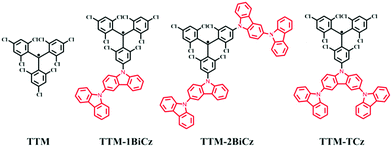
Multicarbazolyl substituted TTM radicals: red-shift of fluorescence emission with enhanced luminescence efficiency†
Shengzhi
Dong
 a,
Ablikim
Obolda
a,
Ablikim
Obolda
 a,
Qiming
Peng
a,
Qiming
Peng
 a,
Yadong
Zhang
b,
Seth
Marder
*b and
Feng
Li
a,
Yadong
Zhang
b,
Seth
Marder
*b and
Feng
Li
 *a
*a
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Qianjin Avenue 2699, Changchun, 130012, P. R. China. E-mail: lifeng01@jlu.edu.cn
bCenter for Organic Photonics and Electronics and School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332-0400, USA. E-mail: seth.marder@chemistry.gatech.edu
First published on 31st July 2017
Abstract
A series of multicarbazolyl substituted tris(2,4,6-trichlorophenyl)-methyl (TTM) radical derivatives were synthesized. Red-shifts of the fluorescence emission of the TTM radicals were achieved along with enhanced luminescence efficiency through incorporating substituent groups with strong electron donating ability as well as restricting the rotation of the outer groups. The photostability of the radicals was also significantly enhanced via the incorporation of substituent groups. This work provides a new approach to realize NIR-emitting radicals with high luminescence efficiency.
In recent years, near-infrared (NIR) emissive organic materials have attracted more and more attention because of their potential application in organic light-emitting diodes (OLEDs),1,2 for bioimaging,3,4 in optical telecommunication systems,5,6etc. Traditional organic NIR emissive molecules can be divided into three categories: small organic molecules,2,7,8 polymers1,9,10 and organometallic compounds.11–13 Small molecules are attractive owing to the ability to produce them in a highly pure (monodisperse) state and from inexpensive, earth abundant materials. The design and synthesis of NIR emitters is intrinsically challenging because the small energy gap between the ground and excited states is usually accompanied by efficient non-radiative decay pathways, according to the energy gap law, which tends to lead to low quantum yields for emission.7,14 Luminescent organic neutral radicals as red and NIR emitters are potentially interesting because in some cases they can exhibit a high fluorescence efficiency whose maximum emission can be tuned by appropriate modifications of the chemical structure.15–17
To date, stable luminescent radicals have been divided into three series: perchlorotriphenyl methyl (PTM) radical derivatives,18,19 tris(2,4,6-trichlorophenyl)methyl (TTM) radical derivatives.20,21 and pyridyl-containing triarylmethyl (PyBTM) radicals.22–24 Substantial efforts have been extended to red-shift the emission of radicals. Lambert et al. have synthesized some triphenylamine substituted PTM radical (PTM-TPA) derivatives. They found that the electron donating ability of a triphenylamine group leads to the red-shift of the fluorescence emission of the PTM radicals.19,25 Juliá and coworkers have reported some carbazole substituted TTM radical (TTM-1Cz) derivatives and reached similar conclusions.26,27 We have also examined the relationship between substituent groups and maximum emission wavelengths.17 In general, the red-shift of the emission is accompanied by a significant decrease in the photoluminescence quantum yield (PLQY). Taking PTM-TPA radicals as an example, when the groups connected to the triphenylamine change from chlorine (Cl) to methyl (Me), the maximum emission wavelength shifts from 763 nm to 885 nm but the PLQY reduces sharply from 0.38 to 0.04. In this work, we incorporate multicarbazolyl with a TTM radical to construct three new TTM radical derivatives, namely TTM-1BiCz, TTM-2BiCz and TTM-TCz, as shown in Fig. 1. Multicarbazolyls were selected because of their stronger electron donating ability compared to carbazolyl which may cause a larger bathochromic-shift. It is noted that the emission of TTM-TCz shows the largest bathochromic shift but also possesses the highest PLQY among the three compounds. This phenomenon was systematically studied and some insights into the design of radicals with bathochromic emission as well as high PLQY were obtained.
