 1.8 to 12 nm, indicating that it contains micropores (<2 nm) and mesopores (2–50 nm). Thermogravimetric analysis under N2 (Fig. 1d, black line) suggests that PNT-1 shows very high thermal stability with only 5% weight loss at 620 °C.The luminescence spectra of solid PNT-1 and the two monomers (TBT and 2,6-DP) were measured and are shown in Fig. 2. Compared to the monomers, the luminescence intensity of PNT-1 increased significantly, owing to the increase in the number of π–π conjugate bonds produced by coupling reaction, and the fact that PNT-1 are more stable and reduce the energy consumed by vibration, similar to the AIE effect.42,43 The high luminescence intensity of the PNT-1 material suggests that it is a good candidate as a luminescent probe.
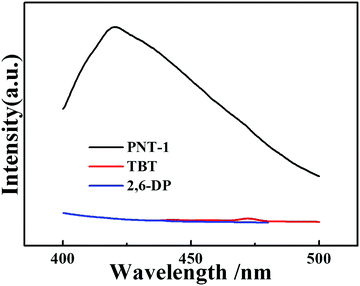 | ||
| Fig. 2 PL emission spectra of solid materials, PNT-1 (black line), TBT (red line) and 2,6-DP (black line). | ||
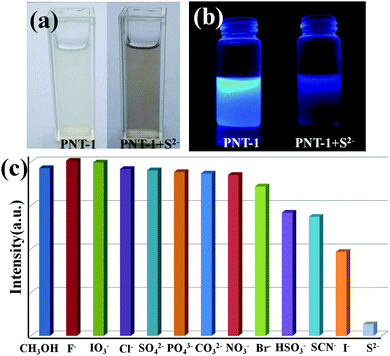
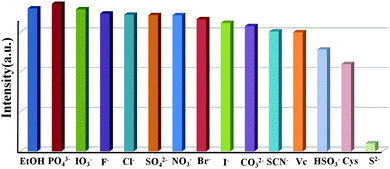
To study the potential property of luminescent PNT-1 for sensing H2S, without loss of generality, we first considered the sensing of PNT-1 for its anion formation, as reported in previous literature.20,21 The solid PNT-1 material was dispersed in CH3OH solution and highly dispersed PNT-1 suspension (2 mg/10 mL) was obtained. Subsequently, quantitatively different methanol solutions of Nax(M) (Mx− = S2−, F−, SO42−, Cl−, Br−, I−, IO3−, SCN−, HSO3−, PO43−, NO3−, and CO32−) were added to the suspension for measurements. As shown in Fig. 3a, after adding S2−, the color of PNT-1 suspension suddenly became deeper and precipitation appeared rapidly, while this special phenomenon did not occur for other anions. The luminescent picture of PNT-1 suspension in Fig. 3b before and after adding S2− can be easily identified by the naked eyes under a UV light (λex = 365 nm). The corresponding luminescence spectra of PNT-1 suspension before and after adding anions were measured and are shown in Fig. S2 (ESI†), in which the peak position of the emission spectrum does not change. Intuitively, the luminescence intensities of PNT-1 suspensions before and after adding different anions are shown in Fig. 3c. Compared to PNT-1 suspension matrix, the luminescence intensity of PNT-1 after adding anions is changed in different degrees. In particular, after adding S2−, the luminescence of PNT-1 is almost quenched completely, suggesting that PNT-1 may be a potential luminescent probe for sensing H2S.