All TTM radical precursors were prepared via a C–N coupling reaction between tris(2,4,6-trichlorophenyl)methyl (HTTM) and multicarbazolyl (S1, ESI†).15,21 The radicals were characterized by FTIR spectroscopy and MALDI-TOF mass spectrometry, and the existence of an unpaired electron was confirmed using electron paramagnetic resonance (EPR) spectra.
The photophysical properties of the radicals were measured in cyclohexane (Fig. 2) and are listed in Table 1. The UV-vis, photoluminescence (PL) and fluorescent decay spectra reveal that incorporating multicarbazolyl into TTM leads to red-shift of the long-wavelength absorption as well as the PL emission and longer fluorescence lifetime. This suggests that incorporation of different electron-donating groups results in various degrees of bathochromic shift, since TTM-2BiCz (681 nm) and TTM-TCz (683 nm) possess more bathochromic fluorescence than TTM-1BiCz (671 nm). Furthermore, the UV-vis and PL spectra of the TTM radicals in different solvents were also measured (Fig. S3 and S4, ESI†) and the data are summarized in Table S1 (ESI†). The results indicate that the PL emission position of TTM changes little in different solvents, while obvious red-shift occurs and the emission intensity decreases sharply as the polarity of the solvents increases for the substituted radicals. This phenomenon is attributed to the charge transfer (CT) between TTM and multicarbazolyls, which could be confirmed by density functional theoretical (DFT) calculations described hereinafter.
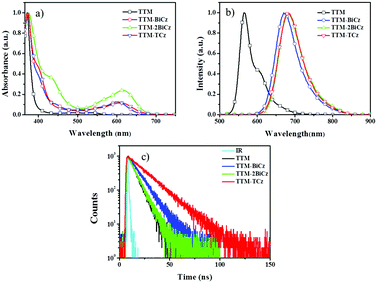 | ||
| Fig. 2 Photophysical spectra of the TTM radical derivatives in cyclohexane: (a) UV-vis; (b) PL; (c) fluorescence lifetime (concentration around 10−4 mol L−1, excited at around 370 nm). | ||
| Adduct | λ abs (nm) | λ em (nm) | τ (ns) | Φ f | f (D1 → D0) | p (Debye) | k f (×106 s−1) | k nr (×106 s−1) |
|---|---|---|---|---|---|---|---|---|
| TTM | 371, 540 | 565 | 6.54 | 0.008 | 0.022 | 0.003 | 1.22 | 152 |
| TTM-1BiCz | 373, 400, 601 | 671 | 9.86 | 0.17 | 0.030 | 7.70 | 17.2 | 84.2 |
| TTM-2BiCz | 374, 400, 617 | 681 | 7.12 | 0.11 | 0.044 | 7.39 | 15.4 | 125 |
| TTM-TCz | 373, 435, 612 | 689 | 17.2 | 0.26 | 0.040 | 7.33 | 15.1 | 43.0 |
The electronic structures of the TTM radicals in the ground state were calculated using DFT (UB3LYP/6-31G(d)) (Fig. 3). Time-dependent DFT (TD-DFT) (UB3LYP/6-31G(d)) results indicate that the fluorescence of the substituted radicals arises from the transition of a D1 excited state, which is marked with red arrows in Fig. 3, since the D1 excited state arises from the highest occupied β orbital (β-HOMO) to the lowest unoccupied β orbital (β-LUMO) (S5, ESI†). Taking TTM-1BiCz for an example, the TD-DFT results show that the first excited state is almost formed by the transition from the β-HOMO to β-LUMO (214B–>215B) (S5, page S21, ESI†). The CT characters of the substituted radicals are confirmed since the electronic distribution is separated between the β-LUMO and β-HOMO.
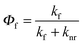 | (1) |
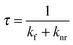 | (2) |
 | ||
| Fig. 3 Molecular orbitals of the TTM radicals in the ground state using DFT methods (UB3LYP/6-31G(d)). (a) TTM, (b) TTM-1BiCz, (c) TTM-2BiCz, and (d) TTM-TCz. | ||
The PLQYs (Φf) of the substituted radicals were measured through excitation at their maximum absorption at around 370 nm in cyclohexane solution and compared with the TTM radical, as shown in Table 1. The results show that TTM-TCz has the highest Φf = 0.26, TTM-1BiCz follows (Φf = 0.17) and TTM-2BiCz is the last (Φf = 0.11); these values are 14 to 33 times higher than that of the unsubstituted TTM radical (Φf = 0.008) under the same conditions. It is very interesting that the PLQY of TTM-TCz is much higher than that of TTM-1BiCz despite the fact that the emission of TTM-TCz is more bathochromic than that of TTM-1BiCz. Hence, to better understand the reasons, the radiative rate constants (kf) and non-radiative rate constants (knr) of the radicals were calculated using the basic photophysical eqn (1) and (2), listed in Table 1. The data demonstrate that both the lower knr and higher kf are the reasons why the PLQYs of the multicarbazolyl substituted TTM radicals are much higher than that of the unsubstituted TTM radical. Moreover, TTM-1BiCz possesses a lower knr compared to TTM-2BiCz. Furthermore, the knr of TTM-TCz is only about half that of TTM-1BiCz, which seems to be the determining factor for the much higher luminescent efficiency observed for TTM-TCz, although the kf of the latter is slightly greater than the former. kf is found to be closely related to the dipole moment (p) of the molecules in the ground state,28 which can be obtained from the results of DFT and is summarized in Table 1. The values of the dipole moment of the substituted TTM radicals are much larger than that of the TTM radical, consistent with the much larger kf of the substituted TTM radicals. In addition, only small differences of p can be observed when the substituted TTM radicals are compared to each other and the variation tendency of the value agrees with that of kf. The oscillator strengths (f) of the transition from the first excited state (β-LUMO → β-HOMO) to the ground state of the substituted TTM radicals were calculated by TD-DFT (UB3LYP/6-31G(d)) and the results vary from 0.03 to 0.04 (Table 1). Therefore, the enhanced luminescence efficiency is mainly related to the big difference of knr, which means the non-radiative deactive routes of TTM-TCz are more efficiently suppressed.
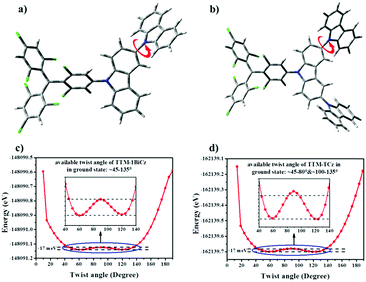
To confirm this hypothesis, the twist potentials of the ground state of TTM-1BiCz and TTM-TCz were calculated by DFT (UB3LYP/6-31G(d)). The outer carbazolyls were supposed to rotate around the other parts of the radicals from 0 to 180° for each 5° (Fig. 4a and b), and the relationships between molecular potential energy and twist angle are shown in Fig. 5c and d. The results show that the available twist angle of TTM-1BiCz in the ground state is about 45–135° under the conditions that the range of the molecular potential energy is limited to 17 meV, while twists of only around 45–80° and 100–135° are allowed for TTM-TCz under the same conditions. In other words, the rotation of TTM-TCz is more restricted than that of TTM-1BiCz because of the incorporation of one more carbazolyl, which leads to the smaller knr of TTM-TCz. Therefore, the restriction of the rotation of outer carbazolyls is the main factor for TTM-TCz with the highest luminescence efficiency among the three compounds, since TTM-TCz possesses more rotatable conjugated groups (one more calbazolyl) but lower knr compared to TTM-1BiCz, and more rotatable conjugated groups are usually supposed to provide more pathways for the non-radiative transition.