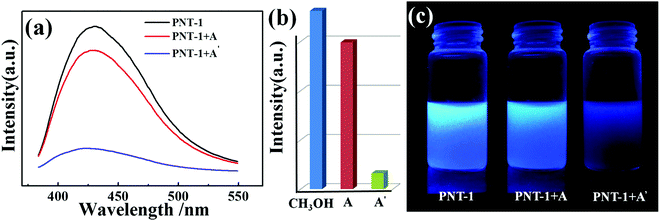
To confirm the high selectivity of PNT-1 as a candidate for sensing S2−, we performed a series of competition experiments to explore the effects of other anions (F−, SO42−, Cl−, Br−, I−, IO3−, SCN−, HSO3−, PO43−, NO3−, and CO32−) on the sensing property of PNT-1 for detecting S2−. In these competition experiments, we measured the luminescence intensities of PNT-1 in the presence of other anions before and after adding S2− in CH3OH solution. The luminescence spectra of PNT-1 in multi-component mixtures (with S2− and without S2−) of other anions were measured and are shown in Fig. 4 and Fig. S3–S12 (ESI†). As shown in Fig. 4, the luminescence intensity of PNT-1 almost has no change after adding anion mixture A (A = SO42− + IO3− + CO32− + F− + Cl− + PO43− + Br− + HSO3− + NO3− + I− + SCN−) to the solution, while the luminescence almost quenches completely after adding S2− to the above solutions. Similar results were obtained under sixty-five different conditions of multi-component mixtures, while the components of these mixtures were contained in A (A = SO42− + IO3− + CO32− + F− + Cl− + PO43− + Br− + HSO3− + NO3− + I− + SCN−) and made up of different combinations in the form of 1-, 2-, 3-, 4-, 5-, 6-, 7-, 8-, 9-, and 10-component mixtures. We then measured the luminescence intensity changes of PNT-1 after adding the mixtures in methanol solutions, as shown in Fig. S3–S12 (ESI†). Clearly, the luminescence intensity of PNT-1 showed no obvious change after adding these sixty different types of anion mixtures to PNT-1 methanol solutions, while the luminescence intensity of PNT-1 almost quenched after adding S2− to the abovementioned solutions. These results indicate that PNT-1 exhibits extremely high selectivity for sensing S2− even in the existence of other anions.
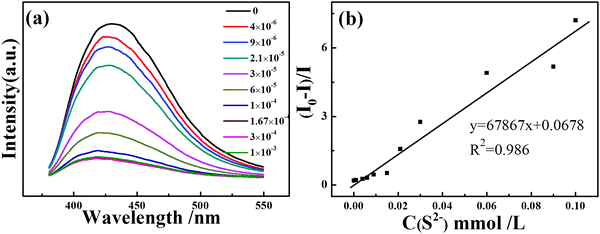
Moreover, the luminescence sensitivity of PNT-1 for detecting H2S was investigated via real-time luminescence response. The methanol solutions of Na2S with different concentrations were gradually added to the PNT-1 suspension, and the corresponding luminescence spectra were measured; the peak position of the emission spectra did not change upon the addition of different concentrations of Na2S solutions (see Fig. 5a). At 30 ppm S2− concentration, the quenching degree reaches about 56.2%, and when the concentration of S2− is 60 ppm, the quenching degree hits about 77%. Then, we calculated the quenching coefficient by Stern–Volmer equation: I0/I = 1 + Ksv [M], where I0 is the luminescence intensity of the luminescent material, and I is the luminescence intensity of the luminescent material after adding the analyte of concentration [M], and Ksv is the quenching coefficient. There is a good linear Stern–Volmer relationship of PNT-1 for sensing S2−, and the Ksv of PNT-1 for detecting S2− reaches 6.79 × 104 (see Fig. 5b), which means that PNT-1 also possesses high sensitivity for S2− detection. We also used the LOD (limited detection concentration) equation: LOD = 3σ/K to calculate the LOD, where σ is standard deviation of the blank luminescent materials (see Fig. S13, ESI†), and K is the Ksv constant of the materials for sensing analytes. The results indicate that the LOD of PNT-1 for detecting S2− reaches 0.053 ppm, which is lower than that of other luminescent probes for sensing S2−, as listed in Table S1 (ESI†).
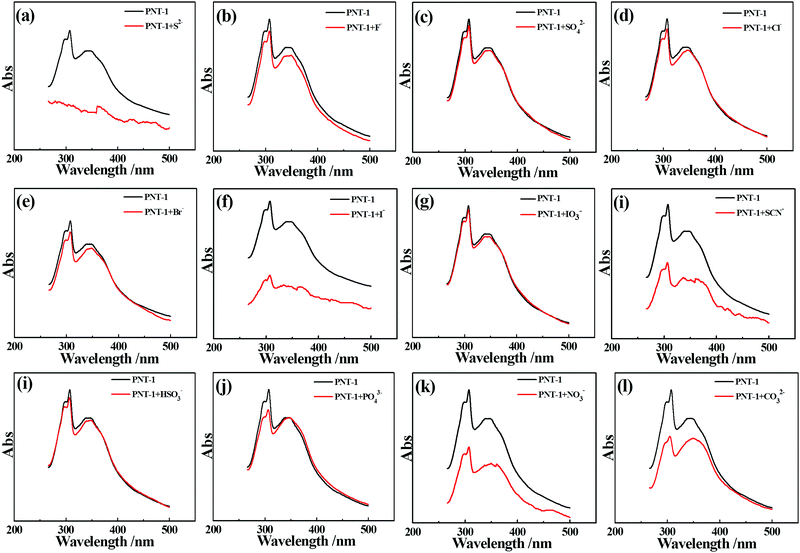
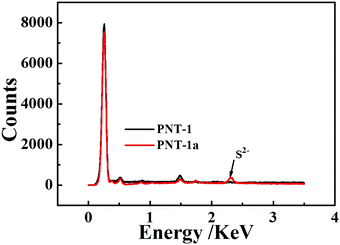
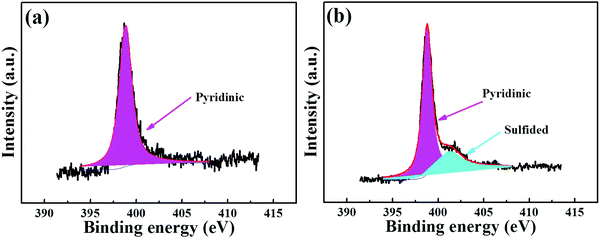
Essentially, the luminescence detection mechanism was also studied by a series of experiments, including UV absorption spectra, XPS, EDS and TGA. The UV absorption spectra of the PNT-1 suspension before and after adding different anions were measured and are shown in Fig. 6. Compared to PNT-1 suspension matrix, the absorption peak and the shape of the spectra of PNT-1 suspension after adding these anions of F−, SO42−, Cl−, Br−, I−, IO3−, SCN−, HSO3−, PO43−, NO3−, and CO32− (Fig. 6b–l) have no significant change except the change of the absorption intensity. However, the absorption spectra of PNT-1 suspension after adding S2− disappear (see Fig. 6a), indicating the appearance of new matter or occurrence of a chemical reaction between PNT-1 and S2−. To prove this observation, we measured the EDS spectra and XPS spectra of PNT-1 before and after adding S2−. After a series of filtration, washing and drying, the obtained solid PNT-1–S2− materials were marked as PNT-1a. EDS data (see Fig. 7) indicate that S2− definitely exists in some special form in PNT-1a after adding S2− to PNT-1, because an obvious peak S2− appears in the PNT-1a EDS spectrum while it is absent in the PNT-1 EDS spectrum. We also further measured the XPS spectra of PNT-1 and PNT-1a, shown in Fig. S14 (ESI†), in which the N1s, C1s and S1s peaks appear in PNT-1a XPS while S1s peak is absent in PNT-1 XPS. Compared to that of PNT-1, the C1s spectrum (see Fig. S15, ESI†) of PNT-1a has no significant change. High-resolution N1s XPS spectra details are shown in Fig. 8. Clearly, PNT-1 only has a peak at the position of 398.6 eV (Fig. 8a), which is in good agreement with the type of nitrogen of PNT-1, i.e., pyridinic N. However, the N1s peak of PNT-1a shows a new peak at 401.8 eV (Fig. 8b), indicating the formation of N–S bond. Therefore, we believed that the pyridinic N in PNT-1 forms an N–S bond with S2− and makes the electron in PNT-1 more stable and not easy to transfer, thus causing the luminescence quenching effect. Moreover, TGA curve of PNT-1a was obtained and is shown in Fig. 1d. Compared to PNT-1, PNT-1a starts to collapse rapidly at 400 °C, and when the temperature reaches 620 °C, about 40% weight is lost, indicating the decrease in its stability. All the UV-spectra, EDS, XPS and TGA results indicate that N–S bond was formed between PNT-1 and S2−, resulting in the luminescence quenching effect of PNT-1 as luminescent probes for sensing S2−.
We also investigated the luminescence stability of PNT-1 when dispersed in solutions. The PL spectra of PNT-1 dispersed in a solvent were measured at different times. As shown in Fig. S16 (ESI†), the PL spectra and luminescence intensity almost do not change with time, indicating that PNT-1 have excellent stability in solvents. Furthermore, the reusability of PNT-1 as a luminescent probe for detecting S2− was also studied. As shown in Fig. S17 (ESI†), the original luminescence intensity of PNT-1 after a series of washing and filtration shows basically no change, and the quenching efficiency for sensing S2− also remains almost the same after repeated usage, suggesting excellent reusability of PNT-1.