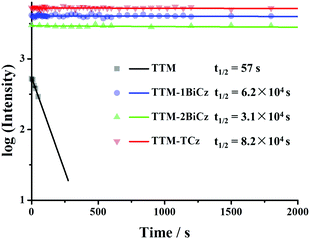 | ||
| Fig. 5 Comparison of photostabilities of TTM radicals under 355 nm pulse laser irradiation (concentration around 10−4 mol L−1 in deoxygenated cyclohexane solution). | ||
In addition to enhanced luminescence efficiency, high stability is essential in consideration of the utility of these molecules for potential applications. Thus the photostabilities of the TTM radicals in deoxygenated cyclohexane solution were tested by using a pulse laser at 355 nm (pulse width: 8 ns, frequency: 10 Hz) with the power intensity of 98.1 kW cm−2 (Fig. 5). As the figure shows, upon irradiation of the laser, the emission intensity of unsubstituted TTM decreased sharply and the approximated half-life (t1/2) of TTM is only 57 s, while for the substituted TTM radicals, barely any changes can be observed after irradiation for several hours. Consequently, the t1/2 of TTM-BiCz, TTM-2BiCz and TTM-TCz is 6.2 × 104 s, 3.1 × 104 s and 8.2 × 104 s, respectively, which is 540 to 1440 times longer than that of TTM. The results suggest that the introduction of multicarbazolyl greatly improves the photostabilities of the TTM radicals. We speculate that the different electron distribution of the excited state induces the improvement in photostability. Because the excited states of unsubstituted TTM radicals are localized pi–pi* states (Fig. 3(a)), photocyclisation occurs upon irradiation. However, the excited states are changed to CT states by the introduction of multicarbazolyl groups (Fig. 3(b)–(d)), thus the possibility of photocyclisation decreases.
Conclusions
In conclusion, several new luminescent multicarbazolyl substituted TTM radical derivatives were designed and synthesized. In comparison with the unsubstituted TTM radical, greatly improved PLQYs and significantly enhanced photostabilities of the substituted radicals were achieved. Above all, the emission of TTM-TCz shows the largest bathochromic shift and simultaneously possesses the highest PLQY. We suggest that the restricted rotation of outer carbazolyls contributes to the increased PLQY for TTM-TCz. The results of this work suggest that incorporation of substituent groups with strong electron donating ability as well as restricted rotation of the outer groups is an important factor to realize NIR-emitting radicals with high luminescence efficiency.Acknowledgements
We are grateful for the financial support from the National Key R&D Program of China (Grant No. 2016YFB0401001), the National Key Basic Research and Development Program of China (973 program, Grant No. 2015CB655003) founded by MOST, and the National Natural Science Foundation of China (Grant No. 51673080).References
- N. Tessler, V. Medvedev, M. Kazes, S. Kan and U. Banin, Science, 2002, 295, 1506–1508 CrossRef PubMed.
- L. Yao, S. Zhang, R. Wang, W. Li, F. Shen, B. Yang and Y. Ma, Angew. Chem., 2014, 126, 2151–2155 CrossRef.
- Y. Gabe, Y. Urano, K. Kikuchi, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2004, 126, 3357–3367 CrossRef CAS PubMed.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- S. Ando, J. Photopolym. Sci. Technol., 2004, 17, 219–232 CrossRef CAS.
- J.-C. G. Bünzli and S. V. Eliseeva, J. Rare Earths, 2010, 28, 824–842 CrossRef.
- T. Liu, L. Zhu, C. Zhong, G. Xie, S. Gong, J. Fang, D. Ma and C. Yang, Adv. Funct. Mater., 2017, 27, 1606384 CrossRef.
- X. Han, Q. Bai, L. Yao, H. Liu, Y. Gao, J. Li, L. Liu, Y. Liu, X. Li and P. Lu, Adv. Funct. Mater., 2015, 25, 7521–7529 CrossRef.
- V. Medvedev, M. Kazes, S. Kan, U. Banin, Y. Talmon and N. Tessler, Synth. Met., 2003, 137, 1047–1048 CrossRef CAS.