In order to confirm the applicability of the PNT-1 material in the actual environment, we assembled PNT-1 into a luminescent test paper. Subsequently, we exposed the luminescent sensing properties of the test paper for different gas atmospheres (e.g. H2S, SO2, NO2, NH3, N2, CO2, CO, H3PO4, H2SO4, ethylic acid, etc.). As shown in Fig. 9, H2S gas can apparently quench the luminescence of the PNT-1 test paper, while other gases (e.g. NO2, NH3, N2, CO2, H3PO4, H2SO4, ethylic acid, etc.) cannot, indicating that the assembled PNT-1 luminescent test paper can be used for selective detection of H2S gas in realistic atmosphere. It is worth mentioning that the process of test paper for sensing H2S gas is in real-time, and the response can be identified by the naked eye under a UV lamp.
To extend its practical application in living cells, we also studied the luminescent sensing property of PNT-1 for detecting S2− in simulated living cells, in which we used PBS buffer solution as the simulated environment of the living cells, and performed a series of measurements about the luminescent sensing property of PNT-1 for detecting S2−in vitro assay. The specific experimental process is presented in the ESI† (Section S1). As shown in Fig. 10, the luminescence intensity of PNT-1 has no significant change after adding the analytes (F−, SO42−, Cl−, Br−, I−, IO3−, SCN−, HSO3−, PO43−, NO3−, CO32−, Cys and Vc), while the luminescent quenching still occurs after adding S2−, which suggests that the PNT-1 as a luminescent probe is still applicable for sensing S2− in simulated living cells. The observation greatly extends the possibility of PNT-1 as efficient luminescent sensors for sensing H2S in practical applications.
4. Conclusions
In summary, we have synthesized a luminescent porous organic polymer nanotubes (PNT-1) material through copolymerization of the monomers of TBT and 2,6-DP. Luminescence measurement suggested that PNT-1 can be used as a luminescent probe for selective sensing of H2S. Furthermore, we used a series of characterizations to study the luminescence quenching mechanism. All the results indicated that the formation of N–S bond between PNT-1 and H2S makes the electron in PNT-1 more stable and therefore leads to the luminescence quenching effect of PNT-1 for sensing H2S. Moreover, we found that the PNT-1 as a luminescent probe is still applicable for sensing H2S in vitro assay system simulated using PBS buffer solution, and the PNT-1-based test paper can definitely achieve the selective sensing of H2S gas in real-time.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Science Fund for Distinguished Young Scholars (No. 21625601) and the Major Project of NSF of China (No. 91334203) and Outstanding Talent Fund from BUCT.References
- C. A. Wagner, J. Nephrol., 2009, 22, 173–176 CAS.
- B. D. Paul and S. H. Snyder, Nat. Rev. Mol. Cell Biol., 2012, 13, 499–507 CrossRef CAS PubMed.
- M. M. Gadalla and S. H. Snyder, J. Neurochem., 2010, 113, 14–26 CrossRef CAS PubMed.
- E. Blackstone, M. Morrison and M. B. Roth, Science, 2005, 308, 518 CrossRef CAS PubMed.
- G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu, S. Zhang, S. Snyder and R. Wang, Science, 2008, 322, 587–590 CrossRef CAS PubMed.
- Y. Kaneko, Y. Kimura, H. Kimura and I. Niki, Diabetes, 2006, 55, 1391–1397 CrossRef CAS PubMed.
- L. Dudragne, Ph. Adam and J. Amouroux, Appl. Spectrosc., 1998, 52, 1321–1327 CrossRef CAS.
- J. E. Doeller, T. S. Isbell, G. Benavides, J. Koenitzer, H. Patel, R. P. Patel, L. J. Jr, V. M. Darley-Usmar and D. W. Kraus, Anal. Biochem., 2005, 341, 40–51 CrossRef CAS PubMed.
- J. Radfordknoery and G. A. Cutter, Anal. Chem., 1993, 65, 976–982 CrossRef CAS.
- D. Tang and P. H. Santschi, J. Chromatogr. A, 2000, 883, 305–309 CrossRef CAS PubMed.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- G. Li, Y. Chen, J. Wang, Q. Lin, J. Zhao, L. Ji and H. Chao, Chem. Sci., 2013, 4, 4426–4433 RSC.
- S. Liu, L. Zhang, T. Yang, H. Yang, K. Y. Zhang, X. Zhao, W. Lv, Q. Yu, X. Zhang and Q. Zhao, ACS Appl. Mater. Interfaces, 2014, 6, 11013–11017 CAS.
- P. Wu, J. Wang, C. He, X. Zhang, Y. Wang, T. Liu and C. Duan, Adv. Funct. Mater., 2012, 22, 1698–1703 CrossRef CAS.