- B. C. Thompson, L. G. Madrigal, M. R. Pinto, T. S. Kang, K. S. Schanze and J. R. Reynolds, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 1417–1431 CrossRef CAS.
- C. Borek, K. Hanson, P. I. Djurovich, M. E. Thompson, K. Aznavour, R. Bau, Y. Sun, S. R. Forrest, J. Brooks and L. Michalski, Angew. Chem., 2007, 119, 1127–1130 CrossRef.
- K. R. Graham, Y. Yang, J. R. Sommer, A. H. Shelton, K. S. Schanze, J. Xue and J. R. Reynolds, Chem. Mater., 2011, 23, 5305–5312 CrossRef CAS.
- O. Fenwick, J. K. Sprafke, J. Binas, D. V. Kondratuk, F. Di Stasio, H. L. Anderson and F. Cacialli, Nano Lett., 2011, 11, 2451–2456 CrossRef CAS PubMed.
- J. V. Caspar, E. M. Kober, B. P. Sullivan and T. J. Meyer, J. Am. Chem. Soc., 1982, 104, 630–632 CrossRef CAS.
- Q. Peng, A. Obolda, M. Zhang and F. Li, Angew. Chem., Int. Ed., 2015, 54, 7091–7095 CrossRef CAS PubMed.
- A. Obolda, X. Ai, M. Zhang and F. Li, ACS Appl. Mater. Interfaces, 2016, 35472–35478 CAS.
- Y. Gao, A. Obolda, M. Zhang and F. Li, Dyes Pigm., 2017, 139, 644–650 CrossRef CAS.
- M. Ballester, J. Riera-Figueras and A. Rodriguez-Siurana, Tetrahedron Lett., 1970, 11, 3615–3618 CrossRef.
- A. Heckmann, S. Dümmler, J. Pauli, M. Margraf, J. Köhler, D. Stich, C. Lambert, I. Fischer and U. Resch-Genger, J. Phys. Chem. C, 2009, 113, 20958–20966 CAS.
- O. Armet, J. Veciana, C. Rovira, J. Riera, J. Castaner, E. Molins, J. Rius, C. Miravitlles, S. Olivella and J. Brichfeus, J. Phys. Chem., 1987, 91, 5608–5616 CrossRef CAS.
- V. Gamero, D. Velasco, S. Latorre, F. López-Calahorra, E. Brillas and L. Juliá, Tetrahedron Lett., 2006, 47, 2305–2309 CrossRef CAS.
- Y. Hattori, T. Kusamoto and H. Nishihara, Angew. Chem., Int. Ed. Engl., 2015, 54, 3731–3734 CrossRef CAS PubMed.
- Y. Hattori, T. Kusamoto and H. Nishihara, RSC Adv., 2015, 5, 64802–64805 RSC.
- Y. Hattori, T. Kusamoto, T. Sato and H. Nishihara, Chem. Commun., 2016, 52, 13393–13396 RSC.
- A. Heckmann, C. Lambert, M. Goebel and R. Wortmann, Angew. Chem., Int. Ed., 2004, 43, 5851–5856 CrossRef CAS PubMed.
- S. Castellanos, D. Velasco, F. López-Calahorra, E. Brillas and L. Julia, J. Org. Chem., 2008, 73, 3759–3767 CrossRef CAS PubMed.
- D. Velasco, S. Castellanos, M. López, F. López-Calahorra, E. Brillas and L. Juliá, J. Org. Chem., 2007, 72, 7523–7532 CrossRef CAS PubMed.
- S. Dong, W. Xu, H. Guo, W. Yan, M. Zhang and F. Li, Effects of Substituents on Luminescent Efficiency of Stable Triaryl Methyl Radicals, 2017, under review.
Footnote |
| † Electronic supplementary information (ESI) available: MS and FTIR spectra; electron paramagnetic resonance (EPR) spectra; additional photophysical and electroluminescent properties; DFT and TD-DFT calculation data. See DOI: 10.1039/c7qm00273d |
| This journal is © the Partner Organisations 2017 |