- C. Liu, J. Pan, S. Li, Y. Zhao, L. Y. Wu, C. E. Berkman, A. R. Whorton and M. Xian, Angew. Chem., Int. Ed., 2011, 123, 10511–10513 CrossRef.
- M. Wang, L. Guo and D. Cao, Sci. China: Chem., 2017, 60, 1090–1097 CrossRef CAS.
- S. Liu, Z. Xiang, Z. Hu, X. Zheng and D. Cao, J. Mater. Chem., 2011, 21, 6649–6653 RSC.
- Y. Y. Cao, X. F. Guo and H. Wang, Sens. Actuators, B, 2017, 243, 8–13 CrossRef CAS.
- Y. Zhang, M. Zhao and D. Chao, Sens. Actuators, B, 2016, 17, 109–114 Search PubMed.
- J. Peng, C. L. Teoh, X. Zeng, A. Samanta, L. Wang, W. Xu, D. Su, L. Yuan, X. Liu and Y. T. Chang, Adv. Funct. Mater., 2016, 26, 191–199 CrossRef CAS.
- X. Liu, B. Du, Y. Sun, M. Yu, Y. Yin, W. Tang, C. Chen, L. Sun, B. Yang and W. Cao, ACS Appl. Mater. Interfaces, 2016, 8, 16379–16385 CAS.
- K. Maiti, A. K. Mahapatra, R. Maji, S. Mondal, S. S. Ali, A. Gangopadhyay, S. K. Manna and S. Mandal, ChemistrySelect, 2016, 1, 5066–5073 CrossRef.
- B. Xiong, R. Zhou, J. Hao, Y. Jia, Y. He and E. S. Yeung, Nat. Commun., 2013, 4, 1708 CrossRef PubMed.
- X. Zhang, Q. Hu, T. Xia, J. Zhang, Y. Yang, Y. Cui, B. Chen and G. Qian, ACS Appl. Mater. Interfaces, 2016, 8, 32259–32265 CAS.
- K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003–18005 CrossRef CAS PubMed.
- M. Tropiano and S. Faulkner, Chem. Commun., 2014, 50, 4696–4698 RSC.
- C. Yu, X. Li, F. Zeng, F. Zheng and S. Wu, Chem. Commun., 2013, 49, 403–405 RSC.
- B. Liu and Y. Chen, Anal. Chem., 2013, 85, 11020–11025 CrossRef CAS PubMed.
- X. Tian, Z. Dong, R. Wang and J. Ma, Sens. Actuators, B, 2013, 183, 446–453 CrossRef CAS.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 125, 1732–1735 CrossRef.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, Angew. Chem., 2009, 121, 508–511 CrossRef.
- R. Banerjee, G. Das, B. P. Biswal, G. K. Sharah, V. Venkatesh, M. Addicoat, T. Heine, G. Kaur and S. Verma, Chem. Sci., 2015, 6, 3931–3939 RSC.
- S. Y. Ding, M. Dong, Y. W. Wang, Y. T. Chen, H. Z. Wang, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed.
- S. Dalapati, S. Jin, J. Gao, Y. Xu, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2013, 135, 17310–17313 CrossRef CAS PubMed.
- Z. Li, Y. Zhang, H. Xia, Y. Mu and X. Liu, Chem. Commun., 2016, 52, 6613 RSC.
- Z. Xiang, R. Mercado, J. M. Huck, H. Wang, Z. Guo, W. Wang, D. Cao, M. Haranczyk and B. Smit, J. Am. Chem. Soc., 2015, 137, 13301–13307 CrossRef CAS PubMed.
- L. Guo, D. Cao, J. Yun and X. Zeng, Sens. Actuators, B, 2017, 243, 753–760 CrossRef CAS.
- N. Sang, C. Zhan and D. Cao, J. Mater. Chem. A, 2015, 3, 92–96 CAS.
- L. Guo, X. Zeng and D. Cao, Sens. Actuators, B, 2016, 226, 273–278 CrossRef CAS.
- Z. Xiang and D. Cao, Macromol. Rapid Commun., 2012, 33, 1184–1190 CrossRef CAS PubMed.
- L. Guo and D. Cao, J. Mater. Chem. C, 2015, 3, 8490–8494 RSC.
- G. Yu, S. Yin, Y. Liu, J. Chen, X. Xu, X. Sun, D. Ma, X. Zhan, Q. Peng and Z. Shuai, J. Am. Chem. Soc., 2005, 127, 6335–6346 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740–1741 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qm00423k |
| ‡ Equally contributed to this work. |
| This journal is © the Partner Organisations 2017 